In most countries, male reproductive health deterioration is regarded as a significant problem [1]. Genital infections, hypogonadism, ejaculatory duct obstruction, varicoceles, or exposure to environmental factors (for example, xenobiotics, ionizing radiation), lifestyle factors (e.g., smoking, alcohol, and obesity), systemic diseases, genetic causes, and abnormal ejaculation are considered as contributing factors for infertility of male [2]. Nevertheless, in about 30–45% of infertile males, the etiology is not still determined and is termed idiopathic infertility [3]. Infertility involves 10-15% of couples in the world, and males account for around 50% of infertility cases [4]. Human spermatozoa may induce oxidative damage because of the high rate of polyunsaturated fatty acids on their plasma membranes [5]. Therefore, cigarette smoking may decrease semen quality in humans. However, male infertility among smokers has not yet been tapped even with the high risk of increased exposure to reactive oxygen species (ROS) due to smoking. ROS, strongly linked to oxidative stress, are oxygen-derived free radicals that involve hydroxyl, superoxide anions, alkoxyl radicals, peroxyl, and hydrogen peroxide. ROS can be produced from endogenous physical processes like seminal leukocytes and mitochondrial respiration or different environmental factors, including toxins, radiation, drugs, smoking, pollution, and diet [6]. ROS can potentially damage the plasma membrane and DNA motility, integrity, and overall quality of semen [7]. So, scavenging excess ROS is essential for normal fertilization and spermatogenesis. The nuclear factor erythroid 2-related factor 2/antioxidant response element (NRF2/ARE) signaling pathway and its regulated antioxidant enzymes significantly contribute to cellular oxidative stress defense during fertilization and spermatogenesis [8]. Antioxidant molecules and enzymes such as glutathione (GSH), superoxide dismutases (SODs), and catalases (CATs) are largely plentiful in sperm cells or in semen plasma [9]. Most of these genes, including glutathione peroxidase (GPX), NRF2, CAT, SOD, glutathione S-transferase (GST), and nitric oxide synthase (NOS), have sequence variants in humans, which in turn can bring about male infertility in different ways [10]. The production of these protective cellular enzymes is induced as they are exposed to ROS, through a regulated mechanism at the transcription level [11].
NRF2 is the vital gene in antioxidant defense, as it is the nuclear transcriptional factor that can generate antioxidant enzymes through ARE element [12]. NRF2 binds to AREs in response to oxidative stress, which mediates transcriptional activation of its responsive genes and modulates in vivo defense mechanisms in reaction to oxidative damage [13]. NRF2 is a transcription factor belonging to the basic leucine zipper protein family and is encoded by the NFE2L2 gene. NRF2 is expressed in all tissues, but it is mostly expressed in the brain, kidney, muscle, lungs, heart, and liver. NRF2 has six highly conserved regions called Neh's (NRF2-ECH homology) domains. When the cells are exposed to oxidative stress and high levels of ROS, the oxidation of key cysteine residues, and the Keap1 protein increase. These constructional changes weaken the binding ability of Keap1 to NRF2 [14]. The induction of antioxidant and cytoprotective enzymes is mediated mostly by NRF2, including CAT, superoxide SOD isoenzymes, and GSTs [15]. The NRF2-ARE signaling pathway is associated with many diseases in animal and human models, such as cerebral ischemia, lung diseases, and eye [16]. Experiments conducted on mice have shown that this gene is also related to spermatogenesis. In the knockout mouse model, NRF2 disruption affects spermatogenesis in an age-dependent manner [8].
Additionally, the NRF2 rs6721961 TT genotype is induced at a higher frequency in heavy smokers suffering from low semen quality compared to those with high semen quality; heavy smokers with this genotype tolerate lower sperm counts and concentrations in comparison to others [17]. NRF2 expression was notably lower in infertile subjects than in controls at the mRNA level. A considerable correlation was detected between the NRF2 mRNA expression level and specific sperm functional parameters, including progressive motility, concentration, vitality, and immotility [18]. The DJ-1 protein that stabilizes NRF2 by targeting 20S proteasomes in cells is associated with the infertility of males [19, 20]. The sperm DJ-1 concentration was lower in moderate asthenozoospermia patients compared to the controls [19]. Thus, expression level of NRF2 and functional polymorphisms as well as its regulators, are found to be associated with defective spermatogenesis in humans.
Human semen has an abundant amount of GSTs [21], belonging to phase II superfamily of antioxidant enzymes contributing to the cellular detoxification of different physiological substances (for example, excessive ROS) or exogenous electrophiles. The process of detoxification is dependent on the family class of genes. Seven GSTs' gene classes can be considered: alpha (α), kappa (k), mu (μ), sigma (ς), pi (π), theta (θ), and zeta (ζ). They are, respectively, coding GSTA, GSTK, GSTM, GSTS, GSTP, GSTT, and GSTZ enzymes [22]. Generally, inhibited GST activity diminishes semen motility through membrane damage. Since unmetabolized toxic substances, accumulating in the testis cellular matrix, deteriorate spermatogenesis, another probable reason for sperm impairment in patients with GSTM1 or GSTT1 null genotypes may be insufficient functioning of fibrosis and seminiferous tubules developed in the testicular tissue Located on 1p13.3 chromosome, GSTM1 includes three alleles at its locus: GSTM1*A, GSTM1*B (causing the lysine 172 replacement by aspartic acid and not altering enzyme activity), and GSTM1 null genotype (gene deletion) [23].
Considering the vital role of free radicals in causing damage and impaired sperm function and the effect of smoking on these parameters, the aim of this study was to evaluate the effect of smoking on the expression of NRF2 and GSTM1 genes and biomarkers of oxidative stress in the sperm of smokers and nonsmokers referring to Isfahan infertility center.
Materials and Methods
Patients
A case-control study was conducted a 15 men smokers (case) and 15 men nonsmokers (control) aged 30-40 years, who had referred to infertility clinics in Isfahan, Iran. People who did not conform with the criteria were excluded. Exclusion criteria were as follows: urinary tract infection, diabetes, thyroid disorders, varicocele, testicular surgery, and consumption of alcoholic beverages. Smokers, patients with clinically diagnosed features of reproductive organ inflammation and symptoms of systemic disease, and patients with body weight disorders were excluded from the study. Research studies on human subjects were carried out based on the Declaration of Helsinki guidelines. The study participants were interviewed once the written informed consent was obtained. The interviewers collected data about lifestyle, medical history, and smoking status of the participants using a structured questionnaire. The smoking dose (packs per day) was multiplied by the duration (years smoked) to obtain smoking pack-years. Heavy smokers were considered as those who were current smokers with a smoking dose ≥ 1 pack per day over 10 years or a smoking dose ≥ 2 packs per day over 5 years. Nonsmokers were those who had never smoked.
Physical examinations were performed for all participants, and they had at least two semen analyses. Unhealthy men or men with a known cause for defective spermatogenesis, including varicocele, obstruction of the vas deferens, infection, chromosomal abnormalities, or microdeletions in the azoospermia factor area on the Y chromosome, were not eligible to take part in the study. Patients diagnosed with severe oligozoospermia (sperm concentration <5 × 106 cells per milliliter), azoospermia, leukopenia, hemospermia, and necrozoospermia [24] were also excluded as there were possibly medical or genetic reasons for the low quality of sperm.
Sample collection and preparation
After at least 48 to 72 h of sexual abstinence, sample collection and semen samples were obtained by masturbation. Semen volume, sperm concentration, pH, motility, vitality, and computer-assisted semen analyses were conducted according to the guidelines of the World Health Organization [24]. Samples were centrifuged for 5 min at 3000 rpm to separate sperm from the semen. The supernatant (containing the plasma) was transferred to a microtube and kept at a temperature of -70˚C.
Total RNA extraction
RNA samples of sperm were extracted using the RNA-Bee kit (Tel-Test, Inc., USA) and phenol-chloroform extraction [25]. The RNA samples were stored at -70˚C until the test time.
Real time-polymerase chain reaction (RT-PCR)
To synthesize cDNA, Reverse Transcription, Oligo DT and dNTP enzymes along with extracted RNA were placed for 5 min at 65˚C and then were placed at 45˚C for 60 min. Finally, to deactivate the enzyme, the temperature was brought to 85˚C for 5 min, and the cDNA obtained product was stored at -20˚C.
Using the Gene Bank for the NRF2 and GSTM1 genes in the studied groups, the RT-PCR technique was applied. For each group, the synthesized cDNA was stored at -20˚C until used. The same reference gene (18 sRNA) and target gene considered the RT-PCR detection system (Life Technology, USA), using Applied Biosystems SYBR® Green PCR Master Mix (2X) (Cat. 4344463). The expression of each target gene was calculated in accordance with the reference gene using the formula 2 -∆∆CT. Gene-specific primers for both GSTM1 and NRF2 genes were designed using Primer software (Table 1).
Assay of oxidative stress markers
To measure lipid peroxidation thiobarbituric acid reactive substances (TBARS), the plasma sample was mixed with trichloroacetic acid (TCA) 20% and then washed with sulfuric acid solution 0.05 M. Then, thiobarbituric acid 2% was added to sodium sulfate 2 M and placed in a boiling water bath for 30 minutes. The products of lipid peroxidation were extracted with n-butanol, and absorbance was measured at 532 nm. [26].
To evaluate the plasma total antioxidant capacity (TAC), the ferric reducing ability of plasma was used. Fe2+–TPTZ complex was evaluated with an absorption maximum at 593 nm [27].
Statistical analysis
SPSS statistical software (version 23.0) was used for statistical analysis. Normally distributed data were determined by a Kolmogorov-Smirnov test and analyzed using independent sample t-tests. This Study was approved by the ethics Committee of Borujerd Branch, Islamic Azad University, Borujerd, Iran.
Table 1: Primer sequences applied in the PCR reaction of semen cDNA samples
| Primer Name |
Sequence 5`→3` |
| GAPDH (F) |
GTCTCCTCTGACTTCAACAGCG |
| GAPDH (R) |
ACCACCCTGTTGCTGTAGCCAA |
| NRF2 (F) |
TTCAGCCAGCCCAGCACATC |
| NRF2 (R) |
CGTAGCCGAAGAAACCTCATTGTC |
| GSTM1 (F) |
TGATGTCCTTGACCTCCACCGT |
| GSTM1 (R) |
GCTGGACTTCATGTAGGCAGAG |
Table 2. The mean of the parameters studied in the seminal fluid of smokers and nonsmokers
| Parameters |
smokers |
Nonsmokers |
P-value |
| Number |
15 |
15 |
- |
| Age (year) |
33.6 ± 6.2 |
35 ± 7.1 |
P> 0.05 |
| Smoking duration (year) |
7 ± 3.2 |
0 |
P< 0.001 |
| Sperm concentration (x 106/ml) |
45.96 ± 8.03 |
97.7 ± 12.8 |
P< 0.001 |
| Semen volume (ml) |
3.5 ± 1.86 |
3.0 ± 1.3 |
P> 0.05 |
| pH |
7.5-8 |
7.5-8 |
- |
| Progressive motility |
25.6 ± 4.3 |
55.9 ± 6.7 |
P < 0.001 |
| Abnormal sperm forms (%) |
27.2 ± 6.2 |
19.3 ± 5.7 |
P > 0.05 |
Data is presented as Mean±SD
Results
Thirty people were included in the study; 15 smokers and 15 nonsmokers. The mean concentration of sperm in smokers was 45.96±8.03 x 106/ml and in nonsmokers, 97.7±12.8 x 106/ml, thus showing a statistically significant difference between smokers and nonsmokers (p<0.001). Significant differences were observed in the progressive sperm motility of smokers (25.6±4.3%) and nonsmokers (55.9%±6.7) (p<0.001). Insignificant differences were identified in sperm parameters, including volume and abnormal sperm forms in the two groups (p>0.05) (Table 2).
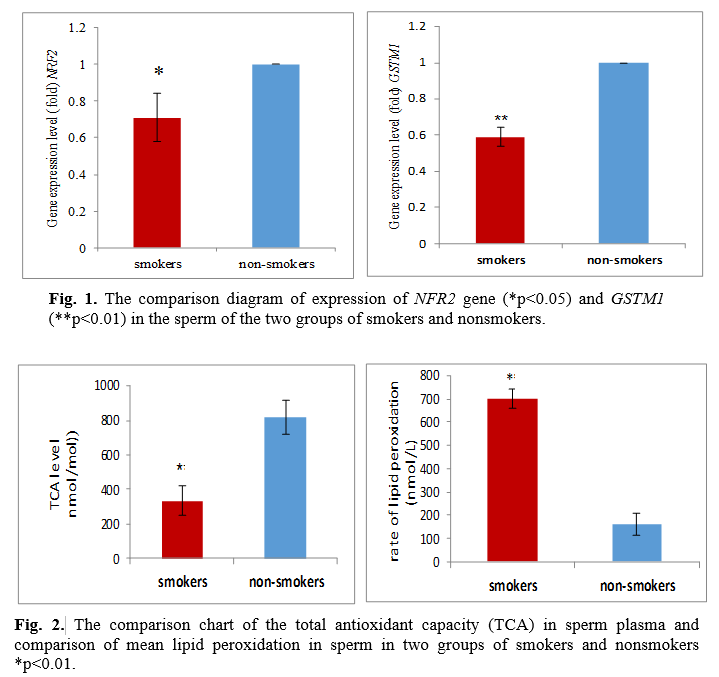
As set out in Fig. 1, the GSTM1 isoenzymes level in the seminal fluid was lower in smokers than in nonsmokers. Differences in these factors were statistically significant in smokers and nonsmokers (NRF2 at p<0.05 for, GSTM1 at p<0.01), and between the expression of the two GSTM1 and NRF2 genes, there was a significant and positive relationship, i.e., reducing the expression of a gene can lower the expression of another gene (r=0.55 and p<0.05). The results revealed that the malondialdehyde (MDA) level, being significantly higher as an indicator of oxidative, damages the seminal fluid of smokers greater than that of nonsmokers. Plasma total antioxidant capacity in smokers and nonsmokers turned out to be 334.48±84.6 nmol/mol and 815.2±98.2 nm/mol, respectively. The difference between the measured values indicates a significant variation between the groups (p<0.01). The rate of lipid peroxidation in the sperm of smokers was 700±42 nmol/L, and in nonsmokers 160.9±45.02 nmol/L. The difference between the measured values indicates a significant difference (p<0.01) between the groups (Fig. 2). The results demonstrated the relationship between MDA level and GSTM1 genes being substantial and negative, and with the NRF2 and GSTM1 genes expression, it proved to be positive so that an increase in one gene increased the other (Table 3). Further, the results showed a significant relationship between smoking duration and total antioxidant capacity with fertility parameters as well as gene expression (Table 4). The relationship between total antioxidant and studied parameters are shown in table 5.
Table 3. Relationship between different gene expression and MDA
| Parameters |
NRF2 |
GSTM1 |
MDA |
| |
P-value |
r |
P-value |
r |
P-value |
r |
| NRF2 |
-- |
-- |
< 0.05 |
0.54 |
> 0.05 |
-0.44 |
| GSTM1 |
< 0.05 |
0.54 |
-- |
-- |
< 0.001 |
-0.85 |
| MDA |
> 0.05 |
-0.44 |
< 0.001 |
-0.85 |
-- |
-- |
Table 4. Relationship between smoking duration with infertility parameters, MDA, total antioxidant capacity and NRF2, and GSTM1 gene expression.
| Parameters |
R |
P value |
| Semen volume in one ejaculation |
-0.16 |
P>0.05 |
| Sperm concentration |
-0.8 |
P<0.001 |
| Abnormal sperm forms |
0.45 |
P< 0.05 |
| Progressive motility |
-0.92 |
P < 0.001 |
| MDA |
0.91 |
P < 0.001 |
| Total antioxidant capacity |
-0.70 |
P < 0.01 |
| NRF2 |
-0.63 |
P < 0.01 |
| GSTM1 |
-0.94 |
P < 0.001 |
Table 5. The relationship between total antioxidant and the studied parameters
| Parameters |
R |
P value |
| Semen volume in one ejaculation |
0.24 |
P>0.05 |
| Abnormal sperm forms |
-0.39 |
P> 0.05 |
| Progressive motility |
0.51 |
P < 0.05 |
| MDA |
0.56 |
P < 0.05 |
| NRF2 |
0.67 |
P < 0.01 |
| GSTM1 |
0.6 |
P < 0.01 |
Discussion
Smoking is regarded as a crucial risk factor for reproductive health [28], but the effects of cigarette smoking on male fertility have not yet been well established [29]. Toxins, including lead present in tobacco, appear to directly impair the process of spermatogenesis itself as well as sperm function through reproductive axis dysfunction or testicular degeneration [30]. It is hard to detect the impact of smoking on semen quality because of complex influences involving a partner's smoking habits, genetic background, and environmental factors. Thus, exploring smoking consequences on the male reproductive system, concerning genetic background, may facilitate perception of the interactions between smoking and genetic variability and their potential synergetic impacts on male fertility, particularly considering the genes involved in antioxidant defense.
The present study discusses the effects of cigarette smoking on sperm traits, the level of expression level, which is effective in antioxidant defense, and the oxidative stress level in smokers' sperm. The results show that smoking can induce destructive effects on sperms. Although several studies have argued that smoking can result in abnormal sperm parameters [31], it is found to be more prevalent in smokers suffering from low semen quality in comparison to smokers with high semen quality and nonsmokers with low semen quality. This manifests a potential synergistic impact of functional polymorphisms of NRF2 and smoking on semen quality of humans. Oxidative stress imposed by smoking has been demonstrated to have a scanty effect on the antioxidant system and DNA damage [32]. Nevertheless, the smoking effects might be amplified if preimposed genetic variations in the antioxidant pathway are involved, as implied by the current results. Many research studies have proved that interactions between environmental factors and genes could be the result of male infertility [33].
Other studies, however, do not endorse such results. Merino et al. reported that smokers have a lower density, normal form, and sperm motility as compared with nonsmokers [27]. Zhang et al. showed that smokers' sperm has lower progressive motility than nonsmokers [31]. As environmental and genetic factors are crucial to human spermatogenesis, perceiving the synergistic influences of cigarette smoking and genetic variation on semen quality may pave the way for the development of new therapeutic and preventive responses to infertility of males.
The results of this study are consistent with the findings of Zhang and Merino [31, 27]. Mostafa et al. showed that although smokers do not experience fertility decline due to cigarette smoking, those who fail to have good sperm quality can reach a higher fertility prognosis by quitting smoking [34]. A significantly higher incidence of morphological influences of spermatozoa has been recognized among smokers with no difference in reproductive hormones and motility in both groups [35]. Smoking considerably decreased concentration of sperm and sperm count, showing that it negatively affected testicular spermatogenesis in humans. According to Nakamura et al., the decline in sperm counts regarding NRF2 gene deficits was more than that of sperm motility in mice. Also, sperm counts decreased significantly when the mice were 4-month old, but that motility did not decline significantly until 6 months of age, confirming the independent impacts of the NRF2 gene on the epididymis and testis [8]. Sperm generation in the testis and sperm maturation in the epididymis are two phases in spermatogenesis. In this regard, the findings of Nakamura and those of the current study show that the NRF2 gene influences both phases. The spermatozoa generation in the testis generates high amounts of ROS. Epididymis sperm cells are sensitive to ROS, causing loss of motility and, subsequently, cell death. Deficiencies in antioxidant capability and increase in exogenous ROS may, therefore, induce lesions both in the epididymis and testis. Further studies, however, are required to illuminate the synergistic impacts of smoking and functional polymorphisms in antioxidant genes on various spermatogenesis stages.
Nadeem et al. reported that cigarette smoking reduces sperm motility, and these complications are directly related to smoking [33]. Smoking may contribute to oxidative stress, generated because of ROS excessive levels coupled with antioxidants deficiency. Although low ROS levels are needed for sperm capacitation, acrosome reaction, hyperactivation, and spermatozoa–oocyte fusion, their excess deluges the neutralizing potential of antioxidants, inducing OS and damaging DNA in the mitochondria and nucleus [37].
In this study, the level of seminal GSTM1 isoenzymes in smokers was significantly lower than in nonsmokers. Hou-Qun Ying ans et al. in 2013 identified a significant relationship between GSTM1 gene expression and infertility [38], which is consistent with the results of ours. The results of this study reveal the malondialdehyde level as an indicator of oxidative damages, being significantly higher in the seminal fluid of smokers.
Total antioxidant capacity and antioxidant gene expression of NRF2 also decreased significantly. Besides, the results proved that infertility indicators such as sperm motility and sperm concentration exert a significant decrease in smokers as compared to nonsmokers. Nakamura et al. found the reduction of this gene is more effective in reducing sperm count than sperm motility [8].
While in the present study, reducing this gene decreased sperm motility more than sperm count, the results of this study revealed a significant and positive relationship between the expression of two GSTM1 and NRF2 genes, i.e., decreasing the expression of a gene reduces the expression of another gene. The results of this study demonstrated a significant relationship between total antioxidant capacity and sperm motility. Moreover, there was a significant relationship between MDA level and total antioxidant capacity, so that by increasing MDA, total antioxidant capacity decreased.
The relationship between MDA and GSTM1 gene expression was also significant and negative. This suggests that increased oxidative stress can reduce the antioxidant gene expression; the reason for this could be the molecular damage to these genes from free radicals. Furthermore, the relationship between total antioxidant capacity and gene expression was significant and positive. The relationship between GSTM1 gene expression and the total antioxidant capacity at p<0.05 and the relationship between NRF2 gene expression and antioxidant capacity was also shown. This positive relationship indicates that the antioxidant gene expression has a great effect on increasing total antioxidant capacity in the body. In this study, the relationship between smoking duration and the studied factors demonstrated that by increasing smoking duration, the concentration in seminal plasma decreased, and the sperm abnormality significantly rose; besides, by increasing smoking duration (year of smoking), sperm motility decreased significantly.
Faraji et al. compared oxidative stress in smokers and nonsmokers and showed that people with a history of over 5 years of the smoking bear a significant reduction in total antioxidant capacity [39]. These results are consistent with those of ours. This effective relationship exhibits that long-term smoking increases the accumulation of free radicals heightens genetic damage, and reduces fertility.
Conclusion
This research showed a novel interaction between smoking and genes in human spermatogenesis, hence providing an explanation for the inconsistency found in different studies regarding the smoking consequences in male fertility. The results may enhance our understanding of the effect of GSTM1 and NRF2 in ROS induced by cigarette smoke and of related impairments in spermatogenesis. Of course, more research is needed to determine the toxic effects of cigarette smoke on sperm parameters.
Conflict of Interest
The authors declared no conflicts of interest.
Acknowledgment
The authors thank all the contributors to this research. Also they would like to acknowledge that since this study was conducted as a pilot, it was run on a limited number of people.
References
[1]. Lassen TH, Iwamoto T, Jensen TK, Skakkebæk NE. Trends in male reproductive health and decreasing fertility: Possible influence of endocrine disrupters. Low fertility and reproductive health in East Asia. Dordrecht: Springer, 2015; p. 117-35
[2]. Abad Gairín C, Gual Frau J, Hannaoui Hadi N, García Peiró A. Antioxidant treatment and prevention of human sperm dna fragmentation: role in health and fertility. Handbook of Fertility, 2015; p. 397-409.
[3]. Jungwirth A, Giwercman A, Tournaye H, Diemer T, Kopa Z, Dohle G, et al. European Association of Urology guidelines on male infertility: The 2012 update. European Urology. 2012; 62(2): 324-32.
[4]. Bashamboo A, Ferraz-de-Souza B, Lourenco D, Lin L, Sebire NJ, Montjean D, Bignon-Topalovic J, Mandelbaum J, Siffroi JP, Christin-Maitre S, Radhakrishna U, Rouba Het al. Human male infertility associated with mutations in NR5A1 encoding steroidogenic factor 1. Am J Hum Genet. 2010; 87: 505-512
[5]. Zalata AA, Christophe AB, Depuydt CE, Schoonjans F, Comhaire FH. The fatty acid composition of phospholipids of spermatozoa from infertile patients. Mol Hum Reprod. 1998; 4:111-18.
[6]. Lavranos G, Balla M, Tzortzopoulou A, Syriou V, Angelopoulou R. Investigating ROS sources in male infertility: a common end for numerous pathways. Reproductive Toxicology. 2012; 34(3): 298-307.
[7]. Chen H, Zhao HX, Huang XF. Does high load of oxidants in human semen contribute to male factor infertility? Antioxidants and Redox Signaling 2012; 16(8): 754-59.
[8]. Nakamura BN, Lawson G, Chan JY, Banuelos J, Cortés MM, Hoang YD, et al. Knockout of the transcription factor NRF2 disrupts spermatogenesis in an age-dependent manner. Free Radical Biology and Medicine 2010; 49(9): 1368-379.
[9]. Meseguer M, Martínez-Conejero J A, Muriel L, Pellicer A, Remohí J, Garrido N. The human sperm glutathione system: a key role in male fertility and successful cryopreservation. Drug Metabolism Letters 2007; 1(2): 121-26.
[10]. Carrell DT, Aston KI. The search for SNPs, CNVs, and epigenetic variants associated with the complex disease of male infertility. Systems Biology in Reproductive Medicine 2011; 57(1-2): 17-26.
[11]. Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2- ARE pathway. Annu Rev Pharmacol Toxicol. 2007; 47: 89-116.
[12]. Holland R, Fishbein JC. Chemistry of the cysteine sensors in kelch-like ECH-associated protein 1. Antioxidants and Redox Signaling. 2010; 13(11): 1749-761.
[13]. Prestera T, Talalay P. Electrophile and antioxidant regulation of enzymes that detoxify carcinogens. Proceedings of the National Academy of Sciences of the United States of America. 1995; 92(19):8965-969.
[14]. Li Y, Paonessa JD, Zhang Y. Mechanism of chemical activation of Nrf2. PLoS One 2012; 7(4): 35122
[15]. McMahon M, Itoh K, Yamamoto M, Chanas SA, Henderson CJ, McLellan LI, et al. The Cap'n'Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001; 61(8): 3299-307.
[16]. Christopher-Hennings J, Dammen M, Nelson E, Rowland R, Oberst R. Comparison of RNA extraction methods for the detection of porcine reproductive and respiratory syndrome virus from boar semen. J Virologic Methods 2006; 136(1-2): 248-53.
[17]. Yu B, Chen J, Liu D, Zhou H, Xiao W, Xia X, et al. Cigarette smoking is associated with human semen quality in synergy with functional NRF2 polymorphisms. Biology of Reproduction 2013; 89(1): 1-5.
[18]. Chen K, Mai Z, Zhou Y, Gao X, Yu B. Low NRF2 mRNA expression in spermatozoa from men with low sperm motility. Tohoku Journal of Experimental Medicine 2012; 228(3):259–266.
[19]. An CN, Jiang H, Wang Q, Yuan RP, Liu JM, Shi WL, et al. Down-regulation of DJ-1 protein in the ejaculated spermatozoa from Chinese asthenozoospermia patients. Fertil Steril. 2011; 96(1): 19-23
[20]. Moscovitz O, Ben-Nissan G, Fainer I, Pollack D, Mizrachi L, Sharon M. The Parkinson's-associated protein DJ-1 regulates the 20S proteasome. Nature Communications 2015; 6(6609): 1-13
[21]. Mann CL, Davies MB, Boggild MD, Aldersea J, Fryer AA, Jones PW, et al. Glutathione S-transferase polymorphisms in MS: Their relationship to disability. Neurology 2000; 54: 552-57
[22]. Bolt HM, Thier R. Relevance of the deletion polymorphisms of the glutathione S-transferases GSTT1 and GSTM1 in pharmacology and toxicology. Curr Drug Metabol. 2006; 7: 613-28
[23]. Shamsi MB, Venkatesh S, Kumar R, Gupta NP, Malhotra N, Singh N, et al. Antioxidant levels in blood and seminal plasma and their impact on sperm parameters in infertile men. Indian J Biochem Biophys. 2010; 47: 38-43
[24]. World Health Organization.WHO laboratory manual for the examination and processing of human semen, 5th ed. Geneva: World Health Organization Press; 2010.
[25]. Aydemir B, Onaran I, Kiziler AR, Alici B, Akyolcu MC. Increased oxidative damage of sperm and seminal plasma in men with idiopathic infertility is higher in patients with glutathione S-transferase Mu-1 null genotype, Asian J Androl. 2007; 9: 108-15.
[26]. Wang B, Cao W, Biswal S, Dore S. Carbon monoxide-activated nrf2 pathway leads to protection against permanent focal cerebral ischemia. Stroke 2011; 42: 2605-610.
[27]. Merino G, Lira SC, Martinez-Chequer JC. Effects of cigarette smoking on semen characteristics of a population in Mexico. Arch Androl. 1998; 41: 11-15.
[28]. Kumar SB, Chawla B, Bisht S, Yadav RK, Dada,R. Tobacco use increases oxidative DNA damage in sperm-possible etiology of childhood cancer. Asian Pac. J. Cancer Prev. 2015; 16: 6967-972.
[29]. The Practice Committee of the American Society for Reproductive Medicine.Smoking and infertility. Fertil Steril. 2008; 90(S 1): 254-59.
[30]. Gandhi J, Hernandez RJ, Chen A, Smith NL, Sheynkin YR, Joshi G, et al. Impaired hypothalamic-pituitary-testicular axis activity, spermatogenesis, and sperm function promote infertility in males with lead poisoning. Zygote. 2017; 25: 103-110.
[31]. Zhang JP, Meng QY, Wang Q, Zhang LJ, Mao YL, Sun ZX. Effect of smoking on semen quality of infertile men in Shandong, China. Asian J. Androl. 2000; 2: 143-46.
[32]. Viloria T, Meseguer M, Martinez-Conejero JA, O'Connor JE, Remohi J, Pellicer A, Garrido N. Cigarette smoking affects specific sperm oxidative defenses but does not cause oxidative DNA damage in infertile men. Fertil Steril. 2010; 94: 631-37.
[33]. Nadeem F, Fahim A, Saira Bugit S. Effects of cigarette smoking on male fertility. Turk J Med Sci. 2012; 42(2): 1400-405
[34]. Mostafa T. Cigarette smoking and male infertility. Journal of Advanced Research. 2010; 1: 179-86.
[35]. Bundhun PK, Janoo G, Bhurtu A, Teeluck AR, Soogund MZS, Pursun M, et al. Tobacco smoking and semen quality in infertile males: a systematic review and meta-analysis. BMC Public Health. 2019; 19: 36.
[36]. Malhotra D, Thimmulappa R, Navas-Acien A, Sandford A, Elliott M, Singh A, et al. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med. 2008; 178: 592-604.
[37]. Bui A, Sharma R, Henkel R, Agarwal A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia 2018; 50(8): e13012.
[38]. Hou-Qun Y, Yue Q, Xiao-Ying P, Shuo-Ran L, and Zhou-Cun A. Association of GSTM1 and GSTT1 Genes with the Susceptibility to Male Infertility: Result from a Meta-Analysis. Genet Test Mol Biomarkers. 2013; 17(7): 535-42.
[39]. Faraji F, Ranjbar A, Eshrati B, Talaie A, Shafie N, Pirasteh S. Comparing the oxidative stress indexes of CVA patients with control group. J Arak Uni Med Sci. 2008; 11 (3): 109-116.