Introduction
The human leukocyte antigen-E (HLA-E) is a non-classical HLA class I gene [1], which has been located between HLA-A and HLA-C loci on chromosome 6 P21.3 [2, 3]. Like other HLA class Ib molecules (HLA-F, -G), HLA-E is distinguished from the classical Ia molecules (HLA-A, -B, -C) in terms of low genetic diversity, low cellular expression and limited tissue distribution [4, 5]. The force of natural selection that led to the low diversity of HLA-E in the process of evolution, is still unknown. Despite the wide transcription of HLA-E in all human tissues [6], the protein expression of this molecule is often limited to endothelial cells (from non-lymphoid organs), T lymphocytes, B lymphocytes, monocytes, macrophages and the trophoblastic tissues in relation to the maternal-fetal tolerance [7- 10]. HLA-E plays a role as a modulator of NK cell activity through interaction with CD94/ NKG2A receptors. On the other hand, this molecule acts as an antigen-presenting molecule and accelerate both innate and adaptive immunity [11, 12].
So far, 25 alleles of HLA-E have been described in the literature (IMGT/HLA Database) [13]. In general, three allelic groups of HLA-E have been defined as different populations, including HLA-E*01:01, E*01:03 and E*01:04 [14]. The differences of these alleles are only in one amino acid, which can influence on the tendency of HLA molecules for binding to peptides [15].The existence of HLA-E*01:04 has not been proven by recent studies [6, 14, 16]. In fact, only the existence of HLA-E*01:01 and HLA-E*01:03 alleles have been proven in all populations [17, 18]. The allele frequencies of HLA-E*01:01 and HLA-E*01:03 are dependent on the ethnicity and the geographical location of the populations [19]. HLA-E*01:01 and HLA-E*01:03 differ by amino acid substitution at codon number 107 in exon 3. The codon number 107 encodes arginine and glycine in HLA-E*0101 and in HLA-E*0103, respectively. These two alleles differ in the surface expression levels, the peptide binding affinity and the stability of the molecule in particular, their thermal stability [2, 20]. A number of studies have shown the relationship between HLA-E alleles and some diseases such as recurrent spontaneous abortion [1, 21], autoimmune diseases such as Behcet's disease [20, 22], Ankylosing spondylitis [23], autoimmune diabete [24], varients of cancer, including cancer of the nose and throat, lymphomas, melanomas, glioblastomas, colorectal and kidney cancer [6, 25, 26], transplantation [27] and type I diabetes [28-30]. Impaired humoral and cell-mediated immunity have been shown in diabetic and hyperglycemia subjects [29]. Also at diabetic patients, different pathways include triacylglyceride synthesis, proteasome degradation, statin pathway, fluoropyrimidine activity, pathogenic infection, adipogenesis and ampk signaling have been changed. Defects in these pathways are associated with some genes such as HLA-B, HLA-E, HLA-J [30]. Diabetes causes high blood sugar levels either due to a lack of insulin (type 1 diabetes) or lack of proper response to insulin. In prediabetes, it is usually due to the cells not responding correctly. In type 2 diabetes, it is usually a combination. Although limited studies have been conducted on the relationship between HLA alleles and type I diabetes [28, 30], but no studies have been done on type 2 diabetes.
The aim of this study was to investigate the frequencies of HLA-E*01:01 and HLA-E*01:03 in a healthy population and in high blood sugar subjects of Iran to survey the possible association between HLA-E alleles and hyperglycemia.
Materials and Methods
Preparation of the samples
Based on the approval of the Medical Ethics Committee of Tarbiat Modares University (reference number; 5192/52 on date 2014), whole blood were prepared from 137 randomly selected Iranian healthy individuals and 86 patients with high blood sugar (fasting blood sugar (FBS) mean= 182.48±47.9 mg/dl) referred to Iranian Blood Transfusion Organization (IBTO). The demographic data of the studied groups were presented in table 1 and diagram 1.
Table 1. Demographic characteristics
| Variable |
Healthy group (n=137) |
High blood sugar group (n=86) |
| Age (yr)* |
46.96±12.94 |
54.81±10.17 |
| Sex |
Male, No. (%) |
58 (42.4%) |
40 (46.5%) |
| Female, No. (%) |
79 (57.6%) |
46 (53.5%) |
*Data presented as Mean±SD
.PNG) DNA extraction and the polymerase chain reaction-sequence specific primer (PCR- SSP)
DNA extraction and the polymerase chain reaction-sequence specific primer (PCR- SSP)
DNA extraction was performed using salting out method and the concentration and purity of the extracted DNA were examined using a Nanodrop spectrophotometer (WPA, UK). Additionally, to ensure the integrity of the DNA molecules and the absence of smears, electrophoresis was carried out on a 1.5% agarose gel. In this study, PCR-SSP and polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) were used to identify the alleles and resolve the ambiguities. PCR reactions were carried out by using 300-500 ng of genomic DNA in a total volume of 50 µl of the reaction mixture containing 0.4 µM of primers for HLA-E*01:01 (exon 3: forward: 5'- GCGAGCTGGG GCCCGTCA -3', reverse: 5'-CCGCCTCAG AGGCATCATTTG-3') and for HLA-E*01:03 primers (exon 3: forward: 5'-TGCGAGCTGGG GCCCGGCG-3', reverse: 5'-CCGCCTCAGA GGCATCATTTG-3'). It is of note that we used primers that have an extra mismatch at 3' terminal. This additional mismatch increases high powerfulness and specificity for intended HLA-E alleles amplification (primers adopted from Liu et al.) [3]. Other ingredients included 1x reaction buffer (Roche, Mannheim, Germany), 2.1 mM MgCL2, 200 µM dNTP and 2.5 U of Taq DNA polymerase (Genet Bio, Korea). The PCR-cycling conditions were as follows: initial denaturation at 98ºC for 5 min., followed by 34 cycles of 96ºC for 40 s, 60ºC for 25 s and 68ºC for 30 s and The program was followed by a final extension step at 72ºC for 5 min All PCR reactions were performed with a thermo cycler machine (Corbett-UK)).
Multiplex PCR-SSP
To ensure accuracy of the results, a multiplex PCR-SSP was used for all the PCR reactions in the presence of beta-actin gene as an internal control with the following primers
: forward: 5'-TCAACCCTACAGTCACCCAT-3' and reverse: 5'-CTACAATTACGAACAGCATTGAG-3.
' PCR reaction medium consisted of 0.4 µM HLA-E*01:01 or 0.4 µM HLA-E*01:03 primers accompanied with 0.2 µM of beta-actin primers, 1x reaction buffer (Roche, Mannheim, Germany), 2.1 mM MgCL2, 200 µM dNTP and 2.5 U of Taq DNA polymerase. According to the primers used in this study, PCR bands with the size of 500 bp, 159 bp and 160 bp were expected for β-actin (as internal control), HLA-E*01:01 and HLA-E*01:03, respectively. The annealing temperature was determined primarily due to the melting temperature of the primers and subsequently adjusted by the experiments.
The PCR-cycling conditions were as follows: initial denaturation at 96ºC for 5 min., followed by 10 cycles of 94ºC for 15s, 62ºC for 30 s and 68ºC for 1 min and another 33 cycles of 94ºC for 30s, 60ºC for 25s and 68ºC for 30s. The program was followed by a final extension step at 72ºC for 6 min. Finally, the PCR products were visualized using electrophoresis on 1.5 % agarose gel included ethidium bromide as a fluorescent DNA intercalator. The sizes of the PCR products were determined using a 50 bp or 100 bp DNA ladder by electrophoresis.
PCR-RFLP
In PCR-RFLP method, a restriction enzyme cuts the amplified DNA. The size of the generated fragments shows the kind of the allele. A fragment of exon 3 was amplified by using primers; forward 5'- GGCTGC GAG CTG GGG CCC GCC -3
'[21] and reverse: 5'- CCGCCTCAGAGGCATCATTTG -3
' [3]. PCR reactions were performed in a volume of 50 µl consisted of 0.2 µM HLA-E primers, 1x reaction buffer (Roche, Mannheim, Germany), 2.1 mM MgCL2, 200 µM dNTP and 2.5 U of Taq DNA polymerase. The PCR-cycling conditions were as follows: initial denaturation at 98ºC for 5 min., followed by 36 cycles of 94ºC for 45s, 61ºC for 45s and 72ºC for 45s. The program was followed by a final extension step at 72ºC for 6 min. The final product of PCR (163 bp) was exposed to MspI (HpaII) restriction enzyme (10U/µl; Thermo Scientific) to cleave DNA at specific sites of exon 3. For each reaction, 15 µl of the PCR product was mixed with 2 µl Tango Buffer, 1 µl enzyme, and 13 µl distilled water and incubated at 37°C for 16 hours. Subsequently, the DNA fragments were separated by gel electrophoresis. Unbroken template bands (163 bp) showed HLA-E*01:01 and the broken fragments (20 and 143 bp) of PCR products indicated the HLA-E*01:03 allele.
Additionally, several samples with heterozygous and homozygous alleles of HLA-E were randomly selected for sequence based typing by Bioneer-Korea. The sequences were analyzed by Blast tool and the softwares of Chromas and Codon code were used to verify the type of HLA-E allele
.
Statistical analysis
The analysis was performed using SPSS 21 software. The statistical tests of c
2 and frequencies were used to analyze the data.
Results
PCR and PCR-SSP results
Diagnosis of HLA-E alleles was done by PCR method using the specific primers of HLA-E*01:01 and HLA-E*01:03. Positive and negative results can be seen in fig. 1A. Because of the uncertainty of the negative results, a multiplex PCR-SSP method was used in the presence of beta-actin primers as an internal control. The results of PCR-SSP were shown for HLA-E*01:01 and HLA-E*01:03 alleles (Fig. 1B, Fig. 1C & Fig. 2).

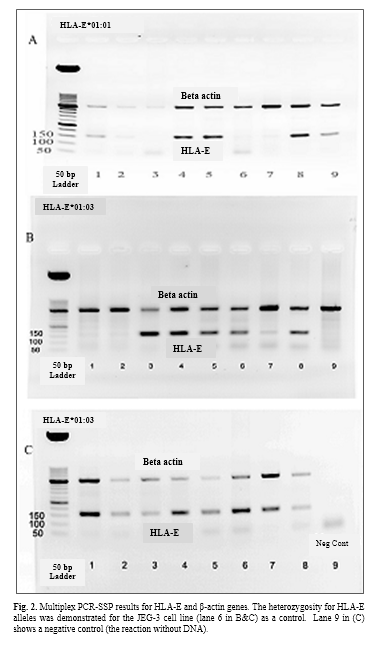 The polymorphism of HLA-E genotype and allele frequencies
The polymorphism of HLA-E genotype and allele frequencies
Gene and allele frequencies of HLA-E at both groups are presented in table 2. The relationship between two alleles of HLA-E gene was determined in healthy and high blood sugar subjects using SPSS and Chi-square test. The frequency of HLA-E*01:03 allele at healthy group was significantly higher (p=0.001, 95% CI= 1.212-1.523) than HLA-E*01:01 allele, but at high blood sugar group no significant differences were observed between two alles (p=0.115, 95% CI= 1.106-1.384).
PCR-RFLP and sequence based typing results
The presence of HpaII restriction site was identified on an amplified fragment of exon 3 of HLA-E gene. This enzyme cuts the HLA-E*0103 allele into two fragments, 143 and 20 bp fragments. The JEG-3 cell line was used as a control. Then, the results were interpreted and evaluated on gel electrophoresis images. The presence of both cut and uncut fragments indicated the presence of both HLA-E*01:01 and HLA-E*01:03 alleles in the sample whereas uncut single bond and cut single bound showed HLA-E*01:01 and HLA-E*01:03 alleles, respectively (Fig. 3).
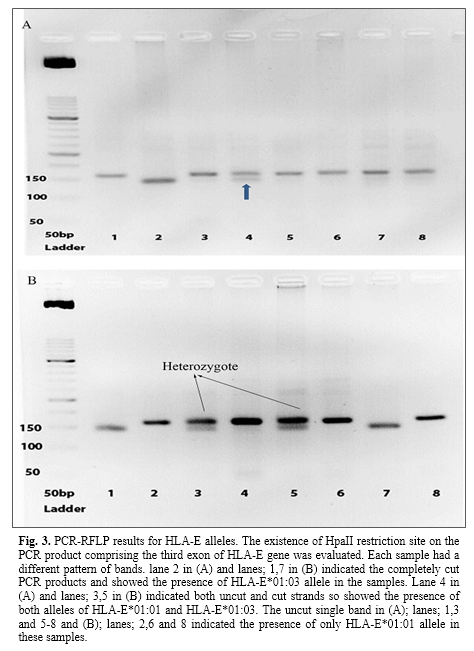
Additionally, the genotype study related to the nucleotide number 382 of exon 3 revealed that, at the healthy group the most frequent genotype was AG (56.93%) and GG (22.63%) and AA (20.44%) followed it, whereas in the higher blood sugar group, the frequent genotype was AG (68.6%) and AA (16.3%) and GG (15.1%) followed it.
Sequence alignment with HLA-E gene was shown by the Basic Local Alignment Search Tool. Furthermore, sequencing-based typing results of HLA-E were analyzed in heterozygous and homozygous samples by Chromas and Codon code softwares. Aligned DNA sequence chromatogram images are shown in fig. 4. The results of a sequence based typing confirmed the results of multiplex PCR-SSP method.
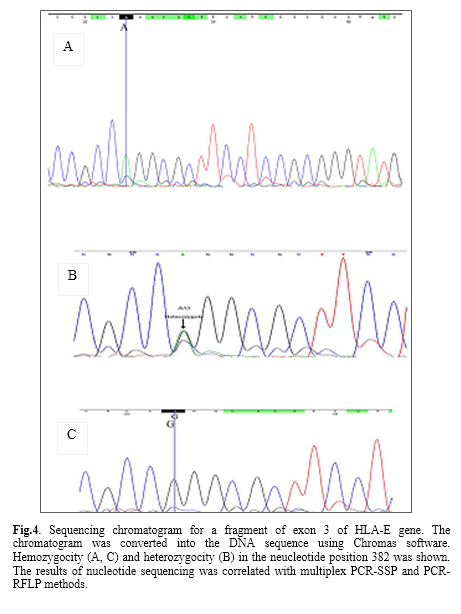 Table 2.
Table 2. The allelic and genotype frequencies of HLA-E in a population of Iran. The most frequent allele and genotype are shown
| High blood sugar group (n=86) |
Healthy group (n=137) |
Allele |
| No. (%) |
No. (%) |
| 87 (50.6) |
134 (48.9%) |
HLA-E*01:01 (nucleotide number 382 in exon 3: A) |
| 85 (49.4) |
140 (51.1) |
HLA-E*01:03 (nucleotide number 382 in exon 3: G) |
| Genotype |
| 14 (16.3) |
28 (20.44) |
AA |
| 59 (68.6) |
78 (56.93) |
AG |
| 13 (15.1) |
31 (22.63) |
GG |
Discussion
The HLA-E gene is important in the modulation of the immune system. The association of the polymorphism of HLA-E alleles and some diseases has been identified [22]. In this study, the polymorphism of the common alleles of HLA-E (HLA-E*01:01 and HLA-E*01:03) were studied in the healthy population and high blood sugar subjects of Iran.
Various methods have been used to investigate the polymorphism of HLA-E alleles such as PCR and sequencing [10], PCR-SSP [11], multiplex PCR [31], PCR-RFLP [32], PCR-SSCP [33] and PCR-SSOP [34]. In this study, a multiplex PCR-SSP technique was used as the main method, due to the specificity of primers. Additionally, PCR-RFLP method was used to resolve ambiguities.
We found that AG genotype (HLA-E*01:01/ HLA-E*01:03) was the most frequent at both normal and high blood sugar groups. In addition, in healthy group GG (HLA-E*01:03/ HLA-E*01:03) genotype was higher than AA. On the contrary, in high blood sugar group, AA (HLA-E*01:01 and HLA-E*01:01) genotype was frequent although no significant difference was observed. The prevalence of HLA-E*01:01 and HLA-E*01:03 alleles at healthy group were 48.9% and 51.1%, and in high blood sugar group were 50.6% and 49.4%, respectively. In comparing between two groups, no significant differences were observed. Despite the relationship between HLA-E expression with diabetes disease [28], our study did not show a relationship between the polymorphism of the studied alleles of HLA-E and high blood sugar.
The frequency of HLA-E alleles in the studied healthy population showed the similarity to the French (HLA-E*01:01=50% and HLA-E*01:03=50%) [31] and South Korean population (HLA-E*01:01=49.1% and HLA-E*01:03= 50.9%) [22]. Whereas, higher differences in HLA-E allele frequencies were reported in some other populations such as Japan (HLA-E*01:01=32% and HLA-E*01:03=68%) [16], southern Chinese Han population (HLA-E*01:01=32.29% and HLA-E*01:03=67.71%) [11] and Mexico (HLA-E*01:01=58.62% and HLA-E*01:03=36.49%) population [35]. It was known that the frequency of HLA alleles was associated with the ethnicity and the geographical location of the population, but the association of HLA-E alleles (HLA-E*01:01 and HLA-E*01:03) with ethnicity was not seen. For example, the results for African people (Zimbabwe) (HLA-E*01:01=50.93% and HLA-E*01:03=49.07%) [31] were similar to French and Korean people.
So, in some unrelated ethnic groups, such as France, Korea and Africa (Zimbabwe), the frequencies of these alleles were similar wherase in other close populations such as the Afro-Caribbean (HLA-E*01:01=53.3% and HLA-E*01:03=46.7%) [36] and Zimbabwe, these alleles were less similar. At present, the relationship between HLA-E alleles and the ethnicities can not be explained. The discovery of new alleles of HLA-E may make it possible to explain the association between HLA-E alleles and the ethnicities in different populations.
Conclusions
It was shown that HLA-E*01:03 was the frequent allele in Iranian population. The relationship between these alleles with high blood sugar was not statistically significant. However, in the high-blood sugar group, the HLA-E*01: 01 allele was more frequent. Our results showed similarities in HLA-E allele frequencies between Iranian healthy people and French and Korean population.
Conflict of Interest
There is no potential conflict of interest to declare.
Acknowledgements
This work was performed with a grant from Tarbiat Modares University. The authors wish to thank the Iranian Blood Transfusion Organization (Tehran, Iran) for the moral and technical support
.
References
[1]. Tripathi P, Naik S, Agrawal S. HLA-E and immunobiology of pregnancy. Tissue Antigens 2006; 67(3): 207-13.
[2]. Moscoso J, Serrano-Vela JI, Pacheco R, Arnaiz-Villena A. HLA-G, -E and –F: allelism, function and evolution. Transpl Immunol. 2006; 17(1): 61-4.
[3]. Liu XX, Pan FH, Tian W. Characterization of HLA-E polymorphism in four distinct populations in Mainland China. Tissue Antigens 2012; 80(1): 26-35.
[4]. Marín R, Ruiz-Cabello F, Pedrinaci S, Méndez R, Jiménez P, Geraghty DE, et al. Analysis of HLA-E expression in human tumors. Immunogenetics 2003; 54(11): 767-75.
[5]. Paul P, Rouas-Freiss N, Moreau P, Cabestre FA, Menier C, Khalil-Daher I, et al. HLA-G, -E, -F Preworkshop: Tools and Protocols for Analysis of Non-Classical Class I Genes Transcription and Protein Expression. Hum Immunol. 2000; 61(11): 1177-195.
[6]. Ravindranath MH, Pham T, El-Awar N, Kaneku H, Terasaki PI. Anti-HLA-E mAb 3D 12 mimics MEM-E/02 in binding to HLA-B and HLA-C alleles: Web-tools validate the immunogenic epitopes of HLA-E recognized by the antibodies. Mol Immunol. 2011; 48(4): 423-30.
[7]. Castelli EC, Mendes-Junior CT, Sabbagh A, Porto IO, Garcia A, Ramalho J, et al. HLA-E coding and 3' untranslated region variability determined by next-generation sequencing in two West-African population samples. Hum Immunol. 2015; 76(12): 945-53.
[8]. Kochan G, Escors D, Breckpot K, Guerrero-Setas D. Role of non-classical MHC class I molecules in cancer immunosuppression. Oncoimmunol. 2013; 2(11): e26491.
[9]. Coupel S, Moreau A, Hamidou M, Horejsi V, Soulillou JP, Charreau B. Expression and release of soluble HLA-E is an immunoregulatory feature of endothelial cell activation. Blood 2007; 109(7): 2806-814.
[10]. Moya-Quiles MR, Martínez-Escribano J, Guerra-Perez N, Muro M, Marín L, Campillo JA, et al. Lack of association between HLA-E polymorphism and primary cutaneous melanoma in Spanish patients. J Dermatol Sci. 2005, 40(1): 62-4.
[11]. Veiga-Castelli LC, Castelli EC, Mendes CTJR, da Silva WAJR, Faucher MC, Beauchemin K, et al. Non-classical HLA-E gene variability in Brazilians: a nearly invariable locus surrounded by the most variable genes in the human genome. Tissue Antigens 2012; 79(1): 15-24.
[12].
Iwaszko M,
Świerkot J,
Kolossa K,
Jeka S,
Wiland P,
Bogunia-Kubik K: Polymorphisms within the human leucocyte antigen-E gene and their associations with susceptibility to rheumatoid arthritis as well as clinical outcome of anti-tumour necrosis factor therapy.
Clin Exp Immunol. 2015; 182(3): 270-77.
[13]. Carvalho dos Santos L, Tureck LV, Wowk PF, Mattar SB, Gelmini GF, Magalhães JC, et al. HLA-E polymorphisms in an Afro-descendant Southern Brazilian population. Hum Immunol. 2013; 74(2): 199-202.
[14]. Grimsley C, Kawasaki A, Gassner C, Sageshima N, Nose Y, Hatake K, et al. Definitive high resolution typing of HLA-E allelic polymorphisms: Identifying potential errors in existing allele data. Tissue Antigens 2002; 60(3): 206-12.
[15]. Paquay MM, Schellekens J, Tilanus MG. A high-throughput Taqman approach for the discrimination of HLA-E alleles. Tissue Antigens 2009; 74(6): 514-19.
[16]. Zheng H, Lu R, Xie S, Wen X, Wang H, Gao X, et al. Human leukocyte antigen-E alleles and expression in patients with serous ovarian cancer. Cancer Sci. 2015; 106(5): 522-28.
[17]. Fürst D, Bindja J, Arnold R, Herr W, Schwerdtfeger R, Müller CH, et al. HLA-E polymorphisms in hematopoietic stem cell Transplantation. Tissue Antigens 2012; 79(4): 287-90.
[18]. Olieslagers TI, Voorter CE, Groeneweg M, Xu Y, Wieten L, Tilanus MG. New insights in HLA-E polymorphism by refined analysis of the full-length gene.
HLA 2017; 89(3): 143-49.
[19]. Fel´ıcio LP, Porto IOP, Mendes-Junior CT, Veiga-Castelli LC, Santos KE, Vianello-Brondani RP, et al. Worldwide HLA-E nucleotide and haplotype variability reveals a conserved gene for coding and 3' untranslated regions. Tissue Antigens 2014; 83(2): 82-93.
[20]. Hosseini E, Schwarer AP, Jalali A, Ghasemzadeh M. The impact of HLA-E polymorphisms on relapse following allogeneic hematopoietic stem cell transplantation. Leuk Res. 2013; 37(5): 516-19.
[21]. Mosaad YM, Abdel-Dayem Y, El-Deek BS, El-Sherbini SM. Association between HLA-E *0101 homozygosity and recurrent miscarriage in Egyptian women. Scand J Immunol. 2011; 74(2): 205-209.
[22]. Park KS, Park JS, Nam JH, Bang D, Sohn S, Lee ES. HLA-E*0101 and HLA-G*010101 reduce the risk of Behcet's disease. Tissue Antigens 2007; 69(2): 139-44.
[23]. Paladini F, Belfiore F, Cocco E, Carcassi C, Cauli A, Vacca A, et al. HLA-E gene polymorphism associates with ankylosing spondylitis in Sardinia. Arthritis Res Ther. 2009; 11(6): R171.
[24]. Jiang H, Canfield SM, Gallagher MP, Jiang HH, Jiang Y, Zheng Z, et al. HLA-E-restricted regulatory CD8(+) T cells are involved in development and control of human autoimmune type 1 diabetes. J Clin Invest. 2010; 120(10): 3641-650.
[25]. Veiga-Castelli LC, de Paula Cruz AS, Inácio MM, Mendes-Junior CT, Vianello-Brondani R, Moreau P, et al. Lack of association between HLA-E polymorphisms and transitional cell carcinoma of the bladder. Tissue Antigens 2013; 82(3): 197-200.
[26].
Douik H,
Romdhane NA,
Guemira F. Are HLA-E*0103 alleles predictive markers for nasopharyngeal cancer risk? Pathol Res Pract. 2016; 212(4): 345-49.
[27]. Mossallam GI, Fattah RA, El-Haddad A, Mahmoud HK. HLA-E polymorphism and clinical outcome after allogeneic hematopoietic stem cell transplantation in Egyptian patients. Hum Immunol. 2015; 76(2-3): 161-65.
[28]. Kutmon M, Evelo CT, Coort SL. A network biology workflow to study transcriptomics data of the diabetic liver. BMC Genomics 2014; 15: 971.
[29]. Chen NK, Chong TW, Loh HL, Lim HK, Gan VH, Wang M, et al. Negative regulatory responses to metabolically triggered inflammation impair renal epithelial immunity in diabetes mellitus. J Mol Med. 2013; 91(5): 587-98.
[30]. Hodgkinson AD, Millward BA, Demaine AG. The HLA-E locus is associated with age at onset and susceptibility to type 1 diabetes mellitus. Hum Immunol. 2000; 61(3): 290-95.
[31]. Di Cristofaro J, Buhler S, Frassati C, Basire A, Galicher V, Baier C, et al. Linkage disequilibrium between HLA-G*0104 and HLA-E*0103 alleles in Tswa Pygmies. Tissue Antigens 2011; 77(3): 193-200.
[32]. Matte C, Lacaille J, Zijenah L, Ward B, Roger M. HLA-G and HLA-E polymorphisms in an indigenous African population. Hum Immunol. 2000; 61(11): 1150-156.
[33]. Kanai T, Fujii T, Keicho N, Tokunaga K, Yamashita T, Hyodo H, et al. Polymorphism of human leukocyte antigen-E gene in the Japanese population with or without recurrent abortion. Am J Reprod Immunol. 2001; 45(3): 168-73.
[34]. Gömez-Casado E, Martínez-Lasot J, Castro MJ, Morales P, Trápaga J, Berciano M, et al. Detection of HLA-E and -G DNA alleles for population and disease studies. Cell Mol Life Sci. 1999; 56(3-4): 356-62.
[35]. Arnaiz-Villena A, Vargas-Alarcon G, Serrano-Vela JI, Reguera R, Martinez-Laso J, Silvera- Redondo C, et al. HLA-E polymorphism in Amerindians from Mexico (Mazatecans), Colombia (Wayu) and Chile (Mapuches): evolution of MHC-E gene. Tissue Antigens 2007; 69(suppl 1): 132-35.
[36]. Antoun A, Jobson S, Cook M, Moss P, Briggs D. Ethnic variability in human leukocyte antigen-E haplotypes. Tissue Antigens 2008; 73(1): 39-45.








































.PNG)




