Introduction
Cancer is the second cause of death after cardiovascular disease in developed countries, and the third (to infectious diseases) in developing countries. In Iran, cancer ranks the 3rd as the cause of death after cardiovascular diseases and accidents [1]. Routine treatment for cancer is surgery, radiotherapy and chemotherapy, which are not very successful in many cases. Besides recurrence, side effects of these interventions are a big problem [2]. In the past decades, cancer drugs researcher have tried to introduce novel anti-tumor drugs, which have better efficiency and fewer side effect. In this regard, heterocyclic compounds having benzopyran ring have drawn much attention [3].
One of the most important heterocyclic groups are chromenes, a subgroup of coumarin, which have been investigated for many years in cancer therapy, and their cell toxicity against many cancers have been proved in different studies. These compounds are present in the nature; many derivatives of them could be synthesized in laboratories by adding different groups into their main heterocyclic structure [4]. Effectiveness of different synthetic chromene derivatives vary based on the type of added groups and the type of cancer cell line. Some of these synthesized chromene derivatives are under investigation to treat solid tumors, some of them are tubulin inhibitors [5], and again others are bcl (or bcl-2) inhibitors [6, 7] that induce apoptosis in cancer cells.
10,11-dihydro chromeno [4,3-b] chromenes were previously considered as anti-tuberculosis agent [8]. In the present study, we produced different derivatives of 10,11-dihydrochromeno [4,3-b] chromenes and investigate their effectiveness on induction of cell death in human acute lymphoblastic leukemia cell line MOLT4
.
Materials and Methods
General information
4-hydroxycoumarine, dimedone, aldehydes, and other necessary chemical compounds were purchased from Fluka Analytical (Germany) and Merck (Germany) companies. The purity of the compounds was routinely checked by thin layer chromatography (TLC) using silica gel 60G (Merck, Germany) with ethylacetate/n-hexane (1:3). In addition, the products were characterized by Fourier-transform infrared spectroscopy (FTIR),
1H nuclear magnetic resonance (NMR) and
13C NMR spectra and by comparison of their physical properties with those reported in the literature. FTIR (ATR) spectra were run on a Bruker, Equinox 55 spectrometer (UK).
1H NMR and
13C NMR spectra were obtained using a BrukerAvans 400 and 500 MHz spectrometers (DRX). Melting points were determined by a Buchi melting point B-540 B.V.CHI apparatus (Sigma-Aldrich, Germany).
Synthesis of 10, 11-dihydrochromeno [4, 3-b] chromene-6, 8 (7H,9H)-dione derivatives
Briefly, a mixture of dimedone (1 mmol, 0.14 g), 4-hydroxycoumarin (1 mmol, 0.16 g), aldehyde (1 mmol) and Zn (OAc)
2 (0.03 g) was heated under solvent-free conditions at 90°C for an appropriate time. The progress of the reaction was followed by TLC (ethyl acetate: n-hexane 30:70). After completion of the reaction, the mixture was dissolved in hot CH
2Cl
2 for isolation of catalyst, and filtered. The solvent of resultant filtrate was evaporated, and the pure product obtained by recrystallization from ethanol. The products, including 10, 10-dimethyl-7-(4-nitrophenyl)-10,11-dihydrochromeno [4, 3-b]chromene-6,8 (7H, 9H)-dione (here arbitrarily named C1), 10, 10-dimethyl-7-(3-hydroxyphenyl)-10, 11-dihydrochromeno [4, 3-b] chromene-6,8 (7H, 9H)-dione (C2), 10, 10-dimethyl-7-(3, 4-dimethoxyphenyl)-10,11-dihydrochromeno [4,3-b] chromene-6,8 (7H, 9H)-dione (C3), and 10, 10-dimethyl-7-(4-hydroxy-3-methoxyphenyl)-10, 11-dihydrochromeno [4,3-b] chromene-6,8 (7H, 9H)-dione (C4) were characterized on the basis of spectroscopic data from FTIR, H NMR and C NMR [9] (Fig. 1). The general synthetic route has been highlighted in scheme 1.
.PNG) Spectroscopic data
10, 10-dimethyl-7 (4-nitropophenyl) -10, 11-dihydrochromeno [4,3-b]chromene-6, 8 (7H, 9H)-dione (C1): White solid, MP=208-210°C:
Spectroscopic data
10, 10-dimethyl-7 (4-nitropophenyl) -10, 11-dihydrochromeno [4,3-b]chromene-6, 8 (7H, 9H)-dione (C1): White solid, MP=208-210°C:
FTIR: ν
max(ATR, neat)
= 2930, 1719, 1651, 1605, 1528, 1458, 1342, 1178, 1098, 1033, 863, 760 cm
-1.
1H-NMR (500 MHz, CDCl
3-d
6): δ = 1.1 (s, 3 H), 1.12 (s, 3 H), 2.18 (d,
J=16.4 Hz, 1H), 2.26 (d,
J=16.4 Hz, 1H), 2.67 (d,
J=18.1 Hz, 1H), 2.73 (d,
J=18.1 Hz, 1H), 4.95 (s, 1 H), 6.90 (d,
J=8.8 Hz, 2H), 7.04 (d,
J=8.8 Hz, 2H), 7.34-7.40 (m, 2H), 7.59 (t,
J=8.2 Hz, 1H), 7.89 (dd,
J=8.2 Hz,
J=1.6 Hz 1H).
13C-NMR (125 MHz, CDCl
3) δ = 27.29, 29.29, 31.21, 32.77, 40.84, 50.71, 114.76, 114.97, 115.05, 115.27, 115.50, 116.96, 122.47, 124.37, 129.80, 129.88, 130.16, 130.25, 132.37, 162.02, 162.34, 162.99, 196.45 ppm.
10, 10-dimethyl-7 (3-hydroxyphenyl)-10, 11-dihydrochromeno [4, 3-b] chromene-6, 8(7H, 9H)-dione (C2): White solid, MP=267-269°C:
FTIR: ν
max(ATR, neat)
= 3400, 2983, 1697, 1665, 1607, 1488, 1364, 1175, 1137, 1058, 897, 767 cm
-1.
1H-NMR (500 MHz, DMSO-d
6): δ = 1 (s, 3 H), 1.1 (s, 3 H), 2.75 (sbr, 2H), 2.37 (d,
J=16.5 Hz, 1H), 4.62 (s, 1H), 6.55 (dd,
J=7.5 Hz,
J=2.1 Hz, 1H), 6.66 (d,
J=7.0 Hz, 1H), 6.71 (s, 1H), 7.03 (t,
J=8.0 Hz, 1H), 7.44 (d,
J=8.5 Hz, 1H), 7.46 (t,
J=7.0 Hz, 1H), 7.69 (t,
J=7.5 Hz, 1H), 7.93 (dd,
J=7.5 Hz,
J=1.5 Hz, 1H), 9.29 (s, 1H).
13C-NMR (125 MHz, DMSO-d
6) δ = 27.58, 29.38, 32.86, 33.63, 50.92, 106.78, 113.97, 114.68, 114.79, 116.38, 117.44, 119.71, 123.40, 125.64, 129.94, 133.65, 144.98, 152.77, 154.46, 157.98, 163.41, 196.73 ppm.
10, 10-dimethyl-7 (3, 4-dimethoxyphenyl)-10, 11-dihydrochromeno[4,3-b]chromene 6, 8(7H, 9H)-dione (C3) White solid, MP=184-185°C:
FTIR: ν
max(ATR, neat)
= 2921, 1721, 1660, 1620, 1513, 1455, 1361, 1262, 1139, 896, 742 cm
-1.
1H-NMR (500 MHz, CDCl
3-d
6): δ = 1.13 (s, 3 H), 1.19 (s, 3 H), 2.30 (d,
J=16.0 Hz, 1H), 2.35 (d,
J=16.0 Hz, 1H), 2.68 (d,
J=17.6 Hz, 1H), 2.74 (d,
J=17.6 Hz, 1H), 3.81 (s, 3 H), 3.89 (s, 3 H), 4.93 (s, 1H), 6.74 (d,
J=8.1 Hz, 1H), 6.82 (dd,
J=8.0 Hz,
J=1.6 Hz, 1H), 7.05 (s, 1H), 7.33-7.39 (m, 2H), 7.58 (t,
J=8.0 Hz, 1H), 7.88 (d,
J=8.0 Hz, 1H).
13C-NMR (125 MHz, CDCl
3) δ = 27.49, 29.26, 32.36, 32.80, 40.85, 50.72, 106.92, 110.93, 112.65, 113.74, 115.20, 116.92, 120.20, 122.41, 124.27, 132.19, 135.36, 148.02, 148.63, 152.56, 153.74, 160.76, 161.91, 196.16 ppm.
10, 10-dimethyl-7-(4-hydroxy-3-methoxyphenyl)-10, 11-dihydrochromeno [4, 3-b] chromene-6, 8(7H, 9H)-dione (C4): White solid, MP=271-273°C:
FTIR: ν
max(ATR, neat)
= 3434, 2993, 1713,
1662, 1608, 1514, 1361, 1272, 1181, 1033, 863, 779. cm
-1.
1H-NMR (500 MHz, DMSO-d
6): δ=1.02 (s, 3H), 1.10 (s, 3 H), 2.20 (d,
J= 16.0 Hz, 1H), 2.34 (d,
J= 16.0 Hz, 1H), 2.76 (s, 2H), 3.70 (s, 3H), 4.61 (s, 1H), 6.61 (d,
J= 8 Hz, 1H), 6.64 (d,
J=8 Hz, 1H), 6.81 (d,
J=1.6 Hz, 1H), 7.43-7.48 (m, 2H), 7.69 (t,
J=7.8 Hz, 1H), 7.92 (d,
J=8 Hz, 1H), 8.88 (s, 1H).
13C-NMR (125 MHz, DMSO-d
6) δ = 26.55, 28.57, 31.94, 32.24, 39.57, 50.02, 55.57, 106.10, 112.81, 113.18, 113.96, 115.15, 116.51, 120.52, 122.55, 124.70, 132.63, 133.80, 145.47, 147.00, 151.83, 153.29, 159.99, 162.43,195.96 ppm.
Cell culture
The cell culture medium Roswell Park Memorial Institute (RPMI)-1640 and fetal bovine serum (FBS) were purchased from Gibco (USA). Penicillin-streptomycin, trypan blue, Ficoll, dimethylsulfoxide (DMSO), phytohemagglutinin (PHA) 1000 mol/mL, and (3-(4,5-dimethyl thiazol-2-yl)-2, 5-diphenyltetrazolium bromide) (MTT) assay kit were obtained from Sigma (Germany). human acute lymphoblastic leukemia cell line (MOLT4) cells were purchased from Pasteur Institute (Tehran, Iran), and cultured in RPMI-1640 supplemented with 10% FBS, 100 units⁄mL penicillin, and 100 mg⁄mL streptomycin. Cells were grown in suspension, at 37 ˚C in humidified air containing 5% CO
2 (16).
Cytotoxicity assay
MOLT4 cells were cultured in 24-well micro plate at a density of 10
6 cells/mL for 42 hours. Then, 20 µL of each synthetic compounds (in 50, 250, 500 and 1000 nM) was dissolved at a concentration of 0.1% in DMSO which was added to each well. The plate was incubated for 24, 48 and 72 h. Cell counting was done by hemacytometer slide, using 0.4% trypan blue in three different times for detection of live vs. dead cells [10].
Cell viability assay
Cell viability was estimated using the MTT reduction assay. MOLT4 cells were plated in 96-well micro plates at a density of 10
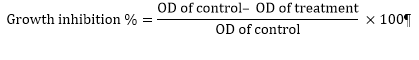
6 cells⁄mL (200 µL⁄well) for 9 hours. Subsequently, 20 µL of synthetic compounds (in 50, 250, 500 and 1000 nM) were added to each well. The cells were incubated for 72 h. Then, the MTT was added to each well at a final concentration of 0.5 mg⁄mL, and plates were incubated for another 4 h at 37˚C. Later, 100 µL DMSO was added to each well. Control wells contained only growth medium. The optical density was measured at 570 nm using a Bio-TEK micro plate reader. The tests were done three times [11]. The percentage of growth inhibition and cell viability compared with the control wells were determined by the following formulas:
.PNG)
The drug concentration which resulted in 50% inhibition of cells in the medium was regarded as half-inhibition concentration (IC
50). IC
50 was calculated by the results from counting cells and MTT assay as follow:
 RNA extraction and cDNA synthesis
RNA extraction and cDNA synthesis
The cells treated with C1 at concentration of 250 nM after 48 h were harvested and treated with RNX Plus solution (CinnaGen, Iran) according to the manufacturer's instructions. Quality and quantity of extracted RNAs was evaluated by UV spectrophotometry (260/280 nm ratio) and gel electrophoresis. The first strand of cDNAs was synthesized using the
reverse transcription system (TaKaRa, Japan) according to the manufacturer's instructions.
Real-Time quantitative polymerase chain reaction (PCR) assay
The expression level of
p53,
bax, and
fas genes in C1 treated cells was investigated by specific primer:
P53 (F):5′-AGAGTCTATAGGCCCACCCC-3′,
P53(R):5′-GCTCGACGCTAGGATCTGAC-3′[12];
Bax (F):5'-GGACGAACTGGACAGTAACATGG-3',
Bax(R):5'-GCAAAGTAGAAAAGGGCGACAAC-3'[13];
Fas(F):5′-TGAAGGACATGGCTTAGAAGTG-3',
Fas (R): 5′-GGTGCAAGGGTCACAGTGTT-3' [14];
The GAPDH gene was used as internal control:
GAPDH (F):5ʹ-GCCACATGCCTCAGACAC-3',
GAPDH (R):5ʹ-GGCAACAATATCCACTTTACCAG-3' [15].
Quantitative real-time PCR was performed with SYBR Green Master Mix. The PCR program was: initial denaturation at 95°C for 10 minutes, followed by 40 cycles of denaturation at 95°C for 10 seconds, annealing/extension at 60°C for 30 seconds, and extension at 72°C for 45 seconds. Expression change was assessed by the 2
-ΔΔCt formula.
Statistical analysis








































.PNG)
.PNG)


.PNG)
.PNG)
.PNG)
