Cartilage is an avascular, aneurysmal, and lymphatic-free connective tissue found in the synovial joints, spine, ribs, outer ears, nose, airways, and children’s and adolescents’ growth plates. There are three significant types of cartilage found in humans: hyaline, fibrous, and elastic. All three types have a low density of cells (chondrocytes) [1] that synthesize and secrete the significant components of the extracellular matrix (ECM) [2]. Besides, cartilage ECM comprises an inimitable family of proteoglycans implicate within a highly hydrated collagen fibrillary network to relocate the biomechanical functions of stipulating structural support and persistence to defor-mation. Chondrocyte-mediated synthesis and assembly of this matrix are aided, in turn, by the synthesis of dozens of additional non-collagenous proteins, proteoglycans, and glycoprotein. The abundance, distribution, and types of collagens and proteoglycans are different in each of the three cartilage types, increasing differences in appearance and biomechanical properties [3]. The cartilage tissue has an extremely high matrix/cell ratio: 2-3% of its mass consists of chondrocytes, and it is the only residing cells in articular cartilage. For the remaining, 65-80% consists of water, 12-21% of collagens being predominantly collagen type II (Coll II), 6-10% of proteoglycans, and approximately 2-3.5% other proteins. The arch-like orientation of Coll II fibrils, being almost horizontal in the superficial zone and almost entirely vertical in the deep zone, gives the articular cartilage its anisotropic nature and transducer mechanical forces throughout the entire tissue.
Additionally, the different zones do not contain the same (ratio of) molecules, having different (levels of) glycosaminoglycan and collagens
. Characteristics such as the calcification of the cartilage near the subchondral bone and lubricant production in the superficial zone are different [3, 4]. Other collagens found in articular cartilage include type III, type X, type XI, type XI, I, and XIV [5].
Cartilage disease and repair
The most prevalent joint disease, osteoarthritis, is characterized by pain and degenerative lesions of the cartilage, subchondral bone, and other joint tissues [6]. Some reasons are causing osteoarthritis to remain incompletely understood. It has become recognized over the years that osteoarthritis is a multifactorial disease. Aging in particular [7] and trauma [8] are the main risk factors identified for the development of osteoarthritis; however, other factors such as genetic predisposition, obesity, inflammation, gender, and hormones, or metabolic syndrome contribute to osteoarthritis development and lead to a more severe outcome [9-11]. Joint damage often leads to joint instability or intra-articular fractures that lead to post-traumatic arthritis [12].
Over the past few years, revitalizing medical strategies have been developed as an alternative to traditional surgical procedures, with the ambitious goal of creating new tissue, showing the most similar features of native cartilage [13]. Tissue composition is deeply connected with function. Thus, the ability to re-create structure is believed to be essential for regeneration. Regenerative medicine deals with developing innovative therapies focused on the repair, regeneration, and replacement of cells, tissues, or organs to restore structure and physiological functions compromised by diseases, trauma, congenital disabilities, or aging [14]. The regeneration of articular cartilage tissue remains one of the main challenges in the orthopedics field [15]. The problem arises from its avascularity, limiting progenitor cell infiltration and leading to the repair process [16]. In the case of osteoporotic lesions involving bone under cartilage cells, a process of repair by undifferentiated mesenchymal stem cells (MSCs) begins in the bone marrow. However, the resulting tissue is fibrocartilage, a poor alternative to the articular cartilage [17]. Besides, the injured joint's actively inflammatory environment has a role in influencing the repair potential [18]. The osteochondral grafting regenerative approach may present problems due to donor site morbidity and graft failure in the autologous procedure or possible disease transmission and short cell viability in the allogenic one [17]. Recent development strategies use various technologies as therapeutic solutions, including cell therapy, tissue engineering, and gene therapy [14].
Cartilage tissue engineering
Tissue engineering is the regeneration and remodeling of tissue
in-vivo to repair, replace, maintain, or enhance organ function. It is also helpful for engineering and growing functional tissue substitute in-vitro for implantation
in-vivo, a biological substitute for damaged or diseased tissues and organs [19, 20]. Successful tissue engineering relies on multiple factors, including obtaining appropriate cells for implantation, directing the development of those cells on a chondrogenic pathway using growth factors or cytokines, supporting the growing cells on a three-dimensional (3D) matrix (optimally biocompatible), and having that matrix remain in the cartilage defect at least until healing is completed [19].
Key concerns are prevalent with each of the elements mentioned above. First, it must be ensured that the implanted cells are immune privileged, or can provide immunosuppressive agents to avoid rejection by the host immune system. Various growth factors, such as the bone morphogenic proteins, are associated with both cartilage and bone development [21]. It is essential to prevent a developmental cascade at a cartilaginous stage, instead of implanting cells progressing to the bone and creating bone islands in the intra-articular joint. Most synthetic polymer matrices tend to degrade with significant acidic pH, which is detrimental to newly implanted cells and other host tissues. Therefore, biocompatible scaffolding is optimally added [19].
Many cells are manipulated
in vitro and eventually implanted to eliminate cartilage defects, including chondrocytes, bone marrow-derived mesenchymal stem/stromal cells (BMSC) adipose,
synovial periosteum-derived periosteum derived stem cells, and cells derived from Wharton’s jelly [19]. To properly manipulate these cells down the correct pathway, “the right signals must be given at the right place and at the right time”. Several growth factors are associated with cartilage regeneration but are not limited to them, bone morphogenetic protein 2, 4 and 7, growth and differentiation factor-5 (GDF-5), insulin-like growth factor-1 (IGF-1), and transforming growth factor Beta (TGFβ). These growth factors are introduced in various ways, including viral vectors, non-viral vectors, nuclear detachment, and direct delivery to the cell environment [20]. The 3-D support structure is the final key to cartilage regeneration.
Poly(lactic-co-glycolic) acid (PLGA) polymer
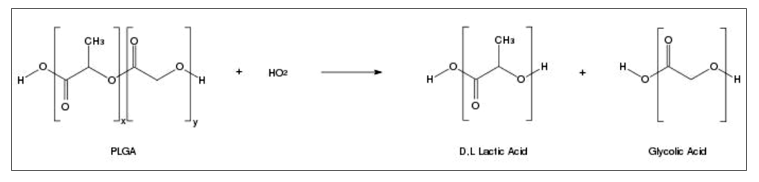
The most generally used biodegradable synthetic polymers for 3-D scaffolds in tissue engineering are saturated poly (α-hydroxy esters), including poly (lactic acid) (PLA) and poly (glycolic acid) (PGA), as well as PLGA copolymers [22]. The chemical properties of these polymers allow hydrolytic degradation through de-esterification. Once degraded, the monomer components of each polymer are removed by natural pathways. PGA is converted to metabolites or eliminated by other mechanisms, and PLA can be cleared through the tricarboxylic acid cycle. Due to these properties, the US FDA has approved PLA and PGA in biomedical products and devices such as destructive sutures. [23, 24]. PGA is a hydrophilic and highly crystalline polymer with a relatively fast degradation rate. Although structurally very similar to PGA, PLA exhibits different chemical, physical, and mechanical properties because of the presence of a pendant methyl group on the alpha carbon. Generally, the copolymer PLGA is preferred compared with its constituent homopolymers for the fabrication of bone and cartilage substitute constructs, as PLGA offers superior control compared with degradation properties by varying the ratio between its monomers. PLGA, for instance, has a wide range of degradation rates, governed by the composition of chains, both hydrophobic/hydrophilic balance and Cristallinity [24, 25]. However, despite biocompatibility, the clinical application of PLGA for cartilage regeneration is impaired by weak conductivity and demonstrates the maximum mechanical properties for administration as load-bearing applications (Table 1). Hence, PLGA is mainly used in combination with other materials such as ceramics, bioactive glass or is opportunistically modified to make PLGA more biomimetic and increase cartilage regeneration [26]. PLGA is a linear copolymer that can be prepared at different ratios between its constituent monomers, lactic and glycolic acid (Figure 1) [27]. Depending on the ratio of lactide to glycolide used for the polymerization, different forms of PLGA can be obtained: these are usually identified regarding the monomers’ ratio used (i.e., PLGA 50:50 identifies a copolymer consisted of 50% lactic acid and 50% glycolic acid) [28].
Different synthesis mechanisms are used to obtain PLGA, and the process parameters influence strongly the physicochemical characteristics of the end product. Among them, the solution poly-condensation of lactic and glycolic acid at temperatures above 120 °C under water-removal conditions allows the synthesis of PLGA with low molecular weight (MW < 10 kDa) [29, 30]. Unlike pure PLA and PGA show poor solubility’s, PLGA can be dissolved by a wide range of common solvents, including chlorinated solvents, tetrahydrofuran, acetone, or ethyl acetate [31, 32], and it can be processed into any shape and size and can encapsulate biomolecules of any size. The physical properties of PLGA have been shown to depend on various factors, including the initial molecular weight of the monomers, the LA: GA ratio, the time of exposure to water, and the storage temperature [33]. Table 1 shows the physicochemical properties and application of different PLGA materials characterized by different lactic: glycolic acid ratios [24].
PLGA degrades by hydrolysis of its ester linkages, through bulk or heterogeneous erosion, in aqueous environments. In details, four steps can be described during its degradation: (i) hydration: water penetrates the amorphous region and disrupts the van der Waals forces and hydrogen bonds, causing a decrease in the glass transition temperature; (ii) initial degradation: cleavage of covalent bonds, with a decrease in the molecular weight; (iii) constant degradation: carboxylic end groups auto catalyze the degradation process, and mass loss begins by massive cleavage of the backbone covalent bonds, resulting in loss of integrity; (iv) solubilization: the fragments are further cleaved to molecules that are soluble in the aqueous environment [34]. After the degradation, lactic and glycolic acid are formed as by-products.
Table 1. Properties of compounds and different percentages of polymers forming poly(lactic-co-glycolic) acid scaffolding
| Degradation time (weeks) |
Crystallinity (%) |
Solvent |
Elongation (%) |
Modulus (GPa) |
Polymer |
| 12–18 |
37 |
Benzene, tetrahydrofuran, dioxane |
- |
2.7 |
Poly(L-lactide) |
| 11–15 |
Amorphous |
Methanol, dimethylformamide |
3–10 |
- |
Poly(D,L-lactide) |
| 5–6 |
Amorphous |
Ethyl acetate, chloroform, acetone, tetrahydrofuran |
3–10 |
2.0 |
Poly(D,L-lactide coglycolide) 85/15 |
| 4–5 |
Amorphous |
Ethyl acetate, chloroform, acetone, dimethylformamide, tetrahydrofuran |
3–10 |
2.0 |
Poly(D,L-lactide-coglycolide) 75/25 |
| 1–2 |
Amorphous |
Ethyl acetate, chloroform, acetone, dimethylformamide, tetrahydrofuran |
3–10 |
2.0 |
Poly(D,L-lactide-coglycolide) 50/50 |
Fig. 1. Hydrolysis of poly lactic-co-glycolic acid
The degradation rates can be influenced by different parameters: (i) the molecular weight: increasing the molecular weight of conventional PLGAs from 10–20 to 100 kDa, degradation rates were reported to range from several weeks to several months; (ii) the ratio of lactic to glycolic acid: PLGA with a higher content of lactic is less hydrophilic, absorb less water and subsequently degrade more slowly, as a consequence of the presence of methyl side groups in PLA making it more hydrophobic than PGA. An exception to this rule is the copolymer 50:50, which exhibits the faster degradation; (iii) stereochemistry: mixtures of D and L lactic acid monomers are most commonly used for PLGA fabrication, as the rate of water penetration is higher in amorphous D, L regions, leading to accelerated PLGA degradation; and (iv) end-group function-alization: polymers that are end-capped with esters (as opposed to the free carboxylic acid) demonstrate longer degradation half-lives [35, 36]. Moreover, the shape of the device strongly affects PLGA degradation behavior depending on the accessibility of water. Besides, acidic surrounding media accelerate PLGA degradation due to autocatalysis [37]. For biomedical applications, PLGA has been used in various forms, such as porous scaffolds, microspheres, and nanospheres described in detail in the following paragraphs.
PLGA porous scaffolds
The scaffold is a 3-D construction where the cells can attach properly and grow potentially [38]. Different kinds of biomaterial are used for constructing the scaffolds. The ideal biomaterial should be biocompatible, non-toxic, non-stimulatory of inflammatory cells, non-immunogenic [39]. It should also have some special appearance that supports the cell to stick together, reproduce, differentiate into characteristic phenotypes, such as mechanical support for cartilage-engineered tissue, and have pores that cause the release of nutrients and waste products. Moreover, these materials must be degradable and provide the reconstruction as new cartilage forms and remodel the principal structure. They must be resistant to decadence at physiological pH and body temperature [40, 41]. The perfect scaffold for cartilage tissue engineering is the one with high porosity and pore-to-pore interconnectivity. High porosity provides adequate space for in-vitro cell adhesion, with, and restructuring of cells [41]. The interconnected porous organization facilitate migration, spread of physiological nutrients and gasses to the cells, and discharge metabolic waste and side-products from cells [42, 43]. Investigations of cartilage tissue engineering have been primarily focused on two loadings: straight confined or unconfined compression and hydrostatic pressure. The direct dynamic compression administered in chondrocyte-seeded scaffolds usually generates enhanced ECM production and proliferation and improves the compressive characteristics of the engineered tissue [44]. The scaffolds needed for cartilage repair have been made using several types of materials with both natural and synthetic polymer bases in a variety of forms [45]. Synthetic polymers are mainly favored because they are quite flexible in modifying the physical, mechanical, and chemical properties; consequently, the ultimate scaffold can be simply processed into the desired form and dimensions [22, 41]. A significant number of synthetic polymers have formerly been successfully incorporated into cartilage tissue engineering. At present, a small number of synthetic polymers are clinically evaluated for their potential use in cartilage repair. The principal disadvantage of the administration of artificial polymers is that their cells mostly do not maintain the chondrocyte phenotype and create a low-quality ECM [46]. On the other hand, natural polymers are cost-effective, environment-friendly, highly biodegradable, less toxic, and renewable, and take low manufacturing and disposal costs. Besides, they have essential control properties that greatly determine the success of cartilage tissue regeneration, including regeneration, biological signaling, cell response degradation, and cell adhesion [22].
Produce 3-D porous scaffolds
Solvent casting/particulate leaching
One way to create pores involves using a water-soluble porogen, such as salt (NaCl) [47]. The first step in this process is to dissolve the polymer ]Poly (l-lactic acid) (PLLA or PLGA)] in chloroform or methylene chloride and then cast it onto a petri dish filled with the porogen. After evaporation of the solvent, the polymer/salt composite is leached in water for two days to remove the porogen. The amount of salt can control the resulting scaffold’s porosity added, while the pore size is dependent on the size of the salt crystals [22]. With 70 weight percent salt and above, the pores exhibited high interconnectivity[48]. Foams fabricated in this manner have been used extensively with various cell types and have shown no adverse effects on new tissue formation [22, 49]. However, due to concerns that the side of the foam exposed to air had a different morphology (rougher) than that exposed to the petri dish, a modification of this technique has been developed [50, 51]. In this instance, the polymer composite components/compression salt is formed cylindrically at the right high melting point (PLLA) or glass transfer temperature (PLGA). Afterward, the cylinder is cut into thick discs before rinsing in water. It permits more accurate control of the thickness of the scaffold and enhances the uniformity of the floor plane.
However, polymer thermal degradation during the compression mold production stage can be a concern. Using particle washing methods, the researchers made pores of PLLA and PLGA scaffolding with a maximum porosity of 87% and porous wells using porous hydrocarbons as well as pores with a diameter of more than 100 micrometers. After combining the porogen and polymer (dissolved in methylene chloride or chloroform) in the dough, the composite is packaged in the Teflon form. The mold is immersed in a hydrocarbon solvent (pentane or hexane) to eliminate the wax without dissolving the PLLA / PLGA. The residual foam dries for a few days to extract any solvent. Using this technique, thick samples (maximum 2.5 cm) are created with intertwined pores. This method also permits incorporating the particle phase to the paste to enhance the stability or electrical conductivity of the definitive structure. With any procedure of pouring solvents/washing particles, organic solvents are used, which in many cases does not prevent the addition of drugs to scaffolding during fabrication. Besides, the rinsing stage for water-soluble porogen outstandingly enhances scaffold provision time. However, in applications where prefabricated cell polymer structures are appropriate, promising results using a wide range of cell types makes these scaffolds very appealing [52].
Gas foaming
To eliminate the need for organic solvents in the pore-making process, a new technique involving gas as a porogen has been introduced. The process begins with solid discs of PGA, PLLA, or PLGA using compression molding with a heated mold. The discs are placed in a chamber and exposed to high-pressure CO2 (5.5 MPa) for three days, at which time the pressure is rapidly decreased to atmospheric pressure. Porosities of up to 93% and pore sizes of up to 100 µm can be obtained using this technique, but the pores are largely unconnected, especially on the surface of the scaffold [52]. While this fabrication method requires no leaching step and uses no harsh chemical solvents, the high temperatures involved in the disc formation prohibit the incorporation of cells or bioactive molecules, and the unconnected pore structure makes cell seeding and migration within the foam difficult [52, 53]. Researchers recently developed another approach to using gas as a porogen. This technique includes both gas foaming and particulate leaching aspects [54]. Ammonium bicarbonate is added to the polymer solution in methylene chloride or chloroform. The resulting mixture is very sticky and can be formed by hand or with a mold. The solvent is then evaporated, and the composite or vacuum is dried or immersed in warm water. Drying the vacuum causes the ammonium bicarbonate to reach excellent condition, while immersion in water leads to the simultaneous evolution of the gas and the washing of the particles. The second method is preferred because it does not cause non-polar outer skin, as seen in dried vacuum scaffolding. Using this method, a porosity of 90% and a 200-500 micrometers hole are obtained [52].
Phase separation
Additional techniques proposed for the fabrication of porous polymer scaffolds are based on the concepts of phase separation rather than an incorporation of a porogen. They include emulsification/freeze-drying [55] and liquid-liquid phase separation [56]. PLGA is dissolved in methylene chloride, and then distilled water is added to emulsify. The polymer/water mixture is poured into the mold and extinguished by placing it in liquid nitrogen. After extinguishing, the scaffolds are dried at -55˚ C and used to remove scattered water and polymer solvents. Large porosity scaffolds (up to 95%), but small pores [13-35 micrometers) are made using this technique [52]. They depend on parameters such as the ratio of the polymer solution to the water and the viscosity of the emulsion because these values affect the stability of the emulsion before it is extinguished [55]. Therefore, with further adjustment, it is possible that pore size could be increased. However, although this technique is advantageous as it does not require an extra washing/leaching step, using organic solvents remains a concern for the inclusion of cells and bioactive molecules. This, combined with the small pore sizes obtained, indicates that this fabrication method currently has limited usefulness in the field of tissue engineering [52]. Both PLLA and PLGA scaffolds have been formulated using this technique [57, 58]. This section reviews the characteristics of the PLGA scaffold along with the growth factors and the cells involved in cartilage tissue engineering (Table 2).
PLGA composite/hybrid scaffold
PLGA, which belongs to one of the synthetic scaffolds, has been widely investigated to serve as the substitute for tissue regeneration and approved by the Food and Drug Administration (FDA) of the United State for certain clinical applications. However, PLGA does not present a favorable surface for cell adhesion, proliferation, and differentiation because of the hydrophobic surface properties and lack of specific cell-recognizable signals [64]. To overcome this drawback, an alternative approach is to create a hybrid scaffold using a multifunctional biological protein and PLGA. Because the hybrid scaffold can be used to create a biomimetic cellular environment by balancing the structural and bio-functional elements, the advent of a biosynthetic hybrid scaffold signifies a major achievement in tissue engineering [22, 65].
Table 2. Overview of investigated PLGA scaffold in cartilage tissue engineering
| Author |
Year |
Scaffolds
type |
lactide/
glycolide ratio |
Fabrication PLGA Scaffold |
Growth factor |
Model/
Cells |
Outcome |
Uematsu K
[59] |
2005 |
PLGA |
Not stated |
SC/PL |
CM |
In-vivo/
rabbit BMSCs |
Provided architectural support and cue to differentiate the MSCs to hyaline cartilage |
Park J
[60] |
2011 |
PLGA |
75:25 |
SC/PL |
CM+ dexamethasone+ TGF-β1 |
in-vitro-
in-vivo/
hADSCs |
Chondrogenic differentiation of hADSCs in vitro without chondrogenic factors. Maintained chondrogenic differentiation of hADSCs in subcutaneous pockets of athymic mice |
Zhang Y
[57] |
2012 |
PLGA |
Not stated |
PS |
CM |
in-vitro-
in-vivo/
Porcine chondrocytes |
Formed thicker cartilage with a more homogeneous structure, stronger mechanical property, and higher cartilage matrix, scaffolds showed better cartilage formation in terms of size, wet weight, and homogeneity in nude mice |
Caminal M
[61] |
2016 |
PLGA |
50:50
75:25 |
SC/PL |
CM |
in-vivo/
bovine chondrocytes, BMSCs and
ADSCs |
BM emerges as a preferential source of MSC for novel cartilage resurfacing therapies of osteochondral defects using PLGA scaffolds. |
Paduszynski P
[62] |
2016 |
PLGA |
85:15 |
SC/PL |
CM + TGF-β3 |
in-vitro/
Wharton’s jelly MSCs |
increase of the genes expression Coll II and AGG, the chondrogenic capacity of WJ-MSCs |
Zhao CF
[63] |
2019 |
PLGA |
75:25 |
Not stated |
CM+ Icariin |
In-vivo/
rabbit chondrocytes |
PLGA and Icariin maintained the functional morphology of articular cartilage and inhibited the resorption of subchondral bone trabeculae |
SC/PL= Solvent casting/ particle leaching; PS: Phase separation; CM= Chondrogenic medium; BMSCs= Bone marrow stem cells; hADSCs= Human adipose-derived stem cells; ADSCs= Adipose-derived stem cells; TGFβ= Transforming growth factor Beta
Table 3. Overview of investigated PLGA composite scaffold in cartilage tissue engineering.
| Author |
Year |
Scaffolds
type |
Lactide/
glycolide ratio |
Fabrication PLGA scaffold |
Growth factor |
Model
cells |
Outcome |
Yoo HS.
[67] |
2005 |
PLGA/ Hyaluronic acid |
50:50
65:35 |
GF/ particle leaching |
CM |
In-vitro/
Bovine chondrocytes |
Enhanced cellular attachment, an increase of the
glycosaminoglycan and total collagen |
Wei Y
[68) |
2009 |
PLGA/fibrin |
50:50 |
low-temperature deposition |
CM |
in-vitro-
in-vivo/
Rabbit ASCs |
Enhanced cellular viability, increase production of Coll II and proteoglycans, promoted cartilage regeneration |
Xue D
[69] |
2010 |
PLGA/ nanohydroxyapatite |
Not stated |
PS |
CM |
in-vivo/
Rat BMSCs |
Repair defect areas |
Wang W
[70] |
2010 |
PLGA/fibrin |
75:25 |
GPL |
CM + TGF-β1 |
In-vivo/
rabbit BMSCs |
Increase of the cartilage-specific genes, differentiation of BMSCs to chondrocytes, osteochondral restoration |
Zheng Q
[71] |
2010 |
PLGA/fibrin |
Not stated |
traditional freeze-dried |
CM |
In-vitro/
rat BMSCs |
Reinforced the fibrin scaffolds and maintained their interspace improved cell proliferation |
Wang W
[72] |
2011 |
PLGA/fibrin |
75:25 |
GPL |
CM |
In-vitro/
rabbit Chondrocytes |
Maintaining phenotype, an increase of the GAG secretion |
Dai W
[73] |
2013 |
PLGA/ collagen |
90:10 |
SC/PL |
CM |
In-vivo/
bovine chondrocytes |
Increase of the cartilaginous extracellular matrices such as Coll II and AGG enhanced the Production of GAGs into the subcutaneous sites of nude mice |
Li B
[74] |
2013 |
PLGA/fibrin |
75:25 |
GPL |
CM+poly (ethylene oxide)-b-poly(L-lysine)/ TGF-β1 plasmid DNA complexes |
In-vivo/
rabbit BMSCs |
Increase of cartilage age-specific genes, increase of the GAG secretion, restoration of the full-thickness cartilage defects |
Hong HJ
(75] |
2014 |
PLGA/fibrin/ hyaluronan |
Not stated |
PS |
CM |
In-vivo/
rabbit Chondrocytes |
Tracheal reconstruction, favorable mechanical and functional recovery |
Sharifian Z
[49] |
2016 |
PLGA/ Hyaluronic acid |
48:52 |
SC/PL |
CM+ Avocado/ Soybean |
in-vitro/
hADSCs |
Increase of the SOX9, AGG, and Coll gene expression |
Guo W
[58] |
2018 |
PLGA/articular cartilage extracellular matrix (ACECM) |
70:30 |
PS |
CM |
In-vivo/
rabbit BMSCs |
Cartilage defects could be completely regenerated, MSC/scaffold constructs enhanced the structure-specific regeneration of hyaline cartilage in a rabbit model |
JE S
[47] |
2018 |
PLGA/duck’s feet collagen |
Not stated |
SC/PL |
CM |
In-vivo/
mice chondrocytes |
positive impact on the maintenance of cell proliferation, increase of the glycosaminoglycan accumulation |
Ahma T
[76] |
2018 |
PLGA/ atelocollagen |
65:35 |
SC/PL |
CM |
In-vitro/ rabbit chondrocytes |
An increase of the ECM secreion, promoted better cartilaginous tissue formation |
Hashemibeni B
[22]
|
2019 |
PLGA/fibrin |
48:52 |
SC/PL |
CM+ Avocado/ Soybean
MC+ TGF-β3 |
In-vitro/
hADSCs |
ASU can induce chondrogenesis in hADSCs in PLGA/ fibrin scaffold. Increase of unique markers of hyaline cartilage and reduce hypertrophic and fibrosis markers compared to the growth factor of TGF-β3 |
Gorji M
[77] |
2020 |
PLGA/Fibrin nanoparticles |
50:50 |
SC/PL |
CM + Icariin+ TGF-β3 |
In-vitro/
hADSCs |
Increase of the cartilaginous-specific gene expression, a decrease of the Coll I gene expression, differentiation of hADSCs to chondrocytes |
SC/PL= Solvent casting/ particle leaching; GF: Gas foaming, PS= Phase separation; GPL= Gelatin porogen leaching; CM= Chondrogenic medium; BMSCs= Bone marrow stem cells; hADSCs= Human adipose-derived stem cells; ADSCs= Adipose-derived stem cells; TGFβ= Transforming growth factor Beta
The defects of natural and synthetic polymers can be compensated by using composite scaffolds made of two or more polymers and functionalization of the polymers that provide proper conditions for cartilage regeneration. Composites create an amalgamation of different features of various polymers to control biodegradation, cell adhesion, proliferation, and differentiation [43, 45, 66]. This section reviews the specifications of composite/hybrid scaffolds based on PLGA, along with growth factors and cells in cartilage tissue engineering (Table 3).
PLGA-based drug delivery devices
Drugs and proteins are the fastest growing class of drugs used to control properties, reduce toxicity, and reduce the risk associated with treatment. However, the stability and delivery challenges associated with these agents have limited the number of marketed products. Maintaining an adequate shelf-life of peptide and protein drugs often requires solid-state formulation to limit hydrolytic degradation reactions [78]. Prescribing peptides and proteins may also require injectable formulations to prevent gastrointestinal damage and transient metabolism, while the short half-lives of peptides and proteins may require injectable formulations that reduce the dose frequency. To avoid the inconvenient surgical insertion of large implants, injectable biodegradable and biocompatible PLGA particles (microspheres, microcapsules, nanocapsules, nanospheres) could be employed for controlled-release dosage forms [79]. Drugs formulated in such polymeric devices are released either by diffusion through the polymer barrier, or by erosion of the polymer material, or by combining both diffusion and erosion mechanisms. In addition to its biocompatibility, drug compatibility, suitable biodegradation kinetics, and mechanical properties, PLGA can be easily processed and fabricated in various forms and sizes [80]. There are several techniques for making nanoparticles, such as: solvent evaporation (single emulsion process and double emulsion process) [81, 82], phase separation [83], and spray drying [84]. Topical therapy through intra-articular injection is a good strategy because osteoarthritis only affects the joints. However, when small molecular drugs enter the intra-articular space, they are easily and quickly eliminated by blood and lymph vessels. Many drugs were hydrophobic molecules and were classified into category II or IV using Bio-Drug Classification System [85]. The crystal suspension system is formed in the intra-articular space and provides the risk of crystal deposition and crystalline sinusitis. Therefore, a suitable drug delivery system for these drugs was needed to increase solubility and increase their retention time in the joint cavity [86]. Biodegradable and bioeliminable materials have been engineered to prepare drug delivery systems for intra-articular injection. It has a great diversity of benefits, including but not limited to enhancing the stability of encapsulated drugs, decreasing toxicity, reducing adverse effects, improving pharmacokinetics, and targeting specific sites [87-89].
Table 4. The micro and nanoscale particles applied for cartilage repair
| Outcome |
Model/Cell |
Drug |
Preparation
technique |
Particle
diameter |
lactide/
glycolide ratio |
Type DDS |
Year |
Author |
| No significant difference was found in the PLGA MS group compared to the control group |
Rabbit chondrocytes/
Ovalbumin/FCA-induced arthritis model |
Diclofenac |
None stated |
5-10 |
50:50 |
PLGA MS |
2000 |
Tuncay M
[91] |
| PLGA is promising for an effective cure of mono-articular arthritis in rabbits |
Rabbit chondrocytes/
Ovalbumin/FCA-induced arthritis model |
Naproxen |
Solvent evaporation |
10 |
50:50 |
PLGA MS/ albumin |
2001 |
Sibel B
[92] |
| PLGA nanospheres should be more suitable for delivery to inflamed synovial tissue than microspheres; PLGA particulate systems with biocompatibility in the joint can provide local-therapy action in joint disease in a different manner depending on the size of the system |
Rat-joint cavity |
Fluoresceinamine |
emulsion solvent diffusion |
265
26.5 |
50:50 |
PLGA MS
PLGA NS |
2002 |
Eijiro H
[93] |
| Prolonged pharmacological efficacy in the joints of arthritic rabbits. |
Rabbit antigen-induced arthritic model |
Betamethasone |
emulsion solvent diffusion |
300-490 |
50:50 |
PLGA NS |
2002 |
Eijiro H
[94] |
| Labrafil modulates the release rate of donor-acceptor substances such as ibuprofen. |
In-vitro |
Ibuprofen, Labrafil |
solvent evaporation |
39-69 |
50:50 |
PLGA MS |
2004 |
Fernández-Carballido A [95] |
| An increase of the collagen and GAG. This scaffold may be useful to regenerate cartilaginous tissue. |
Rabbit chondrocytes/
mouse subcutaneous |
None |
oil-water emulsion and solvent extraction–evaporation |
30–80 |
50:50 |
PLGA MS |
2005 |
Sun-Woong K
[96] |
PLGA MS/fibrin can improve the elastic modulus of the scaffold while has no side effect on the cell proliferation and GAG
secretion. |
In-vitro/
Rabbit Chondrocytes |
NH2 |
emulsion solvent evaporation |
70–100 |
85:15 |
PLGA MS/fibrin |
2009 |
Haiguang Z
[97] |
Biocompatible, uptake of 1 and 10
μm particles, prolonged action of magnetic particles |
Mouse subcutaneous |
Dexamethasone/ super paramagnetic
iron oxide nanoparticles |
Not stated |
1-10 |
75:25 |
PLGA MS |
2009 |
Nicoleta B
[98] |
| PLGA MS is used to deliver lornoxicam following intra-articular administration for enhancing targeting efficiency. |
Rabbit, Rat |
Lornoxicam |
Solvent solid-in-oil-in-water emulsion |
7.47 |
Not stated |
PLGA MS |
2011 |
Zhang Z
[99] |
| Suppressing papain-induced OA changes, improved GAG and Col II levels |
Rat
papain-induced OA |
Parathyroid hormone |
Not stated |
51-85 |
65:35 |
PLGA MS |
2012 |
Rajalakshmanan
E [100] |
| Possible to incorporate small hydrophilic drug in PLGA |
In-vitro |
Clonidine |
Double emulsion |
10-30 |
Not stated |
PLGA MS |
2012 |
Amélie G
[101] |
| PLGA NP in MS decreases the burst releasse and improves retention in vivo, the feasibility of using NiMs to slow down the burst release, and increases the retention of therapeutic agents in articular joints. |
Rat |
Brucine |
Oil-water emulsion |
12.38 |
50:50 |
PLGA NP in MS/ PVA |
2014 |
Zhipeng C
[102] |
| Increasing the articular cartilage of curcumin-treated animals prevented the structural changes of articular cartilage osteoarthritis. |
MIA-induced OA rat |
Curcumin |
Solvent solid-in-oil-in-water emulsion |
100-200 |
50:50 |
PLGA NPs |
2017 |
Niazvand F
[87] |
| Promoting BMSCs proliferation, cartilage tissue regeneration, and integration between the repaired and surrounding cartilages |
In-vitro/
Mouse BMSCs |
Ketogenic |
Double emulsion-solvent evaporation |
27–55 |
75:25 |
PLGA MS/ collagen |
2018 |
Sun X
[103] |
| siRNA p47phox that is introduced with poly (D, L-lactic-co-glycolic acid) (PLGA) nanoparticles (p47phox si_NPs) can alleviate chondrocyte cell death by reducing ROS production. |
Human chondrocytes/
MIA-induced OA rat |
siRNA p47phox |
Emulsification/solvent evaporation |
Not stated |
50:50 |
PLGA |
2020 |
Shin HJ
[104] |
| Decreasing mitochondrial dysfunction-induced cartilage damage |
Human chondrocytes/
MIA-induced OA rat |
p66shc-siRNA |
Emulsification/ solvent evaporation |
Not stated |
50:50 |
PLGA |
2020 |
Shin HJ
[105] |
| NPs designed to passively target cartilage by tuning physicochemical properties to improve the localization of injectable therapeutics |
Bovine Collagenase
OA model |
Cationic surfactant |
Oil–water emulsion |
260–290 |
50:50 |
PLGA NP/PVA |
2020 |
Brown S
[90] |
PLGA= Poly (lactic-co-glycolic) acid; MP= Microparticle; MS= Microspheres; NP= Nanoparticles, NS= Nanospheres;
FCA= Ovalbumin/F’reund’s Complete Adjuvant P@96252
As a delivery carrier, PLGA is suitable for a wide range of biomolecules and can control substance release behaviors [90]. In this section, several PLGA and interesting drugs have been tested as drug delivery systems (DDS) for cartilage repair and osteoarthritis therapy, and their advantages and disadvantages will be discussed (Table 4).
Conclusions
In this study, fictitious approaches, mechanical properties, laboratory degradation, and modification of PLGA scaffolding are highlighted. These confidants are significant for seed and cell adhesion, ECM secretion,
and regeneration of tissue. The in-vivo characterization of PLGA scaffolds and corresponding cell responses are still rather limited. In summary, much progress for PLGA porous scaffolds, a specific physical form of medical material, has been achieved in the latest decade and the development of regenerative medicine. Future research may concentrate more on a fundamental study on cell-material interaction, detailed evaluation of potentially positive and negative effects, scaffolding, efficient and practical modifi-cations based on those insights, and many considerations toward disparate clinical applications.
PLGA polymers appear to be an excellent DDS for the controlled administration of drugs, peptides, and proteins due to their biocompatibility and biodegradability. In general, PLGA degradation and drug secretion can be accelerated by greater hydrophobicity, increased chemical interactions between hydrolyzed groups, lower crystallization, and higher volume-to-device ratio. All these factors must be considered to regulate the mechanism of drug destruction and secretion for a given program. Besides, studies show that PLGA can be easily formed in drug-carrying devices on all scales, for example, as nanospheres, as microspores, and even as implants with a size of millimeters. They can block a wide range of drugs, peptides, or proteins. Existing DDSs are being optimized and, new DDSs are still being developed. It appears that the ideal DDS for the remedy of intra-articular osteoarthritis has not yet been found. However, many obstacles have been considered in producing DDS care and performance for the clinical utilization of cartilage. Given the evolution of DDS and the increase in the number of drugs that may be released from DDS, more clinical trials are expected to be performed to address the need for osteoarthritis treatment with DDS.
Conflict of Interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Acknowledgment
The authors thank all the contributors of the Department of Anatomy and Molecular Biology, University of Medical Sciences, Yazd, Iran.
References
- Wachsmuth L, Söder S, Fan Z, Finger F, Aigner T. Immunolocalization of matrix proteins in different human cartilage subtypes. Histol. Histopathol. 2006; 21(5): 477-85
- Naba A, Clauser KR, Ding H, Whittaker CA, Carr SA, Hynes RO. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biology 2016; 49(1): 10-24.
- Krishnan Y, Grodzinsky AJ. Cartilage diseases. Matrix Biology 2018; 71(1): 51-69.
- Poole AR, Kojima T, Yasuda T, Mwale F, Kobayashi M, Laverty S. Composition and structure of articular cartilage: a template for tissue repair. Clinical Orthopaedics and Related Research 2001; 391(391): 26-33.
- Eyre DR, Weis MA, Wu J-J. Articular cartilage collagen: an irreplaceable framework. Eur Cell Mater. 2006; 12(1): 57-63.
- Roseti L, Desando G, Cavallo C, Petretta M, Grigolo B. Articular cartilage regeneration in osteoarthritis. Cells 2019; 8(11): 1305-321.
- Valdes AM, Stocks J. Osteoarthritis and ageing. European Medical Journal Rheumatology 2018; 3(1): 116-23.
- Thomas AC, Hubbard-Turner T, Wikstrom EA, Palmieri-Smith RM. Epidemiology of post-traumatic osteoarthritis. Journal of Athletic Training 2017; 52(6): 491-96.
- Berenbaum F, Griffin TM, Liu‐Bryan R. Metabolic regulation of inflammation in osteoarthritis. Arthrit Rheumatol. 2017; 69(1): 9-21.
- Lementowski PW, Zelicof SB. Obesity and osteoarthritis. American Journal of Orthopedics-Belle Mead. 2008; 37(3): 148-51.
- Jiménez G, Cobo-Molinos J, Antich C, López-Ruiz E. Osteoarthritis: Trauma vs Disease. Adv Exp Med Biol. 2018; 1059: 63-83.
- Punzi L, Galozzi P, Luisetto R, Favero M, Ramonda R, Oliviero F, et al. Post-traumatic arthritis: overview on pathogenic mechanisms and role of inflammation. RMD open 2016; 2(2): 279.
- Burguera EF, Gato Calvo L, Pereira CR, Garcia F, Magalhaes J. Regenerative medicine approaches for osteoarthritis. Osteoarthritis; SM Group: Oklahoma, USA. 2016: 1-15.
- Dzobo K, Thomford NE, Senthebane DA, Shipanga H, Rowe A, Dandara C, et al. Advances in regenerative medicine and tissue engineering: Innovation and transformation of medicine. Stem Cells Int. 2018; 1(1): 1-24.
- Zhang W, Ouyang H, Dass CR, Xu J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Research 2016; 4(1): 1-14.
- Jiang Y, Tuan RS. Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat Rev Rheumatol. 2015; 11(4): 206-12.
- Roseti L, Grigolo B. Host environment: Scaffolds and signaling (Tissue Engineering) articular cartilage regeneration: Cells, scaffolds, and growth factors. Bio-orthopaedics: Springer 2017: 87-103.
- Francioli S, Cavallo C, Grigolo B, Martin I, Barbero A. Engineered cartilage maturation regulates cytokine production and interleukin-1β response. Clinic Orthopaedic Relat Res. 2011; 469(10): 2773-784.
- Han F, Wang J, Ding L, Hu Y, Li W, Yuan Z, et al. Tissue engineering and regenerative medicine: achievements, future, and sustainability in Asia. Front Bioengin Biotechnol. 2020; 8(1): 1-35.
- Kessler MW, Grande DA. Tissue engineering and cartilage. Organogenesis 2008; 4(1): 28-32.
- Chubinskaya S, Hurtig M, Rueger DC. OP-1/BMP-7 in cartilage repair. International Orthopaedics 2007; 31(6): 773-81.
- Hashemibeni B, Mardani M, Valiani A, Pourentezari M, Anvari M, Yadegari M, et al. Effects of avocado/soybean on the chondro-genesis of human adipose-derived stem cells cultured on polylactic-co-glycolic acid/fibrin hybrid scaffold. J App Biotechnol Rep. 2019; 6(4): 145-50.
- Jagur-Grodzinski J. Biomedical application of functional polymers. Reactive Functional Polymers 1999; 39(2): 99-138.
- Gentile P, Chiono V, Carmagnola I, Hatton PV. An overview of poly (lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int J Mol Sci. 2014; 15(3): 3640-659.
- Lanao RPF, Jonker AM, Wolke JG, Jansen JA, van Hest JC, Leeuwenburgh SC. Physicochemical properties and applications of poly (lactic-co-glycolic acid) for use in bone regeneration. Tissue Engineering Part B: Reviews 2013; 19(4): 380- 90.
- Tavakoli E, Mehdikhani-Nahrkhalaji M, Hashemi-Beni B, Zargar-Kharazi A, Kharaziha M. Preparation, characterization and mechanical assessment of poly (lactide-co-glycolide)/ hyaluronic acid/fibrin/bioactive glass nano-composite scaffolds for cartilage tissue engineering applications. Procedia Mat Sci. 2015; 11: 124- 30.
- Tavakoli E, Mehdikhani-Nahrkhalaji M, Sarafbidabad M. Poly (lactic-co-glycolic) acid (plga)-based compounds for articular cartilage regeneration. J Rep Pharmaceutic Sci. 2016; 5(2): 94-103.
- Makadia HK, Siegel SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011; 3(3): 1377- 397.
- Zhou S, Deng X, Li X, Jia W, Liu L. Synthesis and characterization of biodegradable low molecular weight aliphatic polyesters and their use in protein‐delivery systems. J App Polymer Sci. 2004; 91(3): 1848- 856.
- Wang ZY, Zhao YM, Wang F, Wang J. Syntheses of poly (lactic acid‐co‐glycolic acid) serial biodegradable polymer materials via direct melt polycondensation and their characterization. J Appl Polymer Sci. 2006; 99(1): 244- 52.
- Prakapenka AV, Bimonte-Nelson HA, Sirianni RW. Engineering poly (lactic-co-glycolic acid)(PLGA) micro-and nano-carriers for controlled delivery of 17β-estradiol. Ann Biomed Engineer. 2017; 45(7): 1697- 709.
- Ansari MJ, Alshahrani SM. Nano-encapsulation and characterization of baricitinib using poly-lactic-glycolic acid co-polymer. Saudi Pharmaceutic J. 2019; 27(4): 491-501.
- Houchin M, Topp E. Physical properties of PLGA films during polymer degradation. J App Polymer Sci. 2009; 114(5): 2848- 854.
- Engineer C, Parikh J, Raval A. Review on hydrolytic degradation behavior of biodegradable polymers from controlled drug delivery system. Trends in Biomaterials Artificial Organs 2011; 25(2): 79-85.
- Wu XS, Wang N. Synthesis, characterization, biodegradation, and drug delivery application of biodegradable lactic/glycolic acid polymers. Part II: biodegradation. Journal of Biomaterials Science, Polymer Edition 2001; 12(1): 21-34.
- Lu L, Peter SJ, Lyman MD, Lai H-L, Leite SM, Tamada JA, et al. In vitro degradation of porous poly (L-lactic acid) foams. Biomaterials 2000; 21(15): 1595- 605.
- Washington MA, Balmert SC, Fedorchak MV, Little SR, Watkins SC, Meyer TY. Monomer sequence in PLGA microparticles: Effects on acidic microclimates and in vivo inflammatory response. Acta Biomaterialia 2018; 65(2): 259-71.
- Chan B, Leong K. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Euro Spine J. 2008; 17(4): 467- 79.
- Rahman MS, Rana MM, Spitzhorn LS, Akhtar N, Hasan MZ, Choudhury N, et al. Fabrication of biocompatible porous scaffolds based on hydroxyapatite/collagen/chitosan composite for restoration of defected maxillofacial mandible bone. Progress Biomat. 2019; 8(3): 137- 54.
- Ahmed TA, Hincke MT. Strategies for articular cartilage lesion repair and functional restoration. Tissue Engineering B: Reviews 2010; 16(3): 305- 29.
- Ahmadi F, Giti R, Mohammadi-Samani S, Mohammadi F. Biodegradable scaffolds for cartilage tissue engineering. Galen Med J. 2017; 6(2): 70-80.
- Stoop R. Smart biomaterials for tissue engineering of cartilage. Injury 2008; 39(1): 77-87.
- Yadegari M, Hashemibeni B, Pourentezari M, Tavakoli E, Anvari M, Valiani A, et al. Evaluation of the mechanical characteristics of PLGA and PLGA/fibrin Scaffolds in providing an appropriate environment for viability and growth of human adipose-derived stem cells. Eur J Analytic Chem. 2018; 13(4): 151- 59
- Bian L, Fong JV, Lima EG, Stoker AM, Ateshian GA, Cook JL, et al. Dynamic mechanical loading enhances functional properties of tissue-engineered cartilage using mature canine chondrocytes. Tissue Engineer A. 2010; 16(5): 1781- 790.
- Hashemibeni B, Pourentezari M, Valiani A, Zamani M, Mardani M. Effect of icariin on the chondrogenesis of human adipose derived stem cells on poly (lactic-co-glycolic) acid/fibrin composite scaffold. Int J Adv Biotechnol Res. 2017; 8(2): 595-605.
- Chen G, Sato T, Ushida T, Hirochika R, Shirasaki Y, Ochiai N, et al. The use of a novel PLGA fiber/collagen composite web as a scaffold for engineering of articular cartilage tissue with adjustable thickness. Journal of Biomedical Materials Research Part A. 2003; 67(4): 1170- 180.
- Song JE, Tripathy N, Cha SR, Jeon SH, Kwon SY, Suh DS, et al. Three-dimensional duck’s feet collagen/PLGA scaffold for chondrification: role of pore size and porosity. Journal of Biomaterials science, Polymer edition 2018; 29(7-9): 932-941.
- Mikos AG, Temenoff JS. Formation of highly porous biodegradable scaffolds for tissue engineering. Electronic J Biotechnol. 2000; 3(2): 23- 4.
- Sharifian Z, Mardani M, Valiani A, Bahramian H, Zamani M, Tavakoli E, et al. Cartilage tissue engineering via avocado/ soybean unsaponifible and human adipose derived stem cells on poly (lactide-co–glycolide)/ hyaluronic acid composite scaffold. Int J Biotechnol. 2016; 7(3): 1142- 154
- Thomson RC, Yaszemski MJ, Powers JM, Mikos AG. Hydroxyapatite fiber reinforced poly (α-hydroxy ester) foams for bone regeneration. Biomaterials 1998; 19(21): 1935- 943.
- Goldstein AS, Zhu G, Morris GE, Meszlenyi RK, Mikos AG. Effect of osteoblastic culture conditions on the structure of poly (DL-lactic-co-glycolic acid) foam scaffolds. Tissue engineer. 1999; 5(5): 421- 33.
- Shastri VP, Martin I, Langer R. Macroporous polymer foams by hydrocarbon templating. Proceed Nation Academy Sci. 2000; 97(5): 1970- 975.
- Singh L, Kumar V, Ratner BD. Generation of porous microcellular 85/15 poly (DL-lactide-co-glycolide) foams for biomedical applications. Biomaterials 2004; 25(13): 2611- 617.
- Nam YS, Yoon JJ, Park TG. A novel fabrication method of macroporous biodegradable polymer scaffolds using gas foaming salt as a porogen additive. J Biomed Mat Res. 2000; 53(1): 1-7.
- Whang K, Thomas C, Healy K, Nuber G. A novel method to fabricate bioabsorbable scaffolds. Polymer 1995; 36(4): 837- 42.
- Nam YS, Park TG. Biodegradable polymeric microcellular foams by modified thermally induced phase separation method. Biomaterials 1999; 20(19): 1783- 790.
- Zhang Y, Yang F, Liu K, Shen H, Zhu Y, Zhang W, et al. The impact of PLGA scaffold orientation on in vitro cartilage regeneration. Biomaterials 2012; 33(10): 2926- 935.
- Guo W, Zheng X, Zhang W, Chen M, Wang Z, Hao C, et al. Mesenchymal stem cells in oriented PLGA/ACECM composite scaffolds enhance structure-specific regeneration of hyaline cartilage in a rabbit model. Stem Cells Int. 2018; 1(1): 1-12.
- Uematsu K, Hattori K, Ishimoto Y, Yamauchi J, Habata T, Takakura Y, et al. Cartilage regeneration using mesenchymal stem cells and a three-dimensional poly-lactic-glycolic acid (PLGA) scaffold. Biomaterials 2005; 26(20): 4273- 279.
- Park JG, Lee JH, Kim JN, Kang JA, Kim KJ, Park KD, et al. Chondrogenic differentiation of human adipose tissue-derived stem cells in functional PLGA scaffolds. Tissue Engineering and Regenerative Medicine 2011; 8(1): 47-54.
- Caminal M, Peris D, Fonseca C, Barrachina J, Codina D, Rabanal R, et al. Cartilage resurfacing potential of PLGA scaffolds loaded with autologous cells from cartilage, fat, and bone marrow in an ovine model of osteochondral focal defect. Cytotechnology 2016; 68(4): 907- 19.
- Paduszyński P, Aleksander-Konert E, Zajdel A, Wilczok A, Jelonek K, Witek A, et al. Changes in expression of cartilaginous genes during chondrogenesis of Wharton’s jelly mesenchymal stem cells on three-dimensional biodegradable poly (L-lactide-co-glycolide) scaffolds. Cel Mol Biol Let. 2016; 21(1): 14-31.
- Zhao CF, Li ZH, Li SJ, Li JA, Hou TT, Wang Y. PLGA scaffold carrying icariin to inhibit the progression of osteoarthritis in rabbits. Royal Society Open Sci. 2019; 6(4): 181877.
- Pang L, Hu Y, Yan Y, Liu L, Xiong Z, Wei Y, et al. Surface modification of PLGA/β-TCP scaffold for bone tissue engineering: hybridization with collagen and apatite. Surface Coatings Technol. 2007; 201(24): 9549- 557.
- Dikovsky D, Bianco-Peled H, Seliktar D. The effect of structural alterations of PEG-fibrinogen hydrogel scaffolds on 3-D cellular morphology and cellular migration. Biomaterials 2006; 27(8): 1496- 506.
- Oliveira JM, Rodrigues MT, Silva SS, Malafaya PB, Gomes ME, Viegas CA, et al. Novel hydroxyapatite/chitosan bilayered scaffold for osteochondral tissue-engineering applications: Scaffold design and its performance when seeded with goat bone marrow stromal cells. Biomaterials 2006; 27(36): 6123-137.
- Yoo HS, Lee EA, Yoon JJ, Park TG. Hyaluronic acid modified biodegradable scaffolds for cartilage tissue engineering. Biomaterials 2005; 26(14): 1925- 933.
- Wei Y, Hu H, Wang H, Wu Y, Deng L, Qi J. Cartilage regeneration of adipose-derived stem cells in a hybrid scaffold from fibrin-modified PLGA. Cell Transplantation 2009; 18(2): 159- 70.
- Xue D, Zheng Q, Zong C, Li Q, Li H, Qian S, et al. Osteochondral repair using porous poly
(lactide‐co‐glycolide)/nano‐hydroxyapatite hybrid scaffolds with undifferentiated mesenchymal stem cells in a rat model. J Biomed Mat Res A. 2010; 94(1): 259- 70.
- Wang W, Li B, Yang J, Xin L, Li Y, Yin H, et al. The restoration of full-thickness cartilage defects with BMSCs and TGF-beta 1 loaded PLGA/fibrin gel constructs. Biomaterials 2010; 31(34): 8964-973.
- Zheng Q, Zhao X. Research of mechanical strength enhanced fibrin-PLGA hybrid scaffold with its effect on proliferation of rMSC. e-Polymers. 2010;10(1):1-9.
- Wang W, Li D, Wang MC, Li YL, Gao CY. A hybrid scaffold of poly (lactide-co-glycolide) sponge filled with fibrin gel for cartilage tissue engineering. Chinese J Polymer Sci. 2011; 29(2): 233-40.
- Dai W, Yao Z, Dong J, Kawazoe N, Zhang C, Chen G. Cartilage tissue engineering with controllable shape using a poly (lactic-co-glycolic acid)/collagen hybrid scaffold. J Bioact Compat Polymer. 2013; 28(3): 247- 57.
- Li B, Yang J, Ma L, Li F, Tu Z, Gao C. Fabrication of poly (lactide‐co‐glycolide) scaffold filled with fibrin gel, mesenchymal stem cells, and poly (ethylene oxide)‐b‐poly (L‐lysine)/TGF‐β1 plasmid DNA complexes for cartilage restoration in vivo. Journal of Biomedical Materials Research Part A. 2013; 101(11): 3097- 108.
- Hong HJ, Chang JW, Park JK, Choi JW, Kim YS, Shin YS, et al. Tracheal reconstruction using chondrocytes seeded on a poly (l‐lactic‐co‐glycolic acid)–fibrin/hyaluronan. Journal of biomedical materials research part A. 2014; 102(11): 4142- 150.
- Tahir AH, Amin MA, Azhim A, Sha’ban M, editors. Evaluation of cartilaginous extracellular matrix production in in vitro” cell-scaffold” construct. in 2018 IEEE-EMBS conference on biomedical engineering and sciences (IECBES) 2018 Dec 3 (pp. 500-504). IEEE.
- Gorji M, Ghasemi N, Setayeshmehr M, Zargar A, Kazemi M, Soleimani M, et al. The effects of fibrin–icariin nanoparticle loaded in poly (lactic-co-glycolic) acid scaffold as a localized delivery system on chondrogenesis of human adipose-derived stem cells. Adv Biomed Res. 2020; 9(1): 6-15.
- Houchin M, Topp E. Chemical degradation of peptides and proteins in PLGA: a review of reactions and mechanisms. J Pharmaceutic Sci. 2008; 97(7): 2395- 404.
- Lee PW, Pokorski JK. PLGA devices: production and applications for sustained protein delivery. Wiley Interdisciplin Rev Nanomed Nanobiotechnol. 2018; 10(5): 1516.
- Anderson JM, Shive MS. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Delivery Rev. 1997; 28(1): 5-24.
- Hernández-Giottonini KY, Rodríguez-Córdova RJ, Gutiérrez-Valenzuela CA, Peñuñuri-Miranda O, Zavala-Rivera P, Guerrero-Germán P, et al. PLGA nanoparticle preparations by emulsification and nanoprecipitation techniques: effects of formulation parameters. RSC Advances 2020; 10(8): 4218- 231.
- Cohen-Sela E, Teitlboim S, Chorny M, Koroukhov N, Danenberg HD, Gao J, et al. Single and double emulsion manufacturing techniques of an amphiphilic drug in PLGA nanoparticles: formulations of mithramycin and bioactivity. J Pharmaceutic Sci. 2009; 98(4): 452- 462.
- Wang T, Zhang C, Zhong W, Yang X, Wang A, Liang R. Modification of three-phase drug release mode of octreotide PLGA microspheres by microsphere-gel composite system. AAPS Pharm Sci Tech. 2019; 20(6): 228-36.
- Arpagaus C. PLA/PLGA nanoparticles prepared by nano spray drying. Journal of Pharmaceutical Investigation 2019; 49(6): 405- 26.
- Sachan NK, Bhattacharya A, Pushkar S, Mishra A. Biopharmaceutical classification system: A strategic tool for oral drug delivery technology. Asian J Pharm. 2014; 3(2): 76-81.
- Kou L, Xiao S, Sun R, Bao S, Yao Q, Chen R. Biomaterial-engineered intra-articular drug delivery systems for osteoarthritis therapy. Drug Delivery 2019; 26(1): 870- 85.
- Niazvand F, Khorsandi L, Abbaspour M, Orazizadeh M, Varaa N, Maghzi M, et al. Curcumin-loaded poly lactic-co-glycolic acid nanoparticles effects on mono-iodoacetate-induced osteoarthritis in rats. Veterinary Research Forum 2017; 8(2): 155- 61.
- Kou L, Bhutia YD, Yao Q, He Z, Sun J, Ganapathy V. Transporter-guided delivery of nanoparticles to improve drug permeation across cellular barriers and drug exposure to selective cell types. Front Pharmacol. 2018; 9(1): 1-16.
- Yao Q, Kou L, Tu Y, Zhu L. MMP-responsive’ smart’drug delivery and tumor targeting. Trends Pharmacol Sci. 2018; 39(8): 766- 81.
- Brown S, Pistiner J, Adjei IM, Sharma B. Nanoparticle properties for delivery to cartilage: the implications of disease state, synovial fluid, and off-target uptake. Mol Pharmaceut. 2017; 16(2): 469- 79.
- Tuncay M, Caliş S, Kaş H, Ercan M, Peksoy I, Hincal A. Diclofenac sodium incorporated PLGA (50: 50) microspheres: formulation considerations and in vitro/in vivo evaluation. Int J Pharmaceut. 2000; 195(1-2): 179- 88.
- Bozdag S, Calis S, Kas H, Ercan M, Peksoy I, Hincal A. In vitro evaluation and intra-articular administration of biodegradable microspheres containing naproxen sodium. J Microencapsul. 2001; 18(4): 443- 56.
- Horisawa E, Hirota T, Kawazoe S, Yamada J, Yamamoto H, Takeuchi H, et al. Prolonged anti-inflammatory action of DL-lactide/glycolide copolymer nanospheres containing betamethasone sodium phosphate for an intra-articular delivery system in antigen-induced arthritic rabbit. Pharmaceutic Res. 2002; 19(4): 403- 10.
- Horisawa E, Kubota K, Tuboi I, Sato K, Yamamoto H, Takeuchi H, et al. Size-dependency of DL-lactide/glycolide copolymer particulates for intra-articular delivery system on phagocytosis in rat synovium. Pharmaceutic Res. 2002; 19(2): 132- 39.
- Fernández-Carballido A, Herrero-Vanrell R, Molina-Martınez I, Pastoriza P. Biodegradable ibuprofen-loaded PLGA microspheres for intraarticular administration: effect of Labrafil addition on release in vitro. Int J Pharmaceutic. 2004; 279(1-2): 33-41.
- Kang SW, Jeon O, Kim BS. Poly (lactic-co-glycolic acid) microspheres as an injectable scaffold for cartilage tissue engineering. Tissue engineer. 2005; 11(3-4): 438-47.
- Zhao H, Ma L, Gao C, Shen J. A composite scaffold of PLGA microspheres/fibrin gel for cartilage tissue engineering: fabrication, physical properties, and cell responsiveness. J Biomed Materials Res B. 2009; 88(1): 240- 49.
- Butoescu N, Jordan O, Burdet P, Stadelmann P, Petri-Fink A, Hofmann H, et al. Dexamethasone-containing biodegradable superparamagnetic microparticles for intra-articular administration: physicochemical and magnetic properties, in vitro and in vivo drug release. Eur J Pharmaceutic Biopharmaceutic 2009; 72(3): 529- 38.
- Zhang Z, Bi X, Li H, Huang G. Enhanced targeting efficiency of PLGA microspheres loaded with Lornoxicam for intra-articular administration. Drug Delivery 2011; 18(7): 536- 44.
- Eswaramoorthy R, Chang CC, Wu SC, Wang GJ, Chang JK, Ho ML. Sustained release of PTH (1–34) from PLGA microspheres suppresses osteoarthritis progression in rats. Acta Biomaterialia 2012; 8(6): 2254- 262.
- Gaignaux A, Réeff J, Siepmann F, Siepmann J, De Vriese C, Goole J, et al. Development and evaluation of sustained-release clonidine-loaded PLGA microparticles. Int J Pharmaceutic. 2012; 437(1-2): 20- 8.
- Chen Z, Liu D, Wang J, Wu L, Li W, Chen J, et al. development of nanoparticles-in-microparticles system for improved local retention after intra-articular injection. Drug Delivery 2014; 21(5): 342- 50.
- Sun X, Wang J, Wang Y, Zhang Q. Collagen-based porous scaffolds containing PLGA microspheres for controlled kartogenin release in cartilage tissue engineering. Artificial Cells, Nanomed, Biotechnol. 2018; 46(8): 1957- 966.
- Shin HJ, Park H, Shin N, Shin J, Gwon DH, Kwon HH, et al. p66shc siRNA Nanoparticles Ameliorate Chondrocytic Mitochondrial Dysfunction in Osteoarthritis. Int J Nanomed. 2020; 15: 2379- 390.
- hin HJ, Park H, Shin N, Kwon HH, Yin Y, Hwang JA, et al. p47phox siRNA-Loaded PLGA nanoparticles suppress ROS/oxidative stress-induced chondrocyte damage in osteoarthritis. Polymers 2020; 12(2): 443- 57.