The liver is an important organ which has a central role in metabolic homeostasis and is responsible for the metabolism, synthesis, storage and redistribution of nutrients, carbohydrates, fats and vitamins [1]. The liver plays a key role in immunological homeostasis and metabolism. This organ is also known to be an important place for generation of free radicals. In addition, free radical production can occur if inflammation is caused by or exposed to toxins [2]. Liver enzymes, including diamine oxidase, aldehyde dehydrogenase, tryptophan dual oxidase, liver dehydrogenase and the cytochrome P450 enzyme system, induce oxidation and production of uncoupling molecules [3, 4]. A high number of free radicals in the liver would be affected by endogenous or exogenous antioxidants and drugs such as acetaminophen. Molecular oxygen has a unique electronic structure that can accept a total of four electrons, and frequently evolve into free radicals formation. Oxygen free radicals, more generally known as reactive oxygen species (ROS), along with reactive nitrogen species represent the most important group of radical species generated in living system. Liver inflammation produces excessive oxygen free radicals to attack host cells, leading to cell damage [5]. Peroxidation of DNA plays an important role in pathogenesis of mutations in the liver cells. The activity of superoxide dismutase, and glutathione peroxidase in chronic liver cirrhosis and hepatitis is significantly low, which is negatively correlated with serum alanine aminotransferase (ALT) level [6]. Lipopolysaccharide (LPS) is a major constituent of the outer membrane of gram-negative bacteria, and the immune system is constantly exposed to low levels of LPS through low-grade bacterial infections [7, 8]. LPS recognition and signaling responses are keys to eliminating invading pathogens. LPS-induced activation of macrophages induces production of bioactive lipids, ROS and in particular, inflammatory cytokines. Although, the LPS response is critical to fighting and clearing bacterial infections, LPS also mediates deleterious host reactions. In addition, LPS can initiate extra production of inflammatory mediators, leading to potentially lethal systemic diseases, for example, septic shock and tissue damage. Inflammation can induce oxidative damage by generating oxidative agents such as the superoxide anion and nitric oxide (NO) [9, 10]. Inducible NO synthase (iNOS) produce NO and NO reacts with superoxide and generate peroxynitrite, particularly in immune cells [11]. Additionally, NO syntheses oxidize the terminal guanidino nitrogen of L-arginine, producing NO and citrulline. Various extracellular stimuli activate nuclear factor kappa-light-chain-enhancer of activated B-cells, an inducible transcription factor. Stimuli include cytokines, LPS and oxidative stress that can cause chronic liver impairment with activation of hepatic stellate cells, recruitment of inflammatory cells, activation of infiltrating immunocompetent cells and initiatation of oxidative stress cascade [12]. Silymarin, a standardized extract obtained from seeds of Silybum marianum (S. marianum), is widely used in treatment of liver diseases of varying origins. Seeds of S. marianum have been shown to treat liver and gall bladder disorders, including hepatitis, cirrhosis and jaundice and to protect the liver poison from chemicals, environmental toxins, snake bites, insect stings, mushroom poisoning and alcohols. Due to its proven hepatoprotective and antioxidant properties, silymarin is being used as a standard agent for hepatoprotective effects induced by LPS [13, 14].
The emergence of silymarin as a natural remedy for liver diseases, coupled with its entry into national institutes of health clinical trial, signifies its hepatoprotective potential. Silymarin is noted for its ability to interfere with apoptotic signaling while acting as an antioxidant. Various in vivo studies were designed to explore the hepatotoxic potential of Doxorubicin, the well-known cardiotoxin, and in particular the possibility of pre-exposures to silymarin to prevent hepatotoxicity by reducing Dox-induced free radical mediated oxidative stress, by modulating expression of apoptotic signaling proteins like Bcl-xL, and by minimizing liver cell death occurring by apoptosis or necrosis [15]. In this way, we decided to evaluate the protective effect of silymarin on LPS-induced liver toxicity in male Wistar rat.
Materials and Methods
Animals and drugs
Totally, 40 male Wistar rats being 12 weeks old and 240±10 g weight were purchased from an animal house. The animals were kept in standard conditions (temperature was 22±2°C and 12 h light/dark cycle). The rats had free access to food and water. The animals were divided into 4 groups (n=10 in each):
- Control: received 1 ml/kg of saline instead of LPS or silymarin
- LPS (daily injection): received 2 mg/kg LPS intraperitoneally with saline instead of silymarin (orally pretreatment in corn oil)
- LPS- silymarin: received 10 mg/kg silymarin before 2 mg/kg LPS
- LPS- silymarin: received 20 mg/kg silymarin before 2 mg/kg LPS
They were injected intraperitoneally with saline instead of LPS and silymarin for two weeks before the biochemical tests. LPS was purchased from Sigma (Sigma Chemical Co). silymarin was kindly provided by Daroupakhsh Company in Iran. In the final stage, urethane was injected to induce a deep anesthesia [16]. Then the livers were removed as liver toxicity index to evaluate the level of oxidative stress including malondialdehyde, catalase and liver enzymes including ALT and aspartate aminotransferase (AST). The Animal Care and Use Committee, and also the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals approved this experimental protocol [17].
Determination of malondialdehyde and catalase activity
Malondialdehyde levels, as an index of lipid peroxidation, were measured in the liver tissues. malondialdehyde reacts with thiobarbituric acid as a thiobarbituric acid reactive substance and produces a red colored complex which has a peak absorbance at 535 nm. two ml thiobarbituric acid/ trichlorooacetic acid/ hydrochloric acid reagent was added to 1 ml homogenate and the solution was incubated in a boiling water bath for 40 min. After cooling, the whole solutions were centrifuged (1000 g for 10 min). The absorbance of supernatant was measured at 535 nm. The malondialdehyde concentration was calculated as follows: C (m)= Absorbance/ (1.65×10
5) [18, 19].
Catalase activity was estimated using the method of Azizi et al. [12]. The principle of the assay is based on determination of the rate constant of hydrogen peroxide decomposition. By measuring the decrease in absorbance at 240 nm per minute, the rate constant of the enzyme was determined. Activities were expressed as k (rate constant) per liter. Catalase is a very important enzyme in protecting the cell from
oxidative damage by ROS [20]. Liver tissues Interleukin (IL)-6 contents determination was performed with a specific rat enzyme-linked immunosorbent assay kit (bioscience Co, San Diego, CA, USA) according to Videla et al [19].
Biochemical blood evaluation
Serum levels of some liver enzymes like ALT and AST were assessed by using commercial colorimetric kits (Hitachi, Japan) [21, 22].
Western blot analysis
For western blot analysis, protein was extracted from the liver tissues for Bax, Bcl
2, caspase-3, caspase-8 and caspase-9. The total protein content was determined using Bradford protein assay kit (Bio-Rad). Levels of Bax, Bcl
2, caspase-3, caspase-8, caspase-9 and β-actin were measured by immune blotting analysis [23]. Briefly, equal amounts of protein extracts (50 μg) were mixed with loading buffer and heated for 8 min at 95˚C, loaded to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and separated by SDS-PAGE on a 12% gel. After electrophoresis, proteins were transferred to polyvinylidene fluoride membrane [24, 25]. The blots were incubated in blocking buffer tris buffered saline with tween 20 for 2 hr at room temperature. The primary antibodies were rabbit monoclonal anti-serum against Bcl
2, caspase-3, caspase-8, caspase-9, Bax and mouse monoclonal anti serum against β-actin (Cell Signaling). Finally, the membranes were incubated with the corresponding secondary antibody. Protein bands were visualized using an enhanced chemiluminescence (Pierce ECL Western blotting substrate) and Alliance gel doc (Alliance 4.7 Gel doc, UK). UV Tec software (UK) was used to semi-quantify protein bands. All protein bands were normalized against β-actin protein in order to achieve the final results.
Statistical analysis
All data were expressed as means ± SEM. The data were compared by one way ANOVA followed by tukey's post hoc comparisons test by prism 5. Differences were considered statistically significant when p<0.05.
Results
Liver inflammation criteria
The liver IL-6 concentration of the LPS group
was significantly higher than that of the control group (p<0.001). It was also identified all pretreatment doses of silymarin having a protective effect against increasing of IL-6 level due to LPS treatment reflected in a lower concentration of IL-6 in the liver tissues (p<0.01 for 10 mg/kg silymarin and p<0.001 for 20 mg/kg silymarin.
Oxidative damage criteria
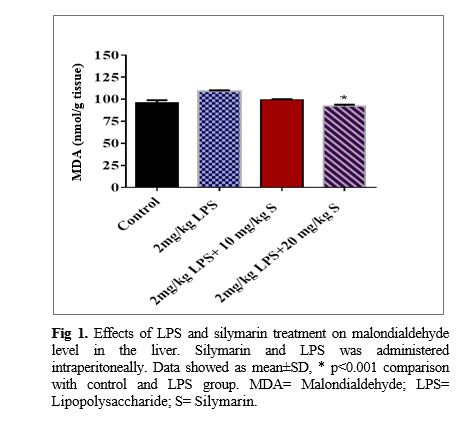
The liver malondialdehyde concentration of the LPS group was significantly higher than that of the control group (p<0.001). It was also shown that both doses including 10 and 20 mg/kg of silymarin have a protective effect against increasing lipid peroxidation due to LPS treatment reflected in a lower concentration of malondialdehyde in the liver tissues (p<0.001; Fig 1). It was also observed and the catalase activity in the liver tissues of LPS group significantly increased more than that of the control group (p<0.001). The findings also revealed that the highest dose of silymarin increases the catalase activity in the liver tissues compared to the LPS (p<0.01).
Liver biochemical criteria
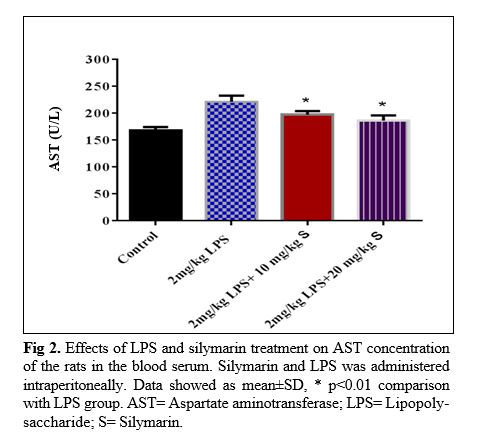
The results of silymarin extract on liver function criteria of the LPS- treated rats: In LPS group, serum AST concentration of the rats was higher than that of the control ones (p<0.01; Fig 2). Treatment of the animals by two doses of silymarin attenuated the serum concentration of AST (p<0.01, p<0.001). LPS also increased serum ALT compared to the control group (p<0.001; Fig 3). All two dose of silymarin prevented increasing of ALT concentration due to LPS administration (p<0.001). Changes in weight and high-density lipoprotein level in various groups of rat are shown in tables 1 and 2 respectively.
Western blot analysis
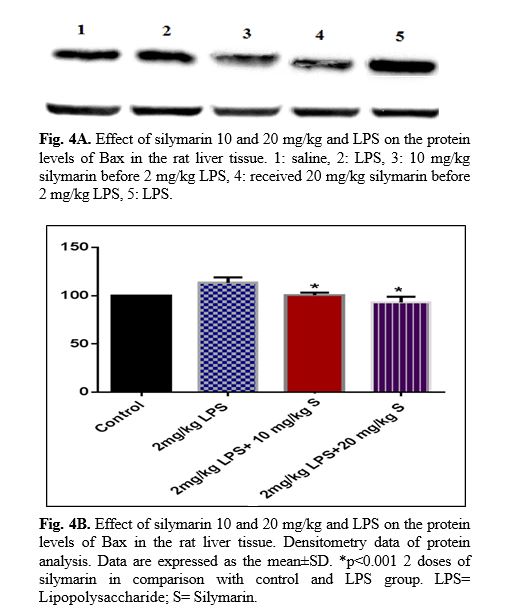
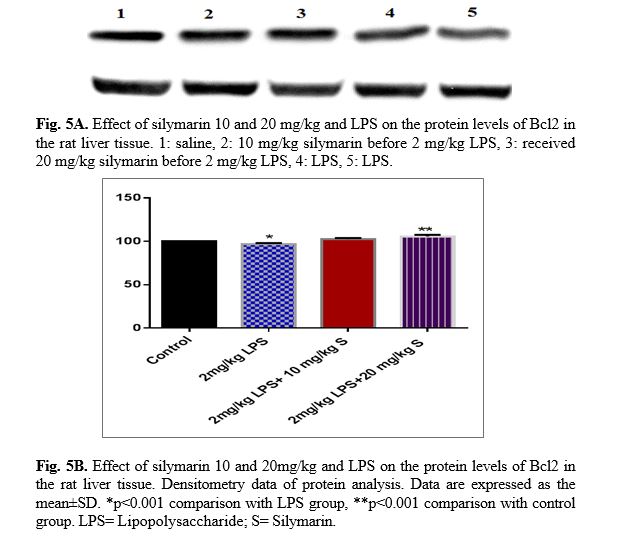
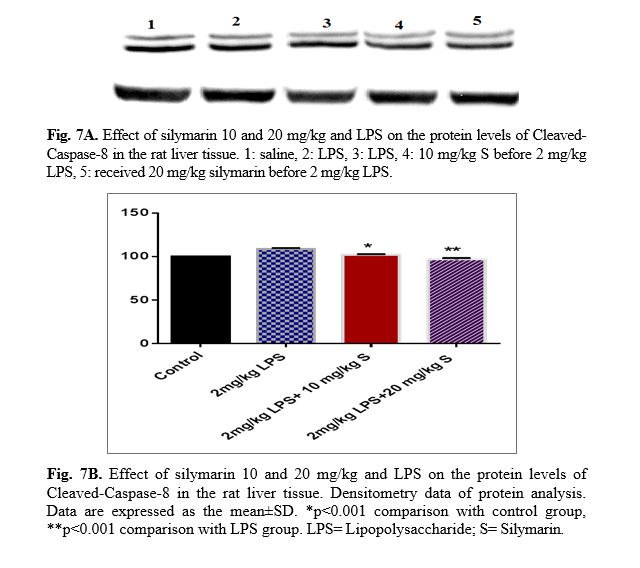
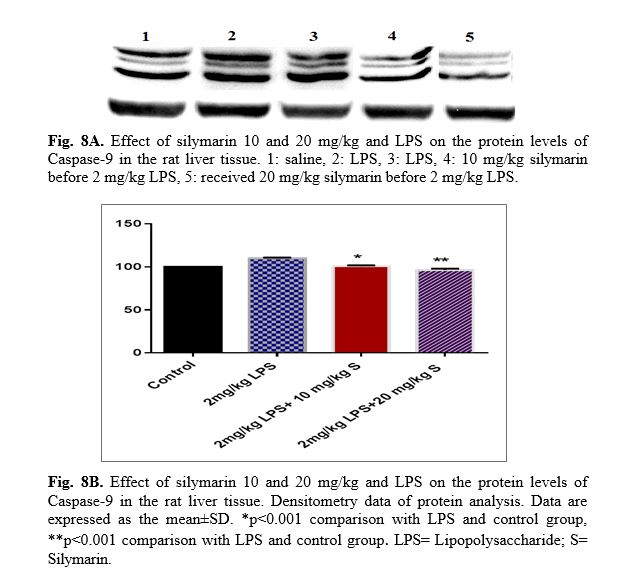
The results of western blot analysis are described as follows: protein expression of Bax/Bcl
2 was up-regulated in LPS group in the liver (p<0.001; Fig 4 and 5). Similarly, protein expressions of caspase-3, caspase-8 and caspase-9 were up-regulated by LPS in the liver (p<0.001; Fig 6-8). The data show significant results in silymarine 10 and 20 mg/kg group and LPS group on the protein levels of Bax in the rat liver tissue. Representative western blots showed specific bands for Bcl2 and β-actin as an internal control. Representative western blots showed specific bands for Caspase-8 and Caspase-9 with β-actin as an internal control. Western blot analysis was conducted on the basis of other studies [27].


 Discussion
Discussion
Acute inflammation results in a general reaction of the organism which is followed by anorexia, loss of body weight, hypoglycemia, and changes in the levels of several plasma proteins produced by the hepatocytes. These conditions can also be reproduced by a number of experimental treatments. In particular, intra- peritoneal injection of LPS is commonly used to reproduce systemic inflammation [28, 29].
Administration of LPS for the animals is used as an experimental model to analyze the mechanism(s) underlying endotoxin-induced acute liver injury since it induces the infiltration of inflammatory cells into the liver and causes acute liver injury. In this study, we showed that LPS induces liver inflammation presented by an increased level of IL-6 in the liver tissues. LPS is also known to enhance the formation of ROS and lipid peroxidation products such as superoxide anions and peroxides and their secondary product, malondialdehyde, in liver [30]. The present data provided evidence that LPS injection increases malondialdehyde and decreases catalase activity. In another study, LPS promoted hepatic oxidative stress in rats. Livers of rats exposed to LPS exhibited elevated levels of lipid peroxidation products, malondialdehyde and hydro peroxides [31]. It has also been reported that the mice lacking iNOS gene are protected against acetaminophen-caused liver injury. Considering these evidences, an overproduction of NO may have a role in hepatic injury as observed in the recent study [32].
The measurement of hepatic enzymes appearing in the blood has been employed as a reliable indicator for assessment of hepatotoxicity. Our data revealed that the liver enzymes including ALT and AST are increased subsequent to inflammation induced by LPS. Consistently, LPS injection was previously reported that was followed by increased levels of AST and ALT, as well as apoptotic and necrotic changes in hepatocytes, which are biochemical and histological parameters of liver damage, respectively. Cytokines have also an important function in the development of hepatic inflammation and fibrosis [33, 34]. The results of the current study exhibited that silymarin improves liver functions in LPS injected rats presented by decreased levels of ALT, AST in the serum. Similar to the results, it has also been previously reported that silymarin ameliorates the development of ALT, AST levels in hepatic injury models [35, 36]. It has been demonstrated that silymarin inhibits LPS-induced production of pro-inflammatory cytokines such as IL-1β in the liver. To evaluate the mechanism of the protective effects of silymarin in hepatic inflammation, we measured the levels of IL-6 in supernatants of liver tissues from each study group [37]. The results projected that the both groups treated with silymarin possessed lower IL-6 level in liver tissue compared to LPS group. The results also explored that the highest dose was more effective than other dose. These findings confirm the previous reports on the antioxidant effect of silymarin active ingredients and compounds (Silybum) [38]. In the current study, our data revealed that Silybum inhibites the LPS-induced production of Interleukin, the activation of malondialdehyde and the caspase3-8-9, thus protecting mice against LPS-induced inflammation [39].
In the present study, we measured protein levels of Bax/Bcl
2 ratio, cleaved caspase-3, cleaved caspase-8 and cleaved caspase-9 for evaluation of apoptosis. Bcl
2 is an anti-apoptotic protein localized in the outer mitochondrial membrane, nuclear membrane and endoplasmic reticulum. Caspases are proteolytic enzymes which trigger cell death by cleaving specific proteins in the
cytoplasm and
nucleus. The initiator caspase-8 and caspase-9 may be stimulated by cell membrane death receptors thus leading to activation of caspase-3 by extrinsic pathways of apoptosis [40].
Conclusion
The results of this research demonstrated that silymarin can exert protective effects against toxic effects of LPS in rat liver such as IL-6 and malondialdehyde. Anti-inflammatory drug can play a protective role in attenuating the liver inflammation induced by LPS injection. LPS also increased expression of Bax/Bcl
2 and caspases ratio as apoptosis parameters. This study suggests silymarin as the most important liver protective compound.
Conflict of Interest
No potential conflict of interest was reported by the authors.
Acknowledgments
The writers of this study would like to express their gratitude to all the hardworking staff of the Microbiology and Biotechnology Research Center for their valuable support. This research was financially sponsored by Shahrekord Islamic Azad University in Shahrekord, Iran (2018-239). The study was financially supported by both the personal as well as Islamic Azad University of Shahrekord, Iran.
















































