







































BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijml.ssu.ac.ir/article-1-361-en.html
Introduction
Age-related macular degeneration (ARMD) is one of the common causes of blindness among the elderly around the world. Two forms of ARMD are nonexudative or dry and exudative or wet. However, exudative-ARMD (E-ARMD) is more destructive and is responsible for more than 80% of the visible damage in such cases. The pathological cause of this disease is the formation of choroidal neovascular membranes below the retina and then, leaking fluid and blood. If it is left untreated, eventually it leads to the formation of a centrally blinding disciform scar in that area. Heredity, genetic factors, and aging of the retinal pigment epithelium are the primary causes of E-ARMD. However, some epigenetic factors such as oxidative stress could accelerate the pathogenesis and disease progression [1, 2]. The macula is mainly at risk of oxidative stress due to its exposure to direct light, high oxygen consumption, and the existence of large amounts of polyunsaturated fatty acids in its cell membranes. As a result, the imbalance between produced reactive oxygen species (ROS) and ROS clearance makes the macula more susceptible to oxidative damage [3, 4]. Evidence shows that a decrease in choroidal blood flow as well as the increase in choriocapillaris pressure result in basal deposits and drusen, calcification, and fragmentation of the membrane [5, 6].
Nitric oxide (NO) acts as a modulator of vascular tone and has a vital role in the regulation of both systemic and ocular blood flow. Endothelium-derived NO, which is involved in normal endothelial function, has anti-atherogenic and anti-proliferative effects on the vascular wall. Its biosynthesis is altered in cardiovascular disease [7, 8]. For example, the accumulation of the endogenous nitric oxide synthase (NOS) inhibitors such as asymmetric dimethylarginine (ADMA) and NG-monomethyl arginine contributes to a decrease in NO generation and results in vascular disease [9].
Protein-arginine methylation is known as one of the post-translational modifications of some proteins. ADMA is a product that comes from the proteolysis of arginine methylated-proteins. It is released into the extracellular space and blood and interferes with L-arginine in the production of NO [9, 10].
The accumulation of ADMA in the body is prevented by renal excretion and through metabolic degradation by dimethylarginine dimethylaminohydrolase (DDAH). Thus, impaired renal function or metabolic activity of DDAH may result in ADMA level elevation, which halts NO synthesis and causing vasoconstriction [11]. The action of DDAH is influenced by oxidative stress. A wide range of pathologic stimuli such as oxidized LDL (OX-LDL), inflammatory, hyperglycemia, hyperhomo-cysteinemia, cytokines, and infectious may induce endothelial oxidative stress. Each of these stimulants undermines DDAH activity in vitro and in vivo [12, 13].
PON1 is an antioxidant enzyme that exclusively attaches to high-density lipoprotein (HDL), which can hydrolyze fatty acid peroxides in LDL as well as atherosclerotic lesions [14]. The phenotypic features of PON1 depend on the genetic, epigenetic, and environmental factors associated with oxidative stress [15, 16]. It can metabolize several substrates including some oxon forms of organophosphate pesticides (referred to its paraoxonase activity) and some aryl esters (related to its arylesterase activity). Therefore, PON1 is a multifunctional enzyme. In this regard, several polymorphisms have been determined for the PON1 gene with different paraoxonase activity levels, but none of them exhibit polymorphism in arylesterase activity. Arylesterase activity could be served as a quantitative estimation of enzyme protein [17, 18]. Thus, the PON1 phenotypic study of the enzyme provides more accurate information on its body state than the genotypic study [18, 19].
Serum ADMA levels and the PON1 activity as oxidant and antioxidant agents, and OX-LDL as a by-product of oxidative stress have been suggested to be promising circulating markers of endothelial dysfunction. Therefore, in the present study, endothelial dysfunction in E-ARMD patients was assessed noninvasively by measuring such blood markers.
Materials and Methods
The subjects of this case-control study (45 E-ARMD patients and 45 healthy people in the age range of 50-80 years) were selected from the Retina clinic (Department of Ophthalmology, Tabriz University of Medical Sciences). All participants were evaluated by a complete ophthalmic examination including the visual acuity (using Snellen-Chart and calculated as the logarithm of the minimal angle of resolution, notated “logMAR”), slit-lamp biomicroscopy, dilated funduscopy (using a Slit-lamp, Haag-Streit, R 900; Haag- Streit AG, Switzerland with a super-field indirect lens), fundus photography, and fundus fluorescein angiography (Imagenet 2000; Topcon TRC 50IX; Topcon Corp., Japan). Selected patients had either a classic form of choroidal neovascularization or a disciform scar. To limit the effect of interference factors on the results, other pathological ophthalmic conditions, such as trauma and angle-closure glaucoma were removed. Also, the subjects in an oxidative state such as smokers, subjects taking antioxidant supplements, and people with disorders such as syndrome metabolic, diabetes, any inflammatory problems, and renal and liver disease were excluded. The Ethics Committee of Tabriz University of Medical Sciences confirmed that the present study was under the Declaration of Helsinki. Informed consent was taken from all participants.
Sampling and biochemical tests: Fasting blood samples were obtained from all participants and analyzed for biochemical parameters using an automated chemical analyzer (Abbott Analyzer; Abbott Laboratories, Abbot Park, IL). LDL was estimated using the Friedewald equation [20]. The rest of the specimens were kept at -70°C for further analysis.
Asymmetric dimethylarginine: Serum ADMA concentrations were determined using Human ADMA enzyme-linked immunosorbent assay (ELISA) Kit (MyBioSource, Canada, Cat.No. MBS264847), according to the constructor's instruction by double antibody sandwich method.
Paraoxonase activity: The basic and salt-stimulated paraoxonase activities were measured colorimetrically (at 405 nm) using paraoxon as a substrate (Sigma-Aldrich, Canada, Cat.No D9286). The reaction mixture contained 2.0 mM paraoxon and 2.0 mM CaCl2 in 0.1 M Tris buffer with pH 8.0. Then, 1 M NaCl was added into the above mixture for estimating the salt-stimulated activity. After adding 20 μL of serum into 700 μL of the reaction mixture, the increase in absorbance was estimated at 405 nm after one minute as the hydrolysis rate of paraoxon. Paraoxonase activity was stated as U/ml (one unit of enzyme hydrolyzes one mole of paraoxon per minute) [21].
Arylesterase activity: It was measured spectrophotometrically (at 270 nm) using phenylacetate (Merck-Schuchardt, Germany) as a substrate. The reaction mixture contained 0.1 M Tris buffer pH 8.0 and 2.0 mM phenylacetate. After adding 10ml of serum into 1cc of the reaction mixture, the increase in absorbance was estimated at 270 nm after one minute. Arylesterase activity was considered as U/l (one unit of enzyme hydrolyzes one mole of phenylacetate per minute) [21].
PON1 phenotypes determination: Double substrate method, which is the ratio of salt-stimulated paraoxonase activity to arylesterase activity was used for PON1 phenotype determination. Based on the obtained ratios, each given participant was phenotyped as AA (low activity), AB (moderate activity), or BB (high activity) [21].
Oxidized low-density lipoprotein: Serum OX-LDL levels were assayed using ELISA kit (Mercodia, Uppsala, Sweden), according to the manufacturer's instruction by double antibody
sandwich method.
Comprehensive lipid indexes: Non-HDL (total cholesterol (TC) minus HDL), TC/HDL, LDL /HDL non-HDL/HDL (atherogenic index, AI), TG/HDL (atherogenic index of plasma, AIP) and TC∗TG∗LDL/HDL (lipoprotein combine index, LCI) are considered to be predictors for atherosclerosis.
Statistical analysis
Variables were expressed as mean ± standard deviation. The Shapiro-Wilk normality test verified the normality of data distribution in phenotypes. Subsequently, parametric tests were used. ANOVA test followed by Tukey’s test for pair-wise comparisons were applied to determine differences between phenotypes. An independent-sample t-test was used to compare the parameters among the two groups. The significance levels were set at P < 0.05.
Results
The clinical characteristics of both groups of patients and control have been compared in Table 1. Both groups were matched in age and sex distribution. There were no significant differences in subclinical factors among patients and healthy people (p> 0.1) except in best-corrected visual acuity (p= 0.001).
Compared with the controls, E-ARMD patients had higher levels of TC and LDL. The values of nontraditional lipid profiles, including non-HDL-C, TC/HDL, LDL/HDL, AI, OX-LDL, and OX-LDL/HDL were all significantly higher in the patient group than in the control group. Not-significantly, the level of LCI was higher in E-ARMD patients. No significant differences were observed in the AIP index among both groups.
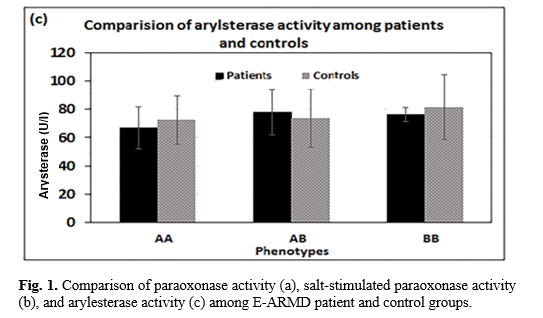
According to Figure 1, serum paraoxonase and also NaCl-induced paraoxonase activities were significantly increased in the order of AA < AB < BB phenotypes (p= 0.01) in both groups, while no significant differences were observed in serum arylesterase activity toward phenylacetate among three phenotypes (p> 0.05).
PON1 phenotypes were determined by the ratio of salt-stimulated paraoxonase activity to the arylesterase activity. The ratios indicated a triple PON1 frequency distribution among participants. The phenotypes were described the ratios below 2.00 as AA phenotype (n=44), the ratios between 2.01 and 4.20 as AB phenotype (n= 34) and the ratio above 4.21 as BB phenotype (n=12). Tukey’s test showed a significant difference in the distribution of the three PON1 phenotypes among two study groups (p=0.01). As it is shown in Fig. 2, the AA phenotype was more distributed among patients compared to healthy subjects (62.2% vs. 35.6%, respectively), while AB and BB phenotypes were more frequent among healthy subjects compared to ARMD patients (46.7% vs. 28.9% and 17.8% vs. 8.9%, respectively).
The serum concentration of ADMA was significantly higher in the patients than the healthy group (Table 1, p=0.02). Table 2 compares ADMA levels among the PON1 phenotypes. In each patient and healthy group, ANOVA multiple comparisons revealed no significant differences among three PON1 phenotypes (p=0.7 and p=0.5, respectively) in mean serum ADMA level, but it tended to be higher in AA and AB phenotypes and lower in BB phenotype.
|
Parameters |
Patients (mean±SD) |
Controls (mean±SD) |
P-value |
|
Number /Sex |
45/(18 M, 27 F) |
45/(18 M, 27 F) |
- |
|
Age (year) |
71 ± 7 |
69 ± 5 |
0.11 |
|
Best corrected visual acuity |
1.18 ± 0.36 |
0.10 ± 0.07 |
0.001 |
|
Pseudophakia |
34 |
29 |
- |
|
Glucose (mg/dl) |
88.0 ± 13 |
85.2 ± 14.9 |
0.58 |
|
Triglycerides (mg/dl) |
144.5 ± 63.8 |
153.7 ± 49.5 |
0.45 |
|
Cholesterol (mg/dl) |
204.0 ± 39.5 |
181.1 ± 36.3 |
0.005 |
|
HDL (mg/dl) |
43.1 ± 5.0 |
44.2 ± 6.3 |
0.3 |
|
LDL (mg/dl) |
131.6 ± 40.2 |
105.1 ± 35.3 |
0.001 |
|
Non-HDL |
4.2 ± 0.66 |
3.54 ± 0.7 |
0.001 |
|
Total Cholesterol/HDL |
4.8 ± 0.6 |
4.1 ± 0.5 |
0.001 |
|
LDL/HDL |
3.09 ± 0.1 |
2.4 ± 0.7 |
0.001 |
|
Atherogenic index |
3.8 ± 0.54 |
3.1 ± 0.5 |
0.006 |
|
Lipoprotein combine index |
27.8 ± 0.6 |
21.4 ± 0.4 |
0.07 |
|
Atherogenic index of plasma |
1.45 ± 0.09 |
1.49 ± 0.07 |
0.5 |
|
Oxidized LDL (U/l) |
52.2 ± 13.8 |
37.8 ± 10.8 |
0.001 |
|
Oxidized LDL /HDL |
1.2 ± 0.1 |
0.8 ± 0.06 |
0.001 |
|
Asymmetric dimethylarginine (μM) |
0.83 ± 0.27 |
0.71 ± 0.21 |
0.02 |
HDL= High-density lipoprotein; LDL= Low-density lipoprotein
Table 2. Comparison of asymmetric dimethylarginine concentrations (μM) among three phenotypes
|
Phenotype Case |
AA |
AB |
BB |
P-value |
|
Patients |
0.86 ± 28 |
0.82 ± 0.26 |
0.72 ± 0.17 |
0.7 |
|
Control |
0.77 ± 0.18 |
0.75 ± 0.22 |
0.70 ± 0.2 |
0.2 |
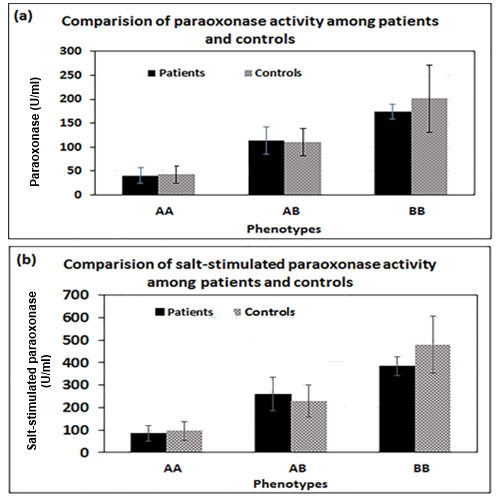


No significant correlation was found between serum OX-LDL and LDL levels in both groups of the case (p>0.07, r=0.27) and control groups (p>0.3, r=0.15). Figure 3 shows the correlations among the levels of total ADMA, OX-LDL, and PON1 activity in patient and control groups. A negative correlation existed between total OX-LDL and PON1 activity in both groups, but the correlation was statistically significant in the patient group (p=0.003). In both groups, the correlations between total levels of OX-LDL and ADMA were positively and statistically significant (P<0.05). No correlation was observed among PON1 activity and total ADMA level in each of patient and control groups (p>0.05), even though it was tended to be negative in both patient and control.
Discussion
It is approved by several studies that oxidative stress increase E-ARMD progression to advanced stages [1, 22]. Thickening of Bruch’s membrane and degradation of RPE and photoreceptors in the macula are indexes of disease progression that cause choroidal neovascularization. Endothelial dysfunction, that is occurred in these disorders, makes the release of some chemoattractant and inflammatory molecules, some growth factors, and a decrease in eNOS expression and activity. Oxidative stress may create a pro-angiogenic condition in the eye by exacerbating these phenomena which induce the formation of choroidal neovascularization [23, 24].
ADMA, as an independent risk factor, seems to be a predictor of cardiovascular complications due to the induction of endothelial dysfunction [25]. Enhanced levels of ADMA have been reported in several diseases with vascular abnormalities in both patients and animal models. Similar to these reports, increased serum ADMA concentrations were also detected in E-ARMD patients compared to the healthy group. Javadiyan et al., in their study on patients with advanced glaucoma, reported a significant increase in the circulation levels of both symmetric and asymmetric dimethyl-arginine (ADMA and SDMA) [26]. Although the mechanism for increasing serum ADMA in E-ARMD and its role in vascular disorders is not well understood, studies on cardiovascular disease have shown its effect on NO metabolism. As a competitive inhibitor factor for the arginine/ NO pathway, ADMA inhibits eNOS by preventing cationic amino acid transporters and the cellular uptake of arginine. Endothelial function associates with eNOS activity and vascular signaling of its product, NO. In arginine depleted cells, eNOS can produce superoxide anion (O2−). O2− inactivates endothelial-derived NO via the formation of peroxynitrite (ONOO−). ONOO− is a potent oxidant that seems to be a major mediator in NO-mediated tissue injury which in overproduction causes damage to biomolecules [27, 28].
A negative correlation among oxidative stress and PON1 activity and also among some PON1 polymorphism has been reported in exudative and non-exudative ARMD patients by several studies [29, 30]. More than 180 gene polymorphisms have been recognized for PON1 that have shown different protective effects against oxidative stress [31]. Also, the activity is affected by other factors such as epigenetics, environment, and lifestyle [15]. Therefore, determining the phenotype provides more precise information than genotypes for correlating PON1activity with susceptibility to E-ARMD disease.
In the present study, the total activity of PON1 was significantly decreased in E-ARMD patients compared to healthy control group. The polymorphic distribution of PON1 among the study population showed that AA, as weak PON1 phenotype, was more frequent among E-ARDM subjects, while AB and BB, as moderate and strong PON1 phenotypes, respectively, were more prevalent among healthy subjects.
PON1 which is associated with HDL prevents the accumulation of lipid peroxides in OX-LDL and inactivates bioactive oxidized phospholipids. High OX-LDL levels were detected in all PON1 phenotypes in E-ARMD which were equal among them. Similar to previous studies, the result showed a significant negative correlation among PON1 activity and OX-LDL levels in patients who suffer from oxidative stress [32, 33]. Regarding the high activity of PON1, such correlation was not significant in healthy subjects. That means an oxidative condition and elevated OX-LDL levels associate with weak PON1 phenotype and low enzyme activity. Elevated blood OX-LDL in strong PON1 phenotype (BB) of E-ARMD might be attributed to the high-oxidative state in E-ARMD disease. Macula characteristics such as the high amount of PUFAs in the cell membrane, high consumption of O2, and exposure to direct light make it more susceptible to oxidative stress which accelerates E-ARMD progression [23, 24]. Elevated vascular ROS generation inactivates endothelial-derived NO by forming peroxynitrite (ONOO−). Peroxynitrite can interact with prostacyclin synthase and inactivate it by nitrosating- both the reduction in NO and prostacyclin activity resulted in the development of hypertension, endothelial dysfunction, and atherosclerosis [7, 22].
Positive correlations were detected among OX-LDL and ADMA levels in both patient and control groups. Oxidative stress indicated by high OX-LDL levels impairs DDAH activity and increases ADMA elaboration in endothelial cells [34, 35]. Therefore, OX-LDL and ADMA have synergistic effects on each other's production. In other words, these two factors are the cause and effect of producing each other. ADMA, as a main endogenous inhibitor of NO synthase, reduces NO production and decreases vasodilator responses. Thus, ADMA may be considered as a marker of endothelial dysfunction and a risk factor for vascular disease.
ADMA, as a competitive inhibitor factor for L-arginine/ NO pathway, inhibits eNOS by preventing cationic amino acid transporters and the cellular uptake of L-arginine. Endothelial function depends on eNOS activity and vascular signaling of its product, NO. In L-arginine depleted cells, eNOS can generate superoxide anion (O2−) that inactivates endothelial-derived NO due to the formation of ONOO−. ONOO−is a potent oxidant that its overproduction can cause oxidative damage to biomolecules and induce injury in multiple cell types including epithelial cells. It seems that peroxynitrite is an important mediator of NO-mediated tissue damage and involves in inflammatory and oxidative stress. Nevertheless, more researches are required to understand the mechanism of ADMA's effect on the progression of vascular disease in E-ARMD [2, 13].
As was mentioned in results, the total ADMA level was higher in the patient group compared to the healthy group, but no significant differences were observed in ADMA levels among PON1 phenotypes. Although it tended to be negative, no correlation was detected among total ADMA and PON1 activity. No previous study evaluated circulating ADMA levels, and PON1 in patients with E-ARMA disease, but Soyman et al. in their research on polycystic ovary syndrome [36] and Locsey et al. in their study on renal transplant patients [37] reported a negative correlation among serum ADMA levels and PON1 activity. They suggested that ADMA levels and PON1 activity are also expected to be indicators of endothelial dysfunction. Our results on E-ARMD indicate that high blood ADMA and low protective effect of PON1contribute independently and indirectly to endothelial dysfunction, which facilitates LDL penetration into the arterial wall and its oxidation into OX-LDL.
Dyslipidemia and oxidative stress are independent risk factors that alone or together can aggravate the development of atherosclerosis. Previous studies suggested that atherosclerosis may be involved in the etiology of E-ARMD [38, 39]. Some lipid indexes were considered as predictors for the development of atherosclerosis lesion including non-HDL, TC/HDL, LDL/HDL, AI, AIP, OX-LDL, OX-LDL/HDL, and LIC. All these estimated indexes were higher in the patients compared to healthy groups except with AIP which was equal in both groups. These data indicate that E-ARMD patients are susceptible to vascular and sclerotic injury. AIP index reflects the interaction between atherogenic and protective lipoprotein. A previous study showed that AIP had an inverse relationship with the diameter of the LDL particle [40]. It represents the concentration of small dense LDL. small dense LDL easily enters into the arterial wall compared with LDL and oxidized to OX-LDL. Although the AIP values were similar in the E-ARMD patient and control groups, high levels of OX-LDL in the patient group were probably due to endothelial dysfunction, high oxidative stress, and decreased PON1 activity.
Conclusions
E-ARMD is recognized as a multifactorial disease. In addition to genetic factors and aging of the retinal pigment epithelium layer, this disease is affected by the body's intrinsic oxidative and antioxidant capacities. Oxidative stress, as a potent modifier, may cause early illness or change the structure of macula and severity of the ARMD phenotype. Thus, this phenomenon is not a direct agent for this disorder but it induces the development and progression of E-ARMD in elderly subjects. High levels of ADMA, as an oxidant agent, and more distribution of weak PON1 phenotypes among the patients indicate high levels of oxidative stress in E-ARMD disease. Therefore, increased blood OX-LDL as an oxidant by-product may indicate and lipid indexes may predict the risk of E-ARMD more precisely than other blood factors. Our results showed that high blood ADMA and low protective effect of PON1 contribute independently and indirectly to endothelial dysfunction. However, more research is required to determine the mechanism of ADMA in the progression of E-ARMD. It was concluded that high-risk individuals could be identified by evaluating the blood levels of the factors involved in oxidative stress, and antioxidant therapies might reduce the incidence and progression of the disease.
Conflict of Interest
No competing interests to be disclosed.
Acknowledgments
This study was supported by the Drug Applied Research Center, Tabriz University of Medical Sciences. Thanks to the members of the Biochemistry Department of Tabriz University of Medical Sciences for their efforts in improving this study.
Reference
[1]. Jarrett SG, Boulton ME. Consequences of oxidative stress in age-related macular degeneration. Mol Aspects Med 2012; 33(4): 399-417.
[2]. Chen Y, Zeng J, Zhao C, Wang K, Trood E, Buehler J, et al. Assessing susceptibility to age-related macular degeneration with genetic markers and environmental factors. Arch Ophthalmol. 2011; 129(3): 344-51.
[3]. Nowak JZ. Oxidative stress, polyunsaturated fatty acids-derived oxidation products and bisretinoids as potential inducers of CNS diseases: focus on age-related macular degeneration. Pharmacologic Rep. 2013; 65(2): 288-304.
[4]. Kersten E, Paun CC, Schellevis RL, Hoyng CB, Delcourt C, Lengyel I, et al. Systemic and ocular fluid compounds as potential biomarkers in age-related macular degeneration. Survey Ophthalmol. 2018; 63(1): 9-39.
[5].Ehrlich R, Harris A, Kheradiya NS, Winston DM, Ciulla TA, Wirostko B. Age-related macular degeneration and the aging eye. Clinic Interven Aging 2008;3(3):473-82.
[6]. Ciulla TA, Harris A, Martin BJ. Ocular perfusion and age‐related macular degeneration. Acta Ophthalmologica Scandinavica 2001; 79(2): 108-15.
[7]. Kvasnicka T. NO (nitric oxide) and its significance in regulation of vascular homeostasis. Vnitrni lekarstvi. 2003; 49(4): 291-6.
[8]. Naruse K, Shimizu K, Muramatsu M, Toki Y, Miyazaki Y, Okumura K, et al. Long-term inhibition of NO synthesis promotes atherosclerosis in the hypercholesterolemic rabbit thoracic aorta. PGH2 does not contribute to impaired endothelium-dependent relaxation. Arteriosclerosis Thrombosis 1994; 14(5): 746-52.
[9]. Böger RH. Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the “L-arginine paradox” and acts as a novel cardiovascular risk factor. J Nutrition. 2004; 134(10): 2842-847.
[10]. Zakrzewicz D, Eickelberg O. From arginine methylation to ADMA: a novel mechanism with therapeutic potential in chronic lung diseases. BMC pulmonary medicine. 2009; 9(1): 5.
[11]. Aldámiz-Echevarría L, Andrade F. Asymmetric dimethylarginine, endothelial dysfunction and renal disease. Int J Mol Sci. 2012; 13(9): 11288-311.
[12]. Zhao C, Li T, Han B, Yue W, Shi L, Wang H, et al. DDAH1 deficiency promotes intracellular oxidative stress and cell apoptosis via a miR-21-dependent pathway in mouse embryonic fibroblasts. Free Radic Biol Med. 2016; 92: 50-60.
[13].Wells SM, Holian A. Asymmetric dimethylarginine induces oxidative and nitrosative stress in murine lung epithelial cells. Am J Respirat Cell Mol Biol. 2007; 36(5): 520-28.
[14]. Atamer A, Kurdas-Ovunc AO, Yesil A, Atamer YJ, Sjogojot GA. Evaluation of paraoxonase, malondialdehyde, and lipoprotein levels in patients with asymptomatic cholelithiasis. Saudi J Gastroenterol. 2014; 20(1): 66.
[15].Holland N, Lizarraga D, Huen KJ. Recent progress in the genetics and epigenetics of paraoxonase: why it is relevant to children’s environmental health. Curr Opinion Pediatr. 2015; 27(2): 240.
[16].van Dijk SJ, Tellam RL, Morrison JL, Muhlhausler BS, Molloy PL. Recent developments on the role of epigenetics in obesity and metabolic disease. Clinical Epigenetics 2015;7(1):66.
[17].Costa LG, Giordano G, Cole TB, Marsillach J, Furlong CE. Paraoxonase 1 (PON1) as a genetic determinant of susceptibility to organophosphate toxicity. Toxicology 2013; 307: 115-22.
[18]. Nakanishi M, Takanami Y, Maruyama T, Murata M, Motohashi Y, Nakano S, et al. The ratio of serum paraoxonase/arylesterase activity using an improved assay for arylesterase activity to discriminate PON1R192 from PON1Q192. J Atheroscleros Thrombos. 2003; 10(6): 337-42.
[19]. Sepahvand SF, Shafiei M, Ghaffari SM, Rahimi‐Moghaddam P, Mahmoudian M. Paraoxonase phenotype distribution in a healthy Iranian population. Basic Clinical Pharmacol Toxicol. 2007; 101(2): 104-7.
[20]. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18(6): 499-502.
[21]. Eckerson HW, Wyte CM, La Du BN. The human serum paraoxonase/arylesterase polymorphism. Am J Human Genet. 1983; 35(6): 1126-138.
[22]. Bellezza I. Oxidative stress in age-related macular degeneration: Nrf2 as therapeutic target. Frontiers in pharmacology. 2018; 9: 1280.
[23]. Marazita MC, Dugour A, Marquioni-Ramella MD, Figueroa JM, Suburo AM. Oxidative stress-induced premature senescence dysregulates VEGF and CFH expression in retinal pigment epithelial cells: implications for age-related macular degeneration. Redox Biol. 2016; 7(1): 78-87.
[24]. Nita M, Grzybowski A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxidat Med Cell Longev. 2016; 3164734.
[25]. Willeit P, Freitag DF, Laukkanen JA, Chowdhury S, Gobin R, Mayr M, et al. Asymmetric dimethylarginine and cardiovascular risk: systematic review and meta-analysis of 22 prospective studies. Journal of the American Heart Association 2015; 4(6): 1833.
[26]. Javadiyan S, Burdon KP, Whiting MJ, Abhary S, Straga T, Hewitt AW, et al. Elevation of serum asymmetrical and symmetrical dimethylarginine in patients with advanced glaucoma. Investigat Ophthalmol Visual Sci. 2012; 53(4): 1923-927.
[27]. Wells SM, Holian A. Asymmetric dimethylarginine induces oxidative and nitrosative stress in murine lung epithelial cells. Am J Respirat Cell Mol Biol. 2007; 36(5): 520-28.
[28]. Chen S, Li N, Deb-Chatterji M, Dong Q, Kielstein JT, Weissenborn K, et al. Asymmetric dimethyarginine as marker and mediator in ischemic stroke. Int J Mol Sci. 2012; 13(12): 15983-6004.
[29]. Baskol G, Karakucuk S, Oner AO, Baskol M, Kocer D, Mirza E, et al. Serum paraoxonase 1 activity and lipid peroxidation levels in patients with age-related macular degeneration. Ophthalmologica 2006; 220(1): 12-6.
[30]. Eren SH, Korkmaz I, Guven FMK, Tekin YK, Ozdemir L. Serum paraoxonase, arylesterase, and glutathione-S-transferase activities and oxidative stress levels in patients with mushroom poisoning. Clinics 2018;73: e16550.
[31].Costa LG, Vitalone A, Cole TB, Furlong CE. Modulation of paraoxonase (PON1) activity. Biochem Pharmacol. 2005; 69(4): 541-50.
[32]. Tsuzura S, Ikeda Y, Suehiro T, Ota K, Osaki F, Arii K, et al. Correlation of plasma oxidized low-density lipoprotein levels to vascular complications and human serum paraoxonase in patients with type 2 diabetes. Metabolism: Clinic Experiment. 2004; 53(3): 297-302.
[33]. Sozer V, Himmetoglu S, Korkmaz GG, Kaya S, Aydin S, Yumuk V, et al. Paraoxonase, oxidized low density lipoprotein, monocyte chemo-attractant protein-1 and adhesion molecules are associated with macrovascular complications in patients with type 2 diabetes mellitus. Minerva Medica. 2014; 105(3): 237-44.
[34]. Matsumoto Y, Ueda S, Yamagishi S, Matsuguma K, Shibata R, Fukami K, et al. Dimethylarginine dimethylaminohydrolase prevents progression of renal dysfunction by inhibiting loss of peritubular capillaries and tubulointerstitial fibrosis in a rat model of chronic kidney disease. J Am Society Nephrol. 2007; 18(5): 1525-533.
[35]. Monsalve E, Oviedo PJ, Garcia-Perez MA, Tarin JJ, Cano A, Hermenegildo C. Estradiol counteracts oxidized LDL-induced asymmetric dimethylarginine production by cultured human endothelial cells. Cardiovascul Res. 2007; 73(1): 66-72.
[36]. Soyman Z, Noyan V, Tulmac M, Yucel A, Sagsoz N, Bayrak T, et al. Serum paraoxonase 1 activity, asymmetric dimethylarginine levels, and brachial artery flow-mediated dilatation in women with polycystic ovary syndrome. Fertility and Sterility 2011; 95(3): 1067-72.
[37]. Locsey L, Seres I, Sztanek F, Harangi M, Padra J, Kovacs D, et al. Relationship between serum paraoxonase and homocysteine thiolactonase activity, adipokines, and asymmetric dimethyl arginine concentrations in renal transplant patients. Transplantation proceedings 2013; 45(10): 3685-687.
[38]. Vingerling JR, Dielemans I, Bots ML, Hofman A, Grobbee DE, de Jong PT. Age-related macular degeneration is associated with atherosclerosis. The Rotterdam study. Am J Epidemiol. 1995; 142(4): 404-409.
[39]. Wong TY, Tikellis G, Sun C, Klein R, Couper DJ, Sharrett AR. Age-related macular degeneration and risk of coronary heart disease: the atherosclerosis risk in communities study. Ophthalmology 2007; 114(1): 86-91.
[40]. Frohlich J, Dobiasova M. Fractional esterification rate of cholesterol and ratio of triglycerides to HDL-cholesterol are powerful predictors of positive findings on coronary angiography. Clinic Chem. 2003; 49(11): 1873-880.
Received: 2020/05/5 | Accepted: 2020/11/28 | Published: 2020/11/30
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







