During recent decades, Acrylamide has been recognized as a risk factor for infertility. It is a white crystalline solid, which is water-and-alcohol-soluble, but insoluble in heptane and benzene. It is an odorless compound reactive that formed Acrylamide with its molecular formula C3H5NO [
1]. Acrylamide monomer is widely used to manufacture polymers and copolymers for use in the mining, paper
, and also the polymer industry, wastewater treatment, adhesives, laboratory gels, and oil field industries [
2]. Recently, the presence of Acrylamide in a wide variety of human food, including heat-treated food products, has been reported. The formation of Acrylamide is increased with high-temperature (≤200
◦C) processing in certain carbohydrate-rich foods such as chips, fried potatoes and bread. Besides, Acrylamide has been reported to be among carcinogen and cytotoxic material [
3]. Exposure to Acrylamide can occur in workplaces or environment through air, water, land, and groundwater during production or use [
4]. Additionally, Acrylamide showed reproductive toxicity, including germ cell mutagenicity and transmitted mutations. Recent report
s indicated that binding of Acrylamide or glycidamide to spermatid protamine causes dominant lethal mutation of testicular germ cells and morphologic abnormalities of sperm but no significant effects on epididymal sperm count or motility [
5,
6]. Some studies indicate that Acrylamide exposure can lead to testicular degeneration and damage in the testis. These changes were more severe with a more extended period of exposure effects of Acrylamide on the reproductive system of animals, including decreased sperm count, increased abnormal sperm morphology
, and severe testicular damages, such as vacuolation and swelling of the round spermatid, and break of DNA during specific germ cell stages [
7,
8]. Besides, animals fed with Acrylamide exhibited significant reductions in fertility, as well as sperm transport in the female reproductive tract
. It is necessary to decrease Acrylamide levels in different foods, and to find ways to decrease the Acrylamide formation as much as possible during the cooking process of different foods [
1].
Icariin (C33H40O15; molecular weight: 676.67), a flavonoid extracted from Herbaepimedii, is considered to be the principal active ingredient responsible for the actions of the plant. Herbaepimedii is known as an effective cure for cardiovascular diseases, osteoporosis (reduced bone mass), and tumors. Furthermore, it can improve the endocrine and immune system functioning [
9,
10]. Herbaepimedii has been used in China as a remedy for the erectile dysfunction, traditionally [
11]. Animal experiments showed that Icariin has an extensive range of effects on reproductive functions in male rats like improving erectile functioning in aged male rats in the way that streptozotocin-induced diabetic rats testosterone production can be increased by an accurate dose of Icariin [
12]. However, an inordinate dose of Icariin can probably cause harmful effects such as tissue and organ oxidative damage [
13].
There was not any published study reviewed the effect of both Acrylamide and Icariin, simultaneously. In this study, the effects of Icariin on histomorphometric changes of testis and prostate induced by Acrylamide were inspected in mice.
Materials and Methods
Chemicals
The Icariin, Acrylamide, and eosin were received from Sigma
_Aldrich Chemical Company. Other chemicals such as Azocarmine B and Aniline orange G were obtained from Santa Cruz Biotechnology.
Animals
The animals were handled according to a protocol approved by the Ethical Committee of Shahid Sadoughi University of Medical Sciences, Yazd, Iran, by the code of IR.SSU.MEDICINE.REC.1397.157.
This experimental study was conducted on 32 male mice, weighing 20-35 gr, which were obtained from the animal house of Shahid Sadoughi University of Medical Sciences, Yazd, Iran. Male mice were received to acclimate under moderated humidity (40-50%), temperature (22-24°C), and light conditions (12-hr light-dark cycle) and had free access to mice food and drinking water.
Experimental design
After 7 days of adaptation to the surroundings, the mice were randomly divided into four groups (n=8): a control group, a sham group that received water, a group receiving an intraperitoneal injection of Acrylamide (10 mg/kg body weight, dissolved in water) [
14] and a group receiving an intraperitoneal injection of Acrylamide (1.5 mg/kg, water solution) + Icariin (1.5 mg/kg, water solution) [
13].
Tissue preparation and histological analysis of testis and prostate
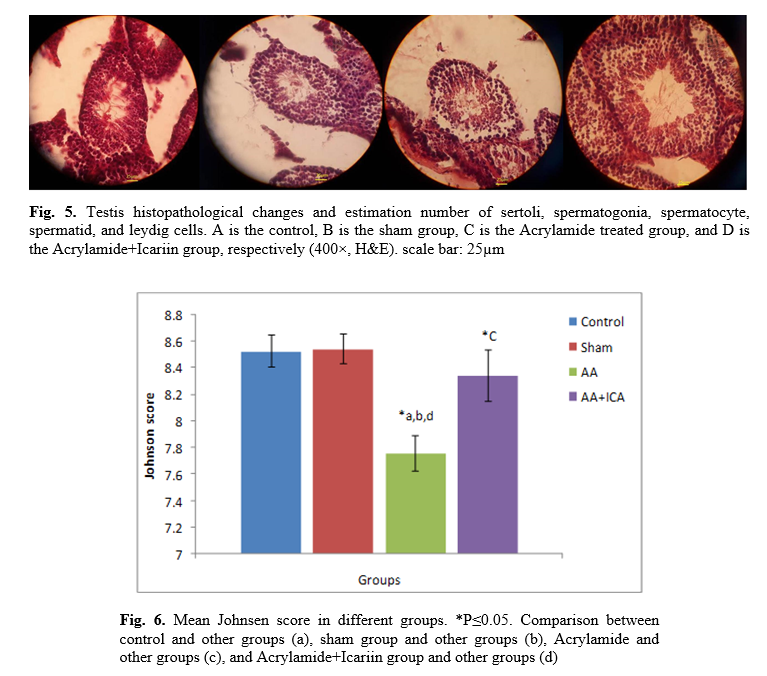
On day 36, all mice were weighed and anesthetized by chloroform; heart blood samples were subsequently taken and analyzed by mouse testosterone enzyme-linked immunosorbent assay kit for the quantitative determination of testosterone in mouse serum for each animal. The testosterone kit was purchased from Sigma Company. An analytic scale weighted the left testis and prostate of each mouse with a precision of 0.001gr. The testes and prostate were fixed in Bouin's and formalin solution, respectively, at room temperature for at least 24 h. It will slowly penetrate the tissue causing chemical and physical changes that harden and preserve the tissue and protect it against subsequent processing steps [
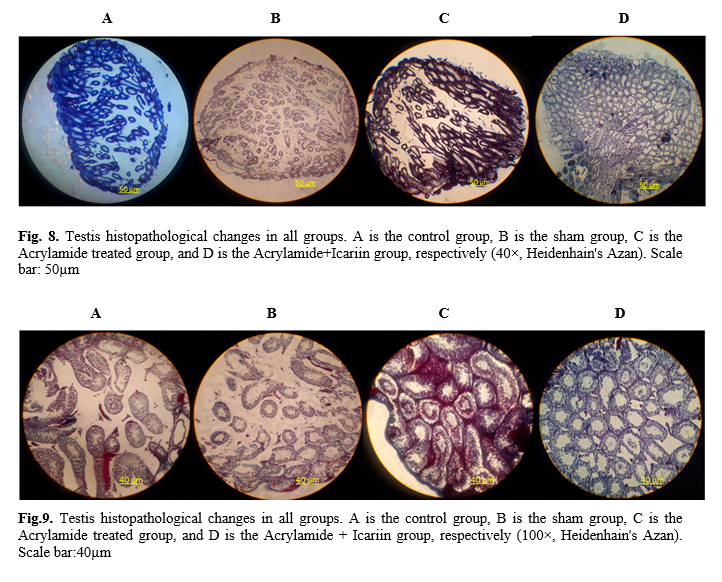
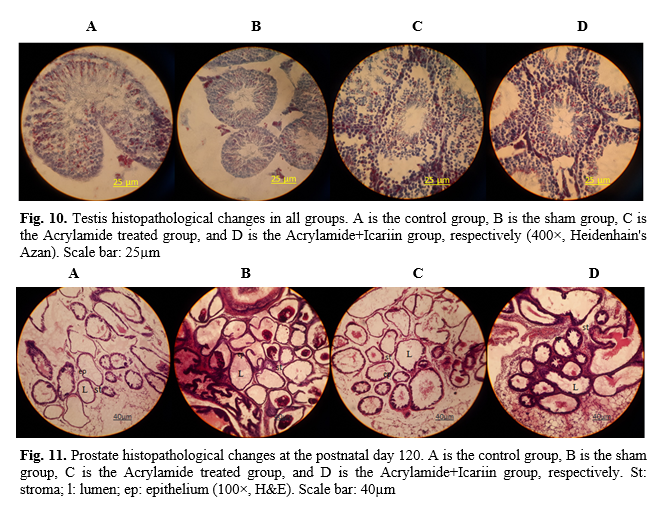
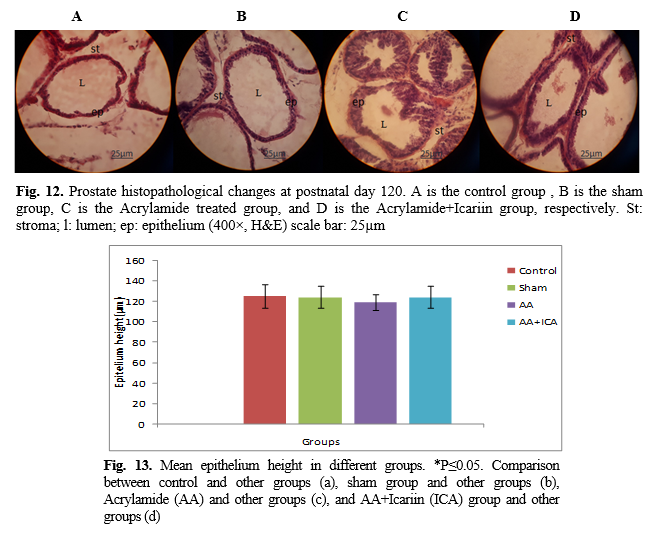
15]. The next steps were dehydration, clearing, and wax infiltration. The tissues were processed for paraffin blocking out and sectioning. Then, 5-µm serial microscopic sections with a certain distance were prepared, were taken from each testis, and serial sections were stained with hematoxylin and eosin and Heidenhain's Azan. The stained sections were mounted and examined under light microscopy (Olympus Japan) with the magnification of 40×, 100×, 400× to evaluate spermatogenesis and histopathological changes of the prostate.
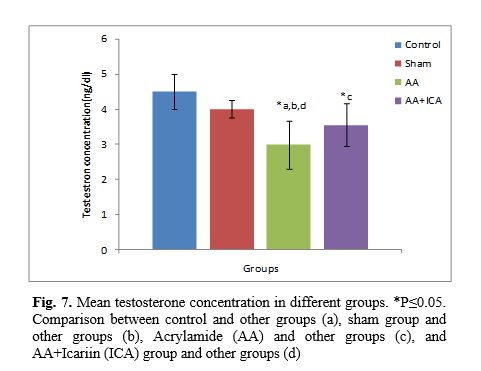
The maturity of the seminiferous epithelium was marked by using a modified Johnsen's score, which was used to categorize the spermatogenesis. This method applies a score of 1-10 for each tubule cross-section that was examined. The germinal epithelium of at least 50-60 tubules was assessed for each testis, and the mean Johnsen's score per mice was calculated [
16-18].
A grade from 1 to 10 was given to each tubule cross-section according to the following criteria: 10 = full spermatogenesis and perfect tubules; 9= many late spermatids and disorganized spermatogenesis; 8= only less than five spermatozoa per tubule, few late spermatids were present; 7= no spermatozoa, no late spermatids but many early spermatids present; 6= only a few early spermatids present, the arrest of spermatogenesis at the spermatid stage, disturbance of spermatid differentiation; 5= no spermatozoa or spermatids but many spermatocytes were present; 4= only a few spermatocytes were present, the arrest of spermatogenesis at the primary spermatocyte stage; 3= only spermatogonia was present; 2= no germinal cells but only sertoli cells were present; 1= tubular sclerosis, no germ cells and no sertoli cells were present.
Heidenhain's Azan staining
A technique used in stereological studies on the testis, using azocarmine B followed by aniline blue to stain nuclei red, cytoplasm pale pink, and the connective tissue blue [
19,
20]. This method differentiates the cells. Thus, cells can be easier investigated and counted.
Heidenhain's Azan Technique
The procedure is as follows:
1) Samples were stained in 1% Azocarmine B solution for about 15 minutes at room temperature. 2) Rinsed in distilled water. 3) Sections were stained in aniline for 2 seconds. 4) The samples were treated with glacial acetic acid for 5 seconds. 5) They were in phosphotungstic acid for 2-3 minutes. 6) They have transferred to an aniline blue-orange G-acetic solution for 5 minutes. 7) They were rinsed with distilled water.
Stereological analysis of testis
The volume of the testis (mm3)
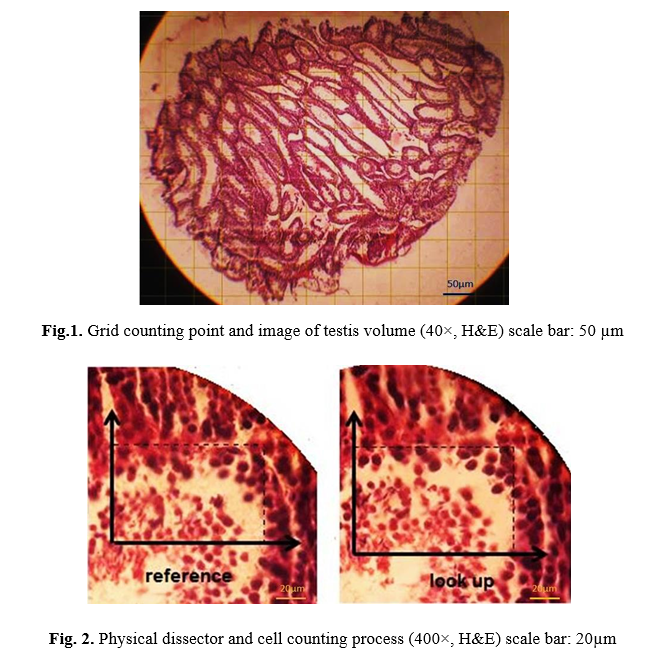
To estimate the total size and volume of the testes in mice, Cavalieri's principle was used because Cavalieri principle runs free from the shape of a solid object. Following histological procedures, the paraffin-embedded testis specimens were sectioned at 5 mm by the microtome with systematic random sampling. Serial sections, with a specific thickness, and a specific distance, have been prepared (5 µm) consecutively from each testis. A point counting grid with a 100 μm distance between two points was used to estimate the area of each section of the testis, and it was randomly located over images (since the point is lacking dimension, a grid with a network of crosses (+) was used (Fig. 1).
Then, hitting points on the surface of the biopsy were counted, and the total volume of the testis was calculated using the following formula:
V= t × a/p × ∑P/m2
V is the total volume of testis, t is defined as means section thickness, a/p; area per point (1000μ), ∑P; counted points in the component of interest, M; is the linear magnification [
21].
There was no significant magnitude or difference in shrinkage between groups, so shrinkage correction was not used in the study.
The number of sertoli, spermatogonia, spermatocyte, spermatid and leydig cells
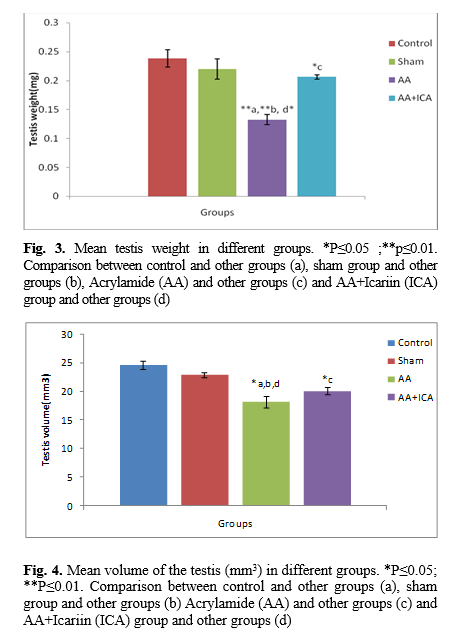
The number of sertoli, spermatogonia, spermatocyte, spermatid, and leydig cells was estimated using the physical dissector counting techniques. A dissector is a 3D sampling probe consists of two parallel planes for counting the number of cells. The size, shape, or spatial position of the cells do not affect this method. Two consecutive sections are then compared. These consecutive sections were chosen randomly. The first chosen section called the reference section and its next section called lookup section, and also called a dissector pair, 30μ separates these used sections as physical dissector pairs. A rule for the dissector counting method is that the distance between two section planes pairs must be about 1/3 of the average interest particle under investigation (in this study cell nuclei) to be estimated. During this method, twenty consecutive dissector pairs from any biopsy were used to the investigation [
22]. Only if the cells were not present in the lookup section, and cells do not touch forbidden lines, they would be counted (Fig. 2).









































 , Maryam Yadegari *
, Maryam Yadegari * 

 , Abbas Shahedi
, Abbas Shahedi 

 , Majid Pourentezari
, Majid Pourentezari 

 , Morteza Anvari
, Morteza Anvari 

 , Azadeh Shahrokhi Raeini
, Azadeh Shahrokhi Raeini 

 , Hengameh Dortaj
, Hengameh Dortaj