Mon, Feb 2, 2026
[Archive]
Volume 4, Issue 4 (November 2017)
IJML 2017, 4(4): 307-315 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Askarikhah B, Dehghani Ashkezari M, Emtiazi H, Hekmatimoghaddam S H. Toxicities of New 10,11-Dihydrochromeno[4,3-b]chromene-6,8(7H,9H)-dione Derivatives on the Cancer Cell Line MOLT4 . IJML 2017; 4 (4) :307-315
URL: http://ijml.ssu.ac.ir/article-1-198-en.html
URL: http://ijml.ssu.ac.ir/article-1-198-en.html
Department of Laboratory Sciences, Faculty of Paramedicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Full-Text [PDF 545 kb]
(959 Downloads)
| Abstract (HTML) (3092 Views)
.PNG)
.PNG)
Discussion
Full-Text: (1046 Views)
Introduction
Cancer is the second cause of death after cardiovascular disease in developed countries, and the third (to infectious diseases) in developing countries. In Iran, cancer ranks the 3rd as the cause of death after cardiovascular diseases and accidents [1]. Routine treatment for cancer is surgery, radiotherapy and chemotherapy, which are not very successful in many cases. Besides recurrence, side effects of these interventions are a big problem [2]. In the past decades, cancer drugs researcher have tried to introduce novel anti-tumor drugs, which have better efficiency and fewer side effect. In this regard, heterocyclic compounds having benzopyran ring have drawn much attention [3].
One of the most important heterocyclic groups are chromenes, a subgroup of coumarin, which have been investigated for many years in cancer therapy, and their cell toxicity against many cancers have been proved in different studies. These compounds are present in the nature; many derivatives of them could be synthesized in laboratories by adding different groups into their main heterocyclic structure [4]. Effectiveness of different synthetic chromene derivatives vary based on the type of added groups and the type of cancer cell line. Some of these synthesized chromene derivatives are under investigation to treat solid tumors, some of them are tubulin inhibitors [5], and again others are bcl (or bcl-2) inhibitors [6, 7] that induce apoptosis in cancer cells.
10,11-dihydro chromeno [4,3-b] chromenes were previously considered as anti-tuberculosis agent [8]. In the present study, we produced different derivatives of 10,11-dihydrochromeno [4,3-b] chromenes and investigate their effectiveness on induction of cell death in human acute lymphoblastic leukemia cell line MOLT4.
Materials and Methods
General information
4-hydroxycoumarine, dimedone, aldehydes, and other necessary chemical compounds were purchased from Fluka Analytical (Germany) and Merck (Germany) companies. The purity of the compounds was routinely checked by thin layer chromatography (TLC) using silica gel 60G (Merck, Germany) with ethylacetate/n-hexane (1:3). In addition, the products were characterized by Fourier-transform infrared spectroscopy (FTIR), 1H nuclear magnetic resonance (NMR) and 13C NMR spectra and by comparison of their physical properties with those reported in the literature. FTIR (ATR) spectra were run on a Bruker, Equinox 55 spectrometer (UK). 1H NMR and 13C NMR spectra were obtained using a BrukerAvans 400 and 500 MHz spectrometers (DRX). Melting points were determined by a Buchi melting point B-540 B.V.CHI apparatus (Sigma-Aldrich, Germany).
Synthesis of 10, 11-dihydrochromeno [4, 3-b] chromene-6, 8 (7H,9H)-dione derivatives
Briefly, a mixture of dimedone (1 mmol, 0.14 g), 4-hydroxycoumarin (1 mmol, 0.16 g), aldehyde (1 mmol) and Zn (OAc)2 (0.03 g) was heated under solvent-free conditions at 90°C for an appropriate time. The progress of the reaction was followed by TLC (ethyl acetate: n-hexane 30:70). After completion of the reaction, the mixture was dissolved in hot CH2Cl2 for isolation of catalyst, and filtered. The solvent of resultant filtrate was evaporated, and the pure product obtained by recrystallization from ethanol. The products, including 10, 10-dimethyl-7-(4-nitrophenyl)-10,11-dihydrochromeno [4, 3-b]chromene-6,8 (7H, 9H)-dione (here arbitrarily named C1), 10, 10-dimethyl-7-(3-hydroxyphenyl)-10, 11-dihydrochromeno [4, 3-b] chromene-6,8 (7H, 9H)-dione (C2), 10, 10-dimethyl-7-(3, 4-dimethoxyphenyl)-10,11-dihydrochromeno [4,3-b] chromene-6,8 (7H, 9H)-dione (C3), and 10, 10-dimethyl-7-(4-hydroxy-3-methoxyphenyl)-10, 11-dihydrochromeno [4,3-b] chromene-6,8 (7H, 9H)-dione (C4) were characterized on the basis of spectroscopic data from FTIR, H NMR and C NMR [9] (Fig. 1). The general synthetic route has been highlighted in scheme 1.
.PNG)
Spectroscopic data
10, 10-dimethyl-7 (4-nitropophenyl) -10, 11-dihydrochromeno [4,3-b]chromene-6, 8 (7H, 9H)-dione (C1): White solid, MP=208-210°C:
FTIR: νmax(ATR, neat) = 2930, 1719, 1651, 1605, 1528, 1458, 1342, 1178, 1098, 1033, 863, 760 cm-1. 1H-NMR (500 MHz, CDCl3-d6): δ = 1.1 (s, 3 H), 1.12 (s, 3 H), 2.18 (d, J=16.4 Hz, 1H), 2.26 (d, J=16.4 Hz, 1H), 2.67 (d, J=18.1 Hz, 1H), 2.73 (d, J=18.1 Hz, 1H), 4.95 (s, 1 H), 6.90 (d, J=8.8 Hz, 2H), 7.04 (d, J=8.8 Hz, 2H), 7.34-7.40 (m, 2H), 7.59 (t, J=8.2 Hz, 1H), 7.89 (dd, J=8.2 Hz, J=1.6 Hz 1H). 13C-NMR (125 MHz, CDCl3) δ = 27.29, 29.29, 31.21, 32.77, 40.84, 50.71, 114.76, 114.97, 115.05, 115.27, 115.50, 116.96, 122.47, 124.37, 129.80, 129.88, 130.16, 130.25, 132.37, 162.02, 162.34, 162.99, 196.45 ppm.
10, 10-dimethyl-7 (3-hydroxyphenyl)-10, 11-dihydrochromeno [4, 3-b] chromene-6, 8(7H, 9H)-dione (C2): White solid, MP=267-269°C:
FTIR: νmax(ATR, neat) = 3400, 2983, 1697, 1665, 1607, 1488, 1364, 1175, 1137, 1058, 897, 767 cm-1. 1H-NMR (500 MHz, DMSO-d6): δ = 1 (s, 3 H), 1.1 (s, 3 H), 2.75 (sbr, 2H), 2.37 (d, J=16.5 Hz, 1H), 4.62 (s, 1H), 6.55 (dd, J=7.5 Hz, J=2.1 Hz, 1H), 6.66 (d, J=7.0 Hz, 1H), 6.71 (s, 1H), 7.03 (t, J=8.0 Hz, 1H), 7.44 (d, J=8.5 Hz, 1H), 7.46 (t, J=7.0 Hz, 1H), 7.69 (t, J=7.5 Hz, 1H), 7.93 (dd, J=7.5 Hz, J=1.5 Hz, 1H), 9.29 (s, 1H). 13C-NMR (125 MHz, DMSO-d6) δ = 27.58, 29.38, 32.86, 33.63, 50.92, 106.78, 113.97, 114.68, 114.79, 116.38, 117.44, 119.71, 123.40, 125.64, 129.94, 133.65, 144.98, 152.77, 154.46, 157.98, 163.41, 196.73 ppm.
10, 10-dimethyl-7 (3, 4-dimethoxyphenyl)-10, 11-dihydrochromeno[4,3-b]chromene 6, 8(7H, 9H)-dione (C3) White solid, MP=184-185°C:
FTIR: νmax(ATR, neat) = 2921, 1721, 1660, 1620, 1513, 1455, 1361, 1262, 1139, 896, 742 cm-1. 1H-NMR (500 MHz, CDCl3-d6): δ = 1.13 (s, 3 H), 1.19 (s, 3 H), 2.30 (d, J=16.0 Hz, 1H), 2.35 (d, J=16.0 Hz, 1H), 2.68 (d, J=17.6 Hz, 1H), 2.74 (d, J=17.6 Hz, 1H), 3.81 (s, 3 H), 3.89 (s, 3 H), 4.93 (s, 1H), 6.74 (d, J=8.1 Hz, 1H), 6.82 (dd, J=8.0 Hz, J=1.6 Hz, 1H), 7.05 (s, 1H), 7.33-7.39 (m, 2H), 7.58 (t, J=8.0 Hz, 1H), 7.88 (d, J=8.0 Hz, 1H). 13C-NMR (125 MHz, CDCl3) δ = 27.49, 29.26, 32.36, 32.80, 40.85, 50.72, 106.92, 110.93, 112.65, 113.74, 115.20, 116.92, 120.20, 122.41, 124.27, 132.19, 135.36, 148.02, 148.63, 152.56, 153.74, 160.76, 161.91, 196.16 ppm.
10, 10-dimethyl-7-(4-hydroxy-3-methoxyphenyl)-10, 11-dihydrochromeno [4, 3-b] chromene-6, 8(7H, 9H)-dione (C4): White solid, MP=271-273°C:
FTIR: νmax(ATR, neat) = 3434, 2993, 1713,
1662, 1608, 1514, 1361, 1272, 1181, 1033, 863, 779. cm-1. 1H-NMR (500 MHz, DMSO-d6): δ=1.02 (s, 3H), 1.10 (s, 3 H), 2.20 (d, J= 16.0 Hz, 1H), 2.34 (d, J= 16.0 Hz, 1H), 2.76 (s, 2H), 3.70 (s, 3H), 4.61 (s, 1H), 6.61 (d, J= 8 Hz, 1H), 6.64 (d, J=8 Hz, 1H), 6.81 (d, J=1.6 Hz, 1H), 7.43-7.48 (m, 2H), 7.69 (t, J=7.8 Hz, 1H), 7.92 (d, J=8 Hz, 1H), 8.88 (s, 1H). 13C-NMR (125 MHz, DMSO-d6) δ = 26.55, 28.57, 31.94, 32.24, 39.57, 50.02, 55.57, 106.10, 112.81, 113.18, 113.96, 115.15, 116.51, 120.52, 122.55, 124.70, 132.63, 133.80, 145.47, 147.00, 151.83, 153.29, 159.99, 162.43,195.96 ppm.
Cell culture
The cell culture medium Roswell Park Memorial Institute (RPMI)-1640 and fetal bovine serum (FBS) were purchased from Gibco (USA). Penicillin-streptomycin, trypan blue, Ficoll, dimethylsulfoxide (DMSO), phytohemagglutinin (PHA) 1000 mol/mL, and (3-(4,5-dimethyl thiazol-2-yl)-2, 5-diphenyltetrazolium bromide) (MTT) assay kit were obtained from Sigma (Germany). human acute lymphoblastic leukemia cell line (MOLT4) cells were purchased from Pasteur Institute (Tehran, Iran), and cultured in RPMI-1640 supplemented with 10% FBS, 100 units⁄mL penicillin, and 100 mg⁄mL streptomycin. Cells were grown in suspension, at 37 ˚C in humidified air containing 5% CO2 (16).
Cytotoxicity assay
MOLT4 cells were cultured in 24-well micro plate at a density of 106 cells/mL for 42 hours. Then, 20 µL of each synthetic compounds (in 50, 250, 500 and 1000 nM) was dissolved at a concentration of 0.1% in DMSO which was added to each well. The plate was incubated for 24, 48 and 72 h. Cell counting was done by hemacytometer slide, using 0.4% trypan blue in three different times for detection of live vs. dead cells [10].
Cell viability assay
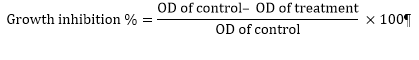
Cell viability was estimated using the MTT reduction assay. MOLT4 cells were plated in 96-well micro plates at a density of 106 cells⁄mL (200 µL⁄well) for 9 hours. Subsequently, 20 µL of synthetic compounds (in 50, 250, 500 and 1000 nM) were added to each well. The cells were incubated for 72 h. Then, the MTT was added to each well at a final concentration of 0.5 mg⁄mL, and plates were incubated for another 4 h at 37˚C. Later, 100 µL DMSO was added to each well. Control wells contained only growth medium. The optical density was measured at 570 nm using a Bio-TEK micro plate reader. The tests were done three times [11]. The percentage of growth inhibition and cell viability compared with the control wells were determined by the following formulas:
.PNG)
The drug concentration which resulted in 50% inhibition of cells in the medium was regarded as half-inhibition concentration (IC50). IC50 was calculated by the results from counting cells and MTT assay as follow:

RNA extraction and cDNA synthesis
The cells treated with C1 at concentration of 250 nM after 48 h were harvested and treated with RNX Plus solution (CinnaGen, Iran) according to the manufacturer's instructions. Quality and quantity of extracted RNAs was evaluated by UV spectrophotometry (260/280 nm ratio) and gel electrophoresis. The first strand of cDNAs was synthesized using the
reverse transcription system (TaKaRa, Japan) according to the manufacturer's instructions. Real-Time quantitative polymerase chain reaction (PCR) assay
The expression level of p53, bax, and fas genes in C1 treated cells was investigated by specific primer:
P53 (F):5′-AGAGTCTATAGGCCCACCCC-3′,
P53(R):5′-GCTCGACGCTAGGATCTGAC-3′[12];
Bax (F):5'-GGACGAACTGGACAGTAACATGG-3',
Bax(R):5'-GCAAAGTAGAAAAGGGCGACAAC-3'[13];
Fas(F):5′-TGAAGGACATGGCTTAGAAGTG-3',
Fas (R): 5′-GGTGCAAGGGTCACAGTGTT-3' [14];
The GAPDH gene was used as internal control:
GAPDH (F):5ʹ-GCCACATGCCTCAGACAC-3',
GAPDH (R):5ʹ-GGCAACAATATCCACTTTACCAG-3' [15].
Quantitative real-time PCR was performed with SYBR Green Master Mix. The PCR program was: initial denaturation at 95°C for 10 minutes, followed by 40 cycles of denaturation at 95°C for 10 seconds, annealing/extension at 60°C for 30 seconds, and extension at 72°C for 45 seconds. Expression change was assessed by the 2-ΔΔCt formula.
Statistical analysis
Cancer is the second cause of death after cardiovascular disease in developed countries, and the third (to infectious diseases) in developing countries. In Iran, cancer ranks the 3rd as the cause of death after cardiovascular diseases and accidents [1]. Routine treatment for cancer is surgery, radiotherapy and chemotherapy, which are not very successful in many cases. Besides recurrence, side effects of these interventions are a big problem [2]. In the past decades, cancer drugs researcher have tried to introduce novel anti-tumor drugs, which have better efficiency and fewer side effect. In this regard, heterocyclic compounds having benzopyran ring have drawn much attention [3].
One of the most important heterocyclic groups are chromenes, a subgroup of coumarin, which have been investigated for many years in cancer therapy, and their cell toxicity against many cancers have been proved in different studies. These compounds are present in the nature; many derivatives of them could be synthesized in laboratories by adding different groups into their main heterocyclic structure [4]. Effectiveness of different synthetic chromene derivatives vary based on the type of added groups and the type of cancer cell line. Some of these synthesized chromene derivatives are under investigation to treat solid tumors, some of them are tubulin inhibitors [5], and again others are bcl (or bcl-2) inhibitors [6, 7] that induce apoptosis in cancer cells.
10,11-dihydro chromeno [4,3-b] chromenes were previously considered as anti-tuberculosis agent [8]. In the present study, we produced different derivatives of 10,11-dihydrochromeno [4,3-b] chromenes and investigate their effectiveness on induction of cell death in human acute lymphoblastic leukemia cell line MOLT4.
Materials and Methods
General information
4-hydroxycoumarine, dimedone, aldehydes, and other necessary chemical compounds were purchased from Fluka Analytical (Germany) and Merck (Germany) companies. The purity of the compounds was routinely checked by thin layer chromatography (TLC) using silica gel 60G (Merck, Germany) with ethylacetate/n-hexane (1:3). In addition, the products were characterized by Fourier-transform infrared spectroscopy (FTIR), 1H nuclear magnetic resonance (NMR) and 13C NMR spectra and by comparison of their physical properties with those reported in the literature. FTIR (ATR) spectra were run on a Bruker, Equinox 55 spectrometer (UK). 1H NMR and 13C NMR spectra were obtained using a BrukerAvans 400 and 500 MHz spectrometers (DRX). Melting points were determined by a Buchi melting point B-540 B.V.CHI apparatus (Sigma-Aldrich, Germany).
Synthesis of 10, 11-dihydrochromeno [4, 3-b] chromene-6, 8 (7H,9H)-dione derivatives
Briefly, a mixture of dimedone (1 mmol, 0.14 g), 4-hydroxycoumarin (1 mmol, 0.16 g), aldehyde (1 mmol) and Zn (OAc)2 (0.03 g) was heated under solvent-free conditions at 90°C for an appropriate time. The progress of the reaction was followed by TLC (ethyl acetate: n-hexane 30:70). After completion of the reaction, the mixture was dissolved in hot CH2Cl2 for isolation of catalyst, and filtered. The solvent of resultant filtrate was evaporated, and the pure product obtained by recrystallization from ethanol. The products, including 10, 10-dimethyl-7-(4-nitrophenyl)-10,11-dihydrochromeno [4, 3-b]chromene-6,8 (7H, 9H)-dione (here arbitrarily named C1), 10, 10-dimethyl-7-(3-hydroxyphenyl)-10, 11-dihydrochromeno [4, 3-b] chromene-6,8 (7H, 9H)-dione (C2), 10, 10-dimethyl-7-(3, 4-dimethoxyphenyl)-10,11-dihydrochromeno [4,3-b] chromene-6,8 (7H, 9H)-dione (C3), and 10, 10-dimethyl-7-(4-hydroxy-3-methoxyphenyl)-10, 11-dihydrochromeno [4,3-b] chromene-6,8 (7H, 9H)-dione (C4) were characterized on the basis of spectroscopic data from FTIR, H NMR and C NMR [9] (Fig. 1). The general synthetic route has been highlighted in scheme 1.
.PNG)
Spectroscopic data
10, 10-dimethyl-7 (4-nitropophenyl) -10, 11-dihydrochromeno [4,3-b]chromene-6, 8 (7H, 9H)-dione (C1): White solid, MP=208-210°C:
FTIR: νmax(ATR, neat) = 2930, 1719, 1651, 1605, 1528, 1458, 1342, 1178, 1098, 1033, 863, 760 cm-1. 1H-NMR (500 MHz, CDCl3-d6): δ = 1.1 (s, 3 H), 1.12 (s, 3 H), 2.18 (d, J=16.4 Hz, 1H), 2.26 (d, J=16.4 Hz, 1H), 2.67 (d, J=18.1 Hz, 1H), 2.73 (d, J=18.1 Hz, 1H), 4.95 (s, 1 H), 6.90 (d, J=8.8 Hz, 2H), 7.04 (d, J=8.8 Hz, 2H), 7.34-7.40 (m, 2H), 7.59 (t, J=8.2 Hz, 1H), 7.89 (dd, J=8.2 Hz, J=1.6 Hz 1H). 13C-NMR (125 MHz, CDCl3) δ = 27.29, 29.29, 31.21, 32.77, 40.84, 50.71, 114.76, 114.97, 115.05, 115.27, 115.50, 116.96, 122.47, 124.37, 129.80, 129.88, 130.16, 130.25, 132.37, 162.02, 162.34, 162.99, 196.45 ppm.
10, 10-dimethyl-7 (3-hydroxyphenyl)-10, 11-dihydrochromeno [4, 3-b] chromene-6, 8(7H, 9H)-dione (C2): White solid, MP=267-269°C:
FTIR: νmax(ATR, neat) = 3400, 2983, 1697, 1665, 1607, 1488, 1364, 1175, 1137, 1058, 897, 767 cm-1. 1H-NMR (500 MHz, DMSO-d6): δ = 1 (s, 3 H), 1.1 (s, 3 H), 2.75 (sbr, 2H), 2.37 (d, J=16.5 Hz, 1H), 4.62 (s, 1H), 6.55 (dd, J=7.5 Hz, J=2.1 Hz, 1H), 6.66 (d, J=7.0 Hz, 1H), 6.71 (s, 1H), 7.03 (t, J=8.0 Hz, 1H), 7.44 (d, J=8.5 Hz, 1H), 7.46 (t, J=7.0 Hz, 1H), 7.69 (t, J=7.5 Hz, 1H), 7.93 (dd, J=7.5 Hz, J=1.5 Hz, 1H), 9.29 (s, 1H). 13C-NMR (125 MHz, DMSO-d6) δ = 27.58, 29.38, 32.86, 33.63, 50.92, 106.78, 113.97, 114.68, 114.79, 116.38, 117.44, 119.71, 123.40, 125.64, 129.94, 133.65, 144.98, 152.77, 154.46, 157.98, 163.41, 196.73 ppm.
10, 10-dimethyl-7 (3, 4-dimethoxyphenyl)-10, 11-dihydrochromeno[4,3-b]chromene 6, 8(7H, 9H)-dione (C3) White solid, MP=184-185°C:
FTIR: νmax(ATR, neat) = 2921, 1721, 1660, 1620, 1513, 1455, 1361, 1262, 1139, 896, 742 cm-1. 1H-NMR (500 MHz, CDCl3-d6): δ = 1.13 (s, 3 H), 1.19 (s, 3 H), 2.30 (d, J=16.0 Hz, 1H), 2.35 (d, J=16.0 Hz, 1H), 2.68 (d, J=17.6 Hz, 1H), 2.74 (d, J=17.6 Hz, 1H), 3.81 (s, 3 H), 3.89 (s, 3 H), 4.93 (s, 1H), 6.74 (d, J=8.1 Hz, 1H), 6.82 (dd, J=8.0 Hz, J=1.6 Hz, 1H), 7.05 (s, 1H), 7.33-7.39 (m, 2H), 7.58 (t, J=8.0 Hz, 1H), 7.88 (d, J=8.0 Hz, 1H). 13C-NMR (125 MHz, CDCl3) δ = 27.49, 29.26, 32.36, 32.80, 40.85, 50.72, 106.92, 110.93, 112.65, 113.74, 115.20, 116.92, 120.20, 122.41, 124.27, 132.19, 135.36, 148.02, 148.63, 152.56, 153.74, 160.76, 161.91, 196.16 ppm.
10, 10-dimethyl-7-(4-hydroxy-3-methoxyphenyl)-10, 11-dihydrochromeno [4, 3-b] chromene-6, 8(7H, 9H)-dione (C4): White solid, MP=271-273°C:
FTIR: νmax(ATR, neat) = 3434, 2993, 1713,
1662, 1608, 1514, 1361, 1272, 1181, 1033, 863, 779. cm-1. 1H-NMR (500 MHz, DMSO-d6): δ=1.02 (s, 3H), 1.10 (s, 3 H), 2.20 (d, J= 16.0 Hz, 1H), 2.34 (d, J= 16.0 Hz, 1H), 2.76 (s, 2H), 3.70 (s, 3H), 4.61 (s, 1H), 6.61 (d, J= 8 Hz, 1H), 6.64 (d, J=8 Hz, 1H), 6.81 (d, J=1.6 Hz, 1H), 7.43-7.48 (m, 2H), 7.69 (t, J=7.8 Hz, 1H), 7.92 (d, J=8 Hz, 1H), 8.88 (s, 1H). 13C-NMR (125 MHz, DMSO-d6) δ = 26.55, 28.57, 31.94, 32.24, 39.57, 50.02, 55.57, 106.10, 112.81, 113.18, 113.96, 115.15, 116.51, 120.52, 122.55, 124.70, 132.63, 133.80, 145.47, 147.00, 151.83, 153.29, 159.99, 162.43,195.96 ppm.
Cell culture
The cell culture medium Roswell Park Memorial Institute (RPMI)-1640 and fetal bovine serum (FBS) were purchased from Gibco (USA). Penicillin-streptomycin, trypan blue, Ficoll, dimethylsulfoxide (DMSO), phytohemagglutinin (PHA) 1000 mol/mL, and (3-(4,5-dimethyl thiazol-2-yl)-2, 5-diphenyltetrazolium bromide) (MTT) assay kit were obtained from Sigma (Germany). human acute lymphoblastic leukemia cell line (MOLT4) cells were purchased from Pasteur Institute (Tehran, Iran), and cultured in RPMI-1640 supplemented with 10% FBS, 100 units⁄mL penicillin, and 100 mg⁄mL streptomycin. Cells were grown in suspension, at 37 ˚C in humidified air containing 5% CO2 (16).
Cytotoxicity assay
MOLT4 cells were cultured in 24-well micro plate at a density of 106 cells/mL for 42 hours. Then, 20 µL of each synthetic compounds (in 50, 250, 500 and 1000 nM) was dissolved at a concentration of 0.1% in DMSO which was added to each well. The plate was incubated for 24, 48 and 72 h. Cell counting was done by hemacytometer slide, using 0.4% trypan blue in three different times for detection of live vs. dead cells [10].
Cell viability assay
Cell viability was estimated using the MTT reduction assay. MOLT4 cells were plated in 96-well micro plates at a density of 106 cells⁄mL (200 µL⁄well) for 9 hours. Subsequently, 20 µL of synthetic compounds (in 50, 250, 500 and 1000 nM) were added to each well. The cells were incubated for 72 h. Then, the MTT was added to each well at a final concentration of 0.5 mg⁄mL, and plates were incubated for another 4 h at 37˚C. Later, 100 µL DMSO was added to each well. Control wells contained only growth medium. The optical density was measured at 570 nm using a Bio-TEK micro plate reader. The tests were done three times [11]. The percentage of growth inhibition and cell viability compared with the control wells were determined by the following formulas:
.PNG)

The drug concentration which resulted in 50% inhibition of cells in the medium was regarded as half-inhibition concentration (IC50). IC50 was calculated by the results from counting cells and MTT assay as follow:

RNA extraction and cDNA synthesis
The cells treated with C1 at concentration of 250 nM after 48 h were harvested and treated with RNX Plus solution (CinnaGen, Iran) according to the manufacturer's instructions. Quality and quantity of extracted RNAs was evaluated by UV spectrophotometry (260/280 nm ratio) and gel electrophoresis. The first strand of cDNAs was synthesized using the
reverse transcription system (TaKaRa, Japan) according to the manufacturer's instructions. Real-Time quantitative polymerase chain reaction (PCR) assay
The expression level of p53, bax, and fas genes in C1 treated cells was investigated by specific primer:
P53 (F):5′-AGAGTCTATAGGCCCACCCC-3′,
P53(R):5′-GCTCGACGCTAGGATCTGAC-3′[12];
Bax (F):5'-GGACGAACTGGACAGTAACATGG-3',
Bax(R):5'-GCAAAGTAGAAAAGGGCGACAAC-3'[13];
Fas(F):5′-TGAAGGACATGGCTTAGAAGTG-3',
Fas (R): 5′-GGTGCAAGGGTCACAGTGTT-3' [14];
The GAPDH gene was used as internal control:
GAPDH (F):5ʹ-GCCACATGCCTCAGACAC-3',
GAPDH (R):5ʹ-GGCAACAATATCCACTTTACCAG-3' [15].
Quantitative real-time PCR was performed with SYBR Green Master Mix. The PCR program was: initial denaturation at 95°C for 10 minutes, followed by 40 cycles of denaturation at 95°C for 10 seconds, annealing/extension at 60°C for 30 seconds, and extension at 72°C for 45 seconds. Expression change was assessed by the 2-ΔΔCt formula.
Statistical analysis
All tests were repeated for three times and the data presented as mean±SD. We used the Student's t-test for statistical analysis (using SPSS software version 15); p<0.05 was considered as level of significant difference. GraphPad Prism 6 software was used for drawing the charts..PNG)
Results
Structure of the synthesized derivatives of dihydrochromeno chromene-6,8-diones
The chemical structures of four derivatives of 10,11-dihydrochromeno[4,3-b]chromene-6,8 (7H,9H)-dione produced in this study are shown in fig. 1. As is seen, each of them has its unique structure and hence could have different effects on cellular processes. The effectiveness of each derivative (C1, C2, C3, and C4) is related to the structure of the groups attached to the main cycles.
IC50 of C1, C2, C3, and C4
In order to find the best derivatives and the best concentration for further experiments, the MTT test was performed on MOLT4 cell lines treated with C1-C4 compounds, and IC50 values after 72 hours exposure were 400±5.2, 250±4.1, 550±3.6, and 500±3.8 nM, respectively (Table 1). The C2 compound had more toxic effect on MOLT4 cells with IC50 of 250 nM. This derivative was chosen for further investigations in this study.
Table 1. IC50 of different synthesized derivatives in MOLT4 cell line (72 h after treatment)
Synthesized derivatives |
IC50 (nM) |
C1 |
400±5.2 |
C2 |
250±4.1 |
C3 |
550±3.6 |
C4 |
500±3.8 |
Cell toxicity of the synthesized compounds
The results showed that all synthetic derivatives had cytotoxic activities in MOLT4 cells with significance differences in IC50 values due to their structural diversity. The best results were achieved using C2, and toxicity was time- and dose-dependent. According to fig. 2, there was inverse relationship between cell viability and concentration of C2.
C2 treatment increases expression of apoptotic genes
MTT assay and microscopic images (the latter not presented here) showed that C2-treated cells underwent apoptosis. For further confirmation, we evaluated expression of apoptotic genes p53, Bax and Fas using real-time PCR. As is shown in fig. 3, all these genes revealed increased expression.
.PNG)
.PNG)
Discussion
During the last decades, chromenes have been considered as important candidates for cancer and inflammation treatment [16].By introduction of different biological activities of natural and synthetic chromenes, more and more studies are focused on these compounds to find the best derivatives of chromenes in each disease. In the present study, for the first time we introduced four novel synthetic derivatives of chromenes, and tested their antitumor activities against MOLT4, an acute lymphoblastic leukemia cell line. Our derivatives, designated as C1, C2, C3 and C4 were tested in various concentrations at 3 different times. IC50 of the derivatives showed that the C2 compound has 50% cell toxicity at concentration of 250 nM at 72 h. The toxicity of C2 was better than other derivatives. Treatment of MOLT4 cells by the C2 compound showed inhibition of cell growth in time- and dose-dependent manner, so further investigations were performed using C2.
Previous reports have shown that chromene derivatives cause cell death in cancer cell lines by induction of apoptosis [17, 18], which would be a reasonable way to fight cancers [19]. Microscopic images showed higher apoptosis as compared with normal saline. So, we tested apoptotic gene expression in MOLT4 cells treated with C2. Real-time quantitative PCR results showed that p53, Bax, and Fas gene expressions have been increased in treated cells, which confirmed the occurrence of apoptosis in them. Apoptotic effects of different derivatives of chromenes are presumably related to the groups attached to the main structure of chromenes. Hydroxyl and methyl groups have been considered as promoting factor for apoptosis in leukemic cell lines P3HR1, K56 and THP-1 [20]. Other reports have shown that chlorine and bromine groups also could induce apoptosis in cancer cell lines [10, 11]. Our results which showed that chromene derivative increase Bax, p53, and Fas gene expressions are consistent with those studies, and relate structure to activity in apoptosis [10, 21].
Conclusion
Among different chromene derivatives synthesized and tested in this investigation, the C2 compound had the best result in terms of induction of cancer cell apoptosis, although other compounds had some anti-tumor effects, too. These derivatives could then be a potential candidate for further investigations.
Conflict of Interest
The authors declare that there is no conflict of interest.
Acknowledgement
There is no acknowledgement to declare.
References
Previous reports have shown that chromene derivatives cause cell death in cancer cell lines by induction of apoptosis [17, 18], which would be a reasonable way to fight cancers [19]. Microscopic images showed higher apoptosis as compared with normal saline. So, we tested apoptotic gene expression in MOLT4 cells treated with C2. Real-time quantitative PCR results showed that p53, Bax, and Fas gene expressions have been increased in treated cells, which confirmed the occurrence of apoptosis in them. Apoptotic effects of different derivatives of chromenes are presumably related to the groups attached to the main structure of chromenes. Hydroxyl and methyl groups have been considered as promoting factor for apoptosis in leukemic cell lines P3HR1, K56 and THP-1 [20]. Other reports have shown that chlorine and bromine groups also could induce apoptosis in cancer cell lines [10, 11]. Our results which showed that chromene derivative increase Bax, p53, and Fas gene expressions are consistent with those studies, and relate structure to activity in apoptosis [10, 21].
Conclusion
Among different chromene derivatives synthesized and tested in this investigation, the C2 compound had the best result in terms of induction of cancer cell apoptosis, although other compounds had some anti-tumor effects, too. These derivatives could then be a potential candidate for further investigations.
Conflict of Interest
The authors declare that there is no conflict of interest.
Acknowledgement
There is no acknowledgement to declare.
References
- Sadjadi A, Nouraei M, Mohagheghi MA, Mousavi Jarrahi A, Malekezadeh R, Parkin DM. Cancer occurrence in Iran in 2002, an international perspective. Asian Pacific J Cancer Preven. 2005; 6(3): 359-63.
- Dadiboyena S, Nefzi A. Synthesis of functionalized tetrasubstituted pyrazolyl heterocycles- A review. Europ J Med Chem. 2011; 46(11): 5258-275.
- Du W. Towards new anticancer drugs: a decade of advances in synthesis of camptothecins and related alkaloids. Tetrahedron 2003; 59(44): 8649-687.
- Okasha RM, Alblewi FF, Afifi TH, Naqvi A, Fouda AM, Al-Dies AM, et al. Design of New Benzo [h] chromene Derivatives: Antitumor Activities and Structure-Activity Relationships of the 2, 3-Positions and Fused Rings at the 2, 3-Positions. Molecules 2017; 22(3): 479.
- Kemnitzer W, Kasibhatla SH, Jiang S, Zhang H, Zhao J, Jia SH, et al., Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell-and caspase-based high-throughput screening assay. 2. Structure–activity relationships of the 7-and 5-, 6-, 8-positions. Bioorganic and Medicinal Chemistry Letters 2005; 15(21): 4745-751.
- Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SM, et al. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proceedings of the National Academy of Sciences 2000; 97(13): 7124-129.
- Doshi JM, Tian D, Xing C. Structure activity relationship studies of ethyl 2-amino-6-bromo-4-(1-cyano-2-ethoxy-2-oxoethyl)-4 H-chromene-3-carboxylate (HA 14-1), an antagonist for antiapoptotic Bcl-2 proteins to overcome drug resistance in cancer. J Med Chem. 2006; 49(26): 7731-739.
- Patil KT, Walekar LS, Undare SS, Kolekar GB, Deshmukh MB, Choudhari PB, et al. Selective synthesis of 10, 11-dihydrochromeno [4, 3-b] chromene-6, 8 (7H, 9H)-dione using copper oxide nanoparticles for potential inhibitors of β-ketoacyl-[acyl-carrier-protein] synthase III of Mycobacterium tuberculosis. NISCAIR-CSIR, India 2016; 55(9): 1151-159.
- Emtiazi H, Amrollahi MA. An efficient and rapid access to the synthesis of tetrahydro chromeno [4, 3-b] chromene-6, 8-dione derivatives by magnesium perchlorate. S Afr J Chem. 2014; 67.
- Naseri MH, Mahdavi M, Davoodi J, Hesami Tackallo SH, Goudarzvand M, Neishabouri SH, et al. Up regulation of Bax and down regulation of Bcl2 during 3-NC mediated apoptosis in human cancer cells. Cancer Cell Int. 2015; 15(1): 55.
- Esmati N, Foroughian M, Saeedi M, Mahdavi M, Khoshneviszadeh M, Firuzi O, et al. Synthesis and Cytotoxic Activity of Some Novel Dihyrobenzo [h] pyrano [3, 2‐c] chromene Derivatives. J Heterocyclic Chem. 2015; 52(1): 97-104.
- Ohtani S, Kagawa S, Tango Y, Umeoka T, Tokunaga N, Tsunemitsu Y, et al. Quantitative analysis of p53-targeted gene expression and visualization of p53 transcriptional activity following intratumoral administration of adenoviral p53 in vivo. Mol Cancer Ther. 2004; 3(1): 93-100.
- Porichi O, Nikolaidou ME, Apostolaki A, Tserkezoglou A, Arnogiannaki N, Kassanos D, et al. BCL-2, BAX and P53 expression profiles in endometrial carcinoma as studied by real-time PCR and immunohistochemistry. Anticancer Res. 2009; 29(10): 3977-982.
- Das H, Koizumi T, Sugimoto T, Chakraborty S, Ichimura T, Hasegawa K, et al. Quantitation of Fas and Fas ligand gene expression in human ovarian, cervical and endometrial carcinomas using real-time quantitative RT-PCR. Br J Cancer 2000; 82(10): 1682-688.
- Poursani EM, Soltani BM, Mowla SJ. Differential expression of OCT4 pseudogenes in pluripotent and tumor cell lines. Cell J. 2016; 18(1): 28-36.
- Thomas N, Zachariah SM. Pharmacological activities of chromene derivatives: an overview. Asian J Pharm Clin Res. 2013; 6(2): 11-15.
- Kemnitzer W, Drewe J, Jiang S, Zhang H, Wang Y, Zhao J, et al. Discovery of 4-Aryl-4 H-chromenes as a new series of apoptosis inducers using a cell-and caspase-based high-throughput screening assay. 1. structure activity relationships of the 4-Aryl group. J Med Chem. 2004; 47(25): 6299-310.
- Rahimi R, Mahdavi M, Pejman S, Zare P, Balalaei S. Inhibition of cell proliferation and induction of apoptosis in K562 human leukemia cells by the derivative (3-NpC) from dihydro-pyranochromenes family. Acta Biochim Pol. 2015; 62: 83-8.
- Hu W, Kavanagh JJ. Anticancer therapy targeting the apoptotic pathway. Lancet Oncol. 2003; 4(12): 721-29.
- Grazul M, Budzisz E. Biological activity of metal ions complexes of chromones, coumarins and flavones. Coordin Chem Rev. 2009; 253(21): 2588-598.
Type of Study: Research |
Subject:
Biochemistry
Received: 2017/09/5 | Accepted: 2017/11/18 | Published: 2017/12/31
Received: 2017/09/5 | Accepted: 2017/11/18 | Published: 2017/12/31
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





