Thu, Dec 4, 2025
[Archive]
Volume 6, Issue 1 (February 2019)
IJML 2019, 6(1): 21-32 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Gholaminejad F, Javadi M, Karami A A, Alizadeh F, Kavianpour M, Khadem Haghighian H. Propolis Supplementation Effects on Semen Parameters, Oxidative Stress, Inflammatory Biomarkers and Reproductive Hormones in Infertile Men with Asthenozoospermia; A Randomized Clinical Trial. IJML 2019; 6 (1) :21-32
URL: http://ijml.ssu.ac.ir/article-1-232-en.html
URL: http://ijml.ssu.ac.ir/article-1-232-en.html
Fereshteh Gholaminejad 
 , Maryam Javadi
, Maryam Javadi 
 , Ali Akbar Karami
, Ali Akbar Karami 
 , Fatemeh Alizadeh
, Fatemeh Alizadeh 
 , Maria Kavianpour
, Maria Kavianpour 
 , Hossein Khadem Haghighian *
, Hossein Khadem Haghighian * 


 , Maryam Javadi
, Maryam Javadi 
 , Ali Akbar Karami
, Ali Akbar Karami 
 , Fatemeh Alizadeh
, Fatemeh Alizadeh 
 , Maria Kavianpour
, Maria Kavianpour 
 , Hossein Khadem Haghighian *
, Hossein Khadem Haghighian * 

Department of Nutrition, Faculty of Health, Qazvin University of Medical Science, Qazvin, Iran.
Full-Text [PDF 533 kb]
(1424 Downloads)
| Abstract (HTML) (2764 Views)
P1: Comparing the mean of sperm quality parameters between the two groups (independent samples t-test)
P2: Comparing the mean of sperm quality parameters in each group before and after the study (paired samples t-test)
Data are presented as Mean±SD
References
Full-Text: (2393 Views)
Introduction
According to World Health Organization (WHO) definition, infertility is the absence of pregnancy after one year or more of sexual relations with no use of contraceptives [1]. Because of lifestyle changes and the presence of various environmental stresses, the incidence of infertility has increased significantly and has become the third most serious health problem following cardiovascular diseases and cancer [2]. According to studies, 10 to 15 percent of couples have infertility problems, out of which 90% can be treated [3]. Based on scientific reports, there are 50 to 80 million infertile couples in the world with about two million new cases per year [1].
Elevated reactive oxygen species (ROS) levels can lead to male infertility. Forty percent of the men had high levels of ROS concentrations as reported in past studies [4]. Because of the presence of polyunsaturated fatty acids in the cell membrane and their oxidation by ROS that leads to changed membrane integrity, sperm motility and sperm viability diminish as well [5]. Infertile men have low antioxidant levels in seminal plasma which can lead to sperm vulnerability against oxidative stress [6].
Propolis is a natural product collected from cracks in the bark of trees by honey bees and enriched by their salivary enzymes [5]. Many biological components such as polyphenolic compounds (i.e., flavonoids), cinnamic acid extracts, different steroids and amino acids have been identified in propolis [7]. Some biological effects such as anti-inflammatory, anticancer, antioxidant, antibiotic and antifungal activities have also been reported from propolis in several papers [5]. Protective properties of propolis against infertility have been reported in several studies, such as ameliorating production, motility, number and quality of sperm, expanding the process of steroi-dogenesis and then testosterone production [8, 9]. Due to increased infertility prevalence and impact of emotional, financial burden of disease on infertile couple’s quality of lives [10], this study was designed to investigate the effect of propolis on sperm parameters, inflammatory factors and sex hormones in men affected by idiopathic infertility.
Elevated reactive oxygen species (ROS) levels can lead to male infertility. Forty percent of the men had high levels of ROS concentrations as reported in past studies [4]. Because of the presence of polyunsaturated fatty acids in the cell membrane and their oxidation by ROS that leads to changed membrane integrity, sperm motility and sperm viability diminish as well [5]. Infertile men have low antioxidant levels in seminal plasma which can lead to sperm vulnerability against oxidative stress [6].
Propolis is a natural product collected from cracks in the bark of trees by honey bees and enriched by their salivary enzymes [5]. Many biological components such as polyphenolic compounds (i.e., flavonoids), cinnamic acid extracts, different steroids and amino acids have been identified in propolis [7]. Some biological effects such as anti-inflammatory, anticancer, antioxidant, antibiotic and antifungal activities have also been reported from propolis in several papers [5]. Protective properties of propolis against infertility have been reported in several studies, such as ameliorating production, motility, number and quality of sperm, expanding the process of steroi-dogenesis and then testosterone production [8, 9]. Due to increased infertility prevalence and impact of emotional, financial burden of disease on infertile couple’s quality of lives [10], this study was designed to investigate the effect of propolis on sperm parameters, inflammatory factors and sex hormones in men affected by idiopathic infertility.
Materials and Methods
Subjects
This double blind randomized controlled clinical trial was accepted by the Ethics Committee of Qazvin University of Medical Sciences and was recorded by the identification code of IRCT2016072519669N2 in clinical trials registry of Iran. A total of 60 asthenozoospermic men seeking infertility treatment at urology clinic of the Velayat Hospital (Qazvin, Iran) during two years were enrolled. The inclusion criteria were: willing to cooperate, age range between 20 and 45, infertility of unknown origin (idiopathic)
based on WHO 1999 criteria [11], normal levels of gonadotropins, testosterone and serum prolactin, asthenospermia with mobility less than 50%. Patients were excluded from the study if any of the following conditions existed: there was a known cause of infertility (such as hormonal disorders, epididymal duct obstruction, and epididymoorchitis), smoking or drugs/ alcohol consumption, diabetes, kidney disease (creatinine doubled or more), chronic liver disease (transaminases more than twice the normal level), infectious diseases with fever and leukocytosis, chromosomal abnormalities, debilitating diseases, sexual system disorders such as varicocele, drugs that stimulate sexual system or interfere with sex hormones, patients undergoing intracytoplasmic sperm injection due to severe spermiogram disorders, presence of other infertility factors, contact with pesticides, heavy metals and solvents, taking antioxidant supplements in the past three months and a body mass index of 30 kg/m2 or higher.
based on WHO 1999 criteria [11], normal levels of gonadotropins, testosterone and serum prolactin, asthenospermia with mobility less than 50%. Patients were excluded from the study if any of the following conditions existed: there was a known cause of infertility (such as hormonal disorders, epididymal duct obstruction, and epididymoorchitis), smoking or drugs/ alcohol consumption, diabetes, kidney disease (creatinine doubled or more), chronic liver disease (transaminases more than twice the normal level), infectious diseases with fever and leukocytosis, chromosomal abnormalities, debilitating diseases, sexual system disorders such as varicocele, drugs that stimulate sexual system or interfere with sex hormones, patients undergoing intracytoplasmic sperm injection due to severe spermiogram disorders, presence of other infertility factors, contact with pesticides, heavy metals and solvents, taking antioxidant supplements in the past three months and a body mass index of 30 kg/m2 or higher.
Study design
At the beginning of the study, patients were randomized to propolis-treated group who received 1500 mg of Iranian propolis daily and placebo group who received matching placebo (a capsule seeming as propolis capsule but containing wheat flour) for 10 weeks (three capsules of 500 mg per day). Propolis was bought from Alamut Company in Qazvin, approved by an expert in Tabriz Faculty of Pharmacy, Iran, and poured into the capsule after being powdered. Each eligible patient received a computer-generated randomization number. Then a randomization table was generated by way of random permuted blocks. The investigator and patients were blinded to the treatment condition. Subjects were given informed consent prior to participation in the study. To maintain and guarantee blinding, propolis and placebo were identical in appearance. Patients' data collected during this trial were kept confidential and locked in a secure area. Randomization codes of the study were opened only after all participants had completed the study protocol. Demographic data, medical history, lifetime history of tobacco use, intake of multivitamin supplements, and lifestyle information were collected from each patient. Weight was measured with the use of digital scales (Soehnle, Germany) with patients minimally clothed. Height was measured with a fixed-to-wall non-stretch tape meter with patients in a standing position. The body mass index was then calculated in kilograms per square meters. Participants were interviewed face to face by trained professional nutritionists.
Preparation of samples
Semen samples were obtained after 3 days of sexual abstinence at urology clinic of the Velayat Hospital (Qazvin, Iran). The semen was held at 37°C to liquefy. After liquefaction, the samples were analyzed according to the WHO protocol [12]. The rest of liquid semen samples were immediately centrifuged at 300 rpm for 10 min. The seminal plasma was divided into several aliquots and kept frozen at -80°C for other biochemical analyses. Venous blood samples were centrifuged at 3,000 g at 4°C for 10 minutes, and serum was aspirated out for hormone assays. The serum was stored at -80°C until assays.
Laboratory methods
Assessment of sperm motility
Motility assessment of sperms was performed according to the WHO 1999 criteria [13]. Sperms were scored for motility as grades a to d, and progressive motility rate was calculated as the percentage of a+b.
Biochemical analysis
C-reactive protein (CRP) levels were measured using turbidimetric immunoassay (BioSystems Co, Barcelona, Spain). Serum levels of tumor necrosis factor (TNF)-α were measured using enzyme-linked immunosorbent assay (ELISA) (DIAsource Co, Belgium). Serum malondial-dehyde levels were detected by tiobarbituric acid method. Colorimetric method was used for analyzing serum total antioxidant capacity (TAC) (Randox Laboratories Ltd, UK). This method has been extensively explained by Khosrowbeygi et al. [14].
Reproductive hormones assay
Serum testosterone (DRG Instruments GmbH, Germany) and prolactin (Padyab Teb Diagnostic, Iran) were assayed using commercial radioimmunoassay kits. These commercial kits had previously been used with an inter-assay and intra-assay variation of lower than 10% (the reference range for testosterone and prolactin is 10 to 35 nmol/L and 92 to 697 pmol/L, respectively.) Luteinizing hormone (LH) was measured by immunochemilumino-metric assay (CUSABIO, Japan), in which intra-assay and interassay coefficients of variation were 3.4% and 3.8%, respectively (the LH normal range is 1.5 to 9.3 IU/L). Follicle-stimulating hormone (FSH) was also measured using immuno-chemiluminometric assay (CUSABIO, Japan) with an intra-assay and inter-assay coefficients of variation of 3.2% and 6.7%, respectively (the FSH normal range is 1.4 to18.1 IU/L).
Statistical analysis
All data were presented as Mean±SD. The distribution of the data was evaluated by the Kolmogorov–Smirnov test. Due to the normal distribution of variables, the independent sample t-test and the paired sample t-test were applied to analyze differences in variables between and within groups, respectively. Statistical computations were calculated using SPSS 16 for windows software (SPSS Inc., Chicago, IL, USA). P<0.05 was considered as statistically significant.
Results
In this study, a total of 60 patients were recruited but only 57 patients completed the whole study including 29 out of 30 in the propolis-treated group and 28 out of 30 in the placebo group (Fig. 1). There were no significant differences in baseline features of the participants between the two groups (Table 1). There were no significant differences in total sperm count, sperm concentration, the percentage of progressive motile sperm, total motility, and sperm morphology between the two groups before treatment (p>0.05, Table 2). However, propolis supplementation, compared to the placebo, significantly increased sperm count, sperm concentration, sperm total motility and live sperm (p<0.05). Within-group analysis indicated that the sperm count, sperm concentration, sperm total motility and live sperm significantly increased after intervention in the propolis-treated group (p<0.05). Other sperm parameters such as ejaculation volume and morphology were not significantly different between the two groups (p>0.05).
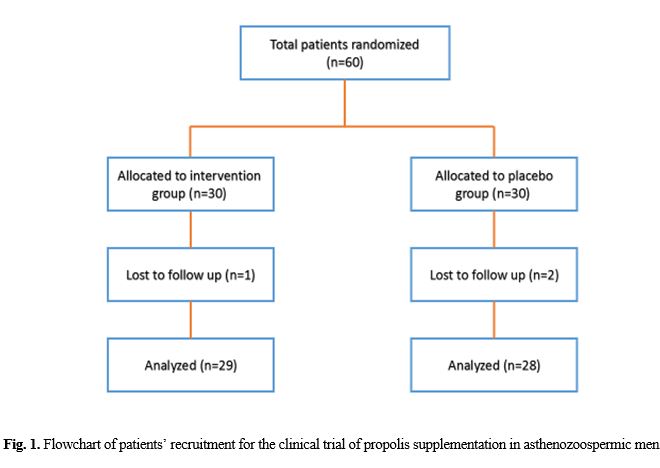

Table 1. Comparison of baseline characteristics of the participants
| Characteristics | Propolis-treated group (N=29) |
Placebo group (N=28) |
P-value | |
| Age (yr) | 31.61 ± 4.18 | 30 ± 3.96 | 0.35* | |
| Duration of Infertility (yr) | 3.93 ± 1.52 | 3.71 ± 0.99 | 0.37* | |
| Smoking history N (%) |
Never smoker | 19 (65.51) | 19 (67.85) | 0.9** |
| Current smoker | 10 (34.48) | 9 (32.14) | ||
| Education status N (%) |
Less than high school | 10 (34.48) | 8 (28.57) | 0.71** |
| High school diploma | 11 (37.93) | 13 (46.42) | ||
| Bachelor’s or higher | 8 (27.58) | 7 (25) | ||
| Weight (Kg) | Base | 85.45 ± 9.03 | 84.86 ± 9.01 | 0.48 |
| End of 12 weeks | 85.33 ± 11.22 | 85.76 ± 10.09 | 0.87 | |
| BMI (Kg/m²) | Base | 27.02 ± 3.04 | 26.52 ± 2.73 | 0.20 |
| End of 12 weeks | 27.15 ± 3.4 | 26.65 ± 3.06 | 0.21 | |
| Physical activity (met-h/week) | Base | 30.63 ± 10.27 | 30.06 ± 9.22 | 0.80 |
| End of 10 weeks | 30.66 ± 10.45 | 30.1 ± 9.74 | 0.21 | |
*Based on statistical analysis with independent samples t-test; **Based on statistical analysis with Chi-square test; yr = year; BMI= Body mass index; N (%)= Number (percent)
Data are presented as Mean±SD
Data are presented as Mean±SD
Table 2. Comparison of sperm quality parameters before and after the intervention within and between two groups
| Variables | Propolis-treated group (N=29) |
Placebo group (N=28) |
P1 | ||
| Ejaculate volume (mL) | Before | 3.45 ± 0.29 | 3.39 ± 0.26 | 0.89 | |
| After | 3.44 ± 0.5 | 3.38 ± 0.47 | 0.901 | ||
| P2 | 0.99 | 0.987 | |||
| Total sperm count (106 sperm/per ejaculate) |
Before | 74.94 ± 4.93 | 75.54 ± 4.42 | 0.42 | |
| After | 86.88±10.41 | 76.36 ± 9.79 | 0.011 | ||
| P2 | 0.002 | 0.261 | |||
| Sperm concentration (106/mL) | Before | 21.77 ± 2.05 | 21.9 ± 1.83 | 0.72 | |
| After | 25.31 ± 5.27 | 22.16 ± 4.96 | <0.001 | ||
| P2 | <0.001 | 0.101 | |||
| Motility grade a + b (%) | Before | 26.82 ± 3.08 | 27 ± 2.74 | 0.8 | |
| After | 32.15 ± 3.82 | 26.06 ± 3.2 | <0.001 | ||
| P2 | <0.001 | 0.104 | |||
| Motility grade a (%) | Before | 3.05 ± 1.26 | 3.07 ± 1.12 | 0.89 | |
| After | 6.29 ± 3 | 3.25 ± 3.56 | <0.001 | ||
| P2 | <0.001 | 0.201 | |||
| Motility grade b (%) | Before | 23.77 ± 2.79 | 23.93 ± 2.48 | 0.47 | |
| After | 25.86 ± 3.23 | 23.69 ± 3.84 | 0.09 | ||
| P2 | 0.099 | 0.326 | |||
| Motility grade c (%) | Before | 6.83 ± 3.2 | 6.88 ± 2.91 | 0.311 | |
| After | 9.1 ± 3.07 | 7.55 ± 3.05 | 0.16 | ||
| P2 | 0.042 | 0.711 | |||
| Motility grade d (%) | Before | 62.46 ± 3.6 | 63.09 ± 3.21 | 0.104 | |
| After | 57.11 ± 4.66 | 62.81 ± 2.94 | 0.003 | ||
| P2 | 0.01 | 0.253 | |||
| Motility grade a+b+c (%) | Before | 33.66 ± 3.9 | 33.88 ± 3.47 | 0.22 | |
| After | 38.98 ± 5.1 | 34.51 ± 2.72 | 0.031 | ||
| P2 | 0.04 | 0.694 | |||
| Normal morphology (%) | Before | 14.99 ± 3.72 | 15.11 ± 3.34 | 0.27 | |
| After | 17.46 ± 4.91 | 14.96 ± 3.29 | 0.052 | ||
| P2 | 0.057 | 0.203 | |||
| Live sperm (%) | Before | 69.12 ± 3.84 | 69.68 ± 3.45 | 0.79 | |
| After | 75.51 ± 5.07 | 68.99 ± 3.39 | 0.021 | ||
| P2 | 0.02 | 0.307 | |||
P1: Comparing the mean of sperm quality parameters between the two groups (independent samples t-test)
P2: Comparing the mean of sperm quality parameters in each group before and after the study (paired samples t-test)
Data are presented as Mean±SD
At the baseline, there were no significant differences in plasma malondialdehyde, TAC, CRP and TNF-α levels between the two groups. After propolis treatment for 10 weeks, plasma TAC levels increased significantly compared to the placebo (2.1±0.06 vs. 1.35±0.09 µmol/l, p<0.001). Plasma malondialdehyde and inflammatory biomarker levels were also affected by propolis supplementation. These factors were significantly decreased in the propolis group, when compared to the placebo group (p<0.05) (Table 3).
Table 3. Comparison of the changes of measures for oxidative stress biomarkers, inflammatory factors and sex hormones before and after the intervention within and between two groups
| Variables | Propolis-treated group (n=29) |
Placebo group (n=28) |
P1 | |
| TAC (mmol/l) | Before | 1.4 ± 0.08 | 1.36 ± 0.08 | 0.901 |
| After | 2.1 ± 0.06 | 1.35 ± 0.09 | <0.001 | |
| P2 | <0.001 | 0.701 | ||
| Malondialdehyde (µmol/l) | Before | 1.08 ± 0.1 | 1.05 ± 0.1 | 0.104 |
| After | 0.83 ± 0.1 | 1.08 ± 0.1 | 0.003 | |
| P2 | 0.001 | 0.198 | ||
| CRP (µmol/l)c | Before | 7.13 ± 2.1 | 6.9 ± 2.03 | 0.065 |
| After | 5.3 ± 1.55 | 6.8 ± 2 | 0.047 | |
| P2 | 0.001 | 0.546 | ||
| TNF α (µmol/l) | Before | 13.12 ± 1.86 | 12.69 ± 1.73 | 0.076 |
| After | 10.74 ± 1.44 | 12.57 ± 1.86 | 0.024 | |
| P2 | 0.001 | 0.511 | ||
| Testosterone (ng/mL) | Before | 14.01 ± 2.95 | 14.12 ± 2.74 | 0.253 |
| After | 15.97 ± 3.35 | 15.08 ± 2.53 | 0.27 | |
| P2 | 0.081 | 0.163 | ||
| FSH (ng/mL) | Before | 5.42 ± 2.1 | 5.37 ± 1.99 | 0.52 |
| After | 4.6 ± 2.11 | 5.43 ± 2.02 | 0.24 | |
| P2 | 0.29 | 0.244 | ||
| LH (ng/mL) | Before | 5.96 ± 2.12 | 6.01 ± 1.97 | 0.84 |
| After | 5.57 ± 1.97 | 5.99 ± 1.88 | 0.29 | |
| P2 | 0.23 | 0.507 | ||
| PRL (ng/mL) | Before | 351.45 ± 32.7 | 354.4 ± 29.02 | 0.27 |
| After | 345.8 ± 32.89 | 355.55 ± 27.26 | 0.08 | |
| P2 | 0.07 | 0.32 | ||
P1: Comparing the mean of oxidative stress biomarkers and inflammatory factors and sex hormones between two groups (independent samples t test)
P2: Comparing the mean of oxidative stress biomarkers and inflammatory factors and sex hormones in each group before and after intervention (paired samples t-test)
TAC =Total antioxidant capacity; CRP= C-reactive protein; TNF α= Tumor necrosis factor α; FSH= Follicle-stimulating hormone; LH= Luteinizing hormone; PRL= Prolactin
Data are presented as Mean±SD
P2: Comparing the mean of oxidative stress biomarkers and inflammatory factors and sex hormones in each group before and after intervention (paired samples t-test)
TAC =Total antioxidant capacity; CRP= C-reactive protein; TNF α= Tumor necrosis factor α; FSH= Follicle-stimulating hormone; LH= Luteinizing hormone; PRL= Prolactin
Data are presented as Mean±SD
There were no significant differences in the baseline levels of LH, FSH, PRL and testosterone between the two groups. Compared to the placebo, the propolis-treated group had an increase in testosterone and decrease in serum FSH, LH and PRL on 10-week treatment. However, this difference was not significant (p>0.05). Within-group analyses indicated that difference in the reproductive hormone levels were not significant after intervention in the propolis-treated group (p>0.05) (Table 3).
Discussion
This study reported, for the first time, the effects of Iranian propolis oral supplement-ation on infertile men with asthenozoospermia. Our knowledge on male fertility, sperm function and evolution of distinctive tackles has been intensively improved in the last decade. Also, our understanding of oxidative stress has resulted in various new treatment methods for ameliorating male infertility. Absence of scientific reports about efficacy of many new antioxidants that are nowadays used for decreasing oxidative stress and boosting sperm function has led to their non-acceptance by food and drug administration.
Several studies have indicated that prevention of reduced sperm motility can be fulfilled with some antioxidants [15]. Our results revealed that a 10-week supplementation with propolis can increase sperm count, sperm concentration, sperm motility and live sperm.
Consistent with our results, Capucho et al. [16] and Rizk et al. [17] reported that propolis extract can increase sperm number in rats. According to these findings, propolis may be regarded as an adjuvant therapy. Testicular tissue exposed to cyclosporine A was protected from oxidation by propolis treatment.
Improved enzymatic activities of oxidative phosphorylation may affect sperm motility. ATP production is dependent on oxidative phosphorylation. Energy for the forward motion of spermatozoa is supplied by ATP [18].
This was also reported by Yousef et al. [8], in rabbits, by daily administration of 50 mg/kg of body weight of propolis orally, for 12 weeks. These results are in line with those shown by Newairy [19], when administering orally, in male rats, 50 mg/kg of body weight of propolis for 70 days. Both investigators found significant amelioration in semen quality of propolis-treated animals compared to controls. Also propolis-treated group showed significant improvement in sperm features and male fertility in animals exposed to chlorpyrifos toxicity [20]. Sperm membrane rich in polyunsaturated fatty acids may be affected by oxidative strikes. Free radical scavenging properties of propolis may protect sperms from adverse effects [21].
One of the main measurements that indicate sperm fertilizing properties is sperm motility [22]. Any negative effect on motility would really affect fertilizing capability [23]. Antioxidant power of flavonoids found in propolis may be related to boosting sperm functions, operating as antioxidant factors, and defending the sperm membrane [24]. Russo et al. [25] identified that propolis prevents oxidative damage in sperm DNA caused by the thiobarbituric acid-reactive substances. It should be noted that the effective dose of propolis has been taken from the study of Russo et al. In that study, the extract of 1.5 grams of propolis increased spermatic mitochondrial activity and thus increased sperm motility. By giving honey containing high levels of flavonoids to rats, Syazana et al. [26], reported that honey can improve sperm count and percentage of normal sperm and can decrease the percentage of sperm head and tail deformity. Therefore, an increase in motility and spermatozoa robustness after propolis supplementation were expected.
de Moraes et al. [27] evaluated the different levels of propolis powder in rabbit diets and their effect on semen functions. Results indicated that propolis in the diet increased normal spermatozoa percentage and decreased spermatozoa abnormalities. The progressive spermatic motility and spermatic concentration were not affected by propolis. Some components, especially flavonoids are likely present in the propolis which affect semen parameters, as pointed out by investigators [8, 25, 17].
The TAC in seminal fluid and blood plasma is firmly linked to male fertility, and proper TAC supplies a good environment for sperm swimming [28]. Considering this fact, Contri et al. [29] reported a clear correlation between sperm parameters and total antioxidant capacity in seminal plasma. Antioxidant defense capacity of semen is reduced in semen processing and cryopreservation [30]. Based on our study, propolis intake significantly increased the level of TAC in plasma. Further, propolis application as antioxidant was awaited to lower malondialdehyde levels and improve the quality of spermatozoa. The result of this research indicated that propolis supplementation 1500 mg lowers malondialdehyde level significantly compared to the placebo group.
Decreased plasma and tissue (liver and kidney) malondialdehyde levels was reported by Kanbur et al. [24] in animals that were administered propolis in association with propetamphos, in comparison to the group that was treated with propetamphos alone. Elementary mechanism of propolis effect may be related to scavenging of free radicals that cause lipid peroxidation.
There are numerous data about anti-inflam-matory properties of propolis [31]. In this study, propolis significantly decreased the levels of CRP and TNF-α. Anti-inflammatory effect of propolis reported in several investigations originates from the presence of flavonoids, mainly galangin [32]. Moreover, flavonoid has been shown to retard cyclo-oxygenase and lipooxygenase activity and the expression of the inducible isoform of cyclo-oxygenase (cyclo-oxygenase-2). Indeed, another component of propolis, caffeic acid phenethyl ester, has anti-inflammatory properties by preventing the release of arachidonic acid from cell membrane, repressing the enzyme activities of cyclo-oxygenase-1 and cyclo-oxygenase-2, and inhibiting the activation of cyclo-oxygenase-2 gene expression [33].
Spermatozoa quality is affected by various spermatogenesis processes in testis including the levels of LH, FSH produced by anterior hypophysis, and testosterone produced by testis Leydig cells [34]. Yousef et al. [35] studied the protective effects of propolis against reproductive toxicity of aluminium chloride (AlCl3) in male rats. Propolis alone decreased the dead and abnormal sperm and thiobarbituric acid reactive substances but increased testosterone. Nevertheless, AlCl3 administration alone lowered the level of testosterone without propolis, but a combination of AlCl3 and propolis recovered the testosterone levels. Guo et al. [36] suggested that aluminium-induced ROS might be a suppressor of testosterone. Evidences demonstrated that ROS play a central role in controlling androgen synthesis. Dobashi et al. [37] observed the inhibition of LH-stimulated steroidogenesis by ROS in Leydig cells. The stress-induced testicular ROS also caused a decrease in steroidogenic enzyme activities [38]. In our study, the propolis-treated group had an increase in testosterone and decrease in serum FSH, LH and PRL at the end of the study. However this difference was not significant.
To date, the effects of propolis or propolis compounds on the sperm parameters have been scarcely studied. All these findings are attributed to the propolis effects in animal studies, none of which were conducted in human investigations. Our study describes a novel result of propolis on the sperm characteristics in asthenozoospermic men.
Conclusion
It can be concluded that intake of propolis improves semen parameters and sperm function. Therefore, it is possible to use 1500 mg of propolis daily in infertile men to significantly increase density, count, and motility of sperm.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgments
This work being the result of the MSc thesis of Fereshteh Gholaminejad, student of Qazvin University of Medical Sciences. (Grant Number: IR.QUMS.REC.1395.42) was financially supported by a grant from Vice-Chancellor for Research Affairs of Qazvin University of Medical Sciences, Qazvin, Iran.
Discussion
This study reported, for the first time, the effects of Iranian propolis oral supplement-ation on infertile men with asthenozoospermia. Our knowledge on male fertility, sperm function and evolution of distinctive tackles has been intensively improved in the last decade. Also, our understanding of oxidative stress has resulted in various new treatment methods for ameliorating male infertility. Absence of scientific reports about efficacy of many new antioxidants that are nowadays used for decreasing oxidative stress and boosting sperm function has led to their non-acceptance by food and drug administration.
Several studies have indicated that prevention of reduced sperm motility can be fulfilled with some antioxidants [15]. Our results revealed that a 10-week supplementation with propolis can increase sperm count, sperm concentration, sperm motility and live sperm.
Consistent with our results, Capucho et al. [16] and Rizk et al. [17] reported that propolis extract can increase sperm number in rats. According to these findings, propolis may be regarded as an adjuvant therapy. Testicular tissue exposed to cyclosporine A was protected from oxidation by propolis treatment.
Improved enzymatic activities of oxidative phosphorylation may affect sperm motility. ATP production is dependent on oxidative phosphorylation. Energy for the forward motion of spermatozoa is supplied by ATP [18].
This was also reported by Yousef et al. [8], in rabbits, by daily administration of 50 mg/kg of body weight of propolis orally, for 12 weeks. These results are in line with those shown by Newairy [19], when administering orally, in male rats, 50 mg/kg of body weight of propolis for 70 days. Both investigators found significant amelioration in semen quality of propolis-treated animals compared to controls. Also propolis-treated group showed significant improvement in sperm features and male fertility in animals exposed to chlorpyrifos toxicity [20]. Sperm membrane rich in polyunsaturated fatty acids may be affected by oxidative strikes. Free radical scavenging properties of propolis may protect sperms from adverse effects [21].
One of the main measurements that indicate sperm fertilizing properties is sperm motility [22]. Any negative effect on motility would really affect fertilizing capability [23]. Antioxidant power of flavonoids found in propolis may be related to boosting sperm functions, operating as antioxidant factors, and defending the sperm membrane [24]. Russo et al. [25] identified that propolis prevents oxidative damage in sperm DNA caused by the thiobarbituric acid-reactive substances. It should be noted that the effective dose of propolis has been taken from the study of Russo et al. In that study, the extract of 1.5 grams of propolis increased spermatic mitochondrial activity and thus increased sperm motility. By giving honey containing high levels of flavonoids to rats, Syazana et al. [26], reported that honey can improve sperm count and percentage of normal sperm and can decrease the percentage of sperm head and tail deformity. Therefore, an increase in motility and spermatozoa robustness after propolis supplementation were expected.
de Moraes et al. [27] evaluated the different levels of propolis powder in rabbit diets and their effect on semen functions. Results indicated that propolis in the diet increased normal spermatozoa percentage and decreased spermatozoa abnormalities. The progressive spermatic motility and spermatic concentration were not affected by propolis. Some components, especially flavonoids are likely present in the propolis which affect semen parameters, as pointed out by investigators [8, 25, 17].
The TAC in seminal fluid and blood plasma is firmly linked to male fertility, and proper TAC supplies a good environment for sperm swimming [28]. Considering this fact, Contri et al. [29] reported a clear correlation between sperm parameters and total antioxidant capacity in seminal plasma. Antioxidant defense capacity of semen is reduced in semen processing and cryopreservation [30]. Based on our study, propolis intake significantly increased the level of TAC in plasma. Further, propolis application as antioxidant was awaited to lower malondialdehyde levels and improve the quality of spermatozoa. The result of this research indicated that propolis supplementation 1500 mg lowers malondialdehyde level significantly compared to the placebo group.
Decreased plasma and tissue (liver and kidney) malondialdehyde levels was reported by Kanbur et al. [24] in animals that were administered propolis in association with propetamphos, in comparison to the group that was treated with propetamphos alone. Elementary mechanism of propolis effect may be related to scavenging of free radicals that cause lipid peroxidation.
There are numerous data about anti-inflam-matory properties of propolis [31]. In this study, propolis significantly decreased the levels of CRP and TNF-α. Anti-inflammatory effect of propolis reported in several investigations originates from the presence of flavonoids, mainly galangin [32]. Moreover, flavonoid has been shown to retard cyclo-oxygenase and lipooxygenase activity and the expression of the inducible isoform of cyclo-oxygenase (cyclo-oxygenase-2). Indeed, another component of propolis, caffeic acid phenethyl ester, has anti-inflammatory properties by preventing the release of arachidonic acid from cell membrane, repressing the enzyme activities of cyclo-oxygenase-1 and cyclo-oxygenase-2, and inhibiting the activation of cyclo-oxygenase-2 gene expression [33].
Spermatozoa quality is affected by various spermatogenesis processes in testis including the levels of LH, FSH produced by anterior hypophysis, and testosterone produced by testis Leydig cells [34]. Yousef et al. [35] studied the protective effects of propolis against reproductive toxicity of aluminium chloride (AlCl3) in male rats. Propolis alone decreased the dead and abnormal sperm and thiobarbituric acid reactive substances but increased testosterone. Nevertheless, AlCl3 administration alone lowered the level of testosterone without propolis, but a combination of AlCl3 and propolis recovered the testosterone levels. Guo et al. [36] suggested that aluminium-induced ROS might be a suppressor of testosterone. Evidences demonstrated that ROS play a central role in controlling androgen synthesis. Dobashi et al. [37] observed the inhibition of LH-stimulated steroidogenesis by ROS in Leydig cells. The stress-induced testicular ROS also caused a decrease in steroidogenic enzyme activities [38]. In our study, the propolis-treated group had an increase in testosterone and decrease in serum FSH, LH and PRL at the end of the study. However this difference was not significant.
To date, the effects of propolis or propolis compounds on the sperm parameters have been scarcely studied. All these findings are attributed to the propolis effects in animal studies, none of which were conducted in human investigations. Our study describes a novel result of propolis on the sperm characteristics in asthenozoospermic men.
Conclusion
It can be concluded that intake of propolis improves semen parameters and sperm function. Therefore, it is possible to use 1500 mg of propolis daily in infertile men to significantly increase density, count, and motility of sperm.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgments
This work being the result of the MSc thesis of Fereshteh Gholaminejad, student of Qazvin University of Medical Sciences. (Grant Number: IR.QUMS.REC.1395.42) was financially supported by a grant from Vice-Chancellor for Research Affairs of Qazvin University of Medical Sciences, Qazvin, Iran.
References
- Sanchez E, Giviziez CR, Sanchez HM, Agostinho P, Barros PS, Approbato MS. Low progesterone levels and ovulation by ultrasound assessment in infertile patients. Jornal brasileiro de reproducao assistida. 2015; 20(1): 13-16.
- Cong J, Li P, Zheng L, Tan J. Prevalence and Risk Factors of Infertility at a Rural Site of Northern China. PloS one. 2016; 11(5): e0155563.
- Pourmasumi S, Mostaghaci M, Sabeti P, Ardian N. Knowledge of infertile couples about assisted reproductive technology in Iran. Women’s Health Gynecol. 2016; 2(3): 1-4.
- Ko EY, Sabanegh ES, Agarwal A. Male infertility testing: reactive oxygen species and antioxidant capacity. Fertil Steril. 2014; 102(6): 1518-527.
- Khaled FA, Yousef MI, Kamel KI. The protective role of propolis against the reproductive toxicity of mono-sodium glutamine in male rabbits. IJCS. 2016; 4(2): 4-9.
- Hoesada I, Nasihun T, Isradji I. The effect of propolis extract on MDA levels (malondial-dehyde) and sperm quality on epididimis. Sains Medika. 2016; 7(1): 9-14.
- Collodel G, Moretti E, Del Vecchio MT, Biagi M, Cardinali R, Mazzi L et al. Effect of chocolate and Propolfenol on rabbit spermatogenesis and sperm quality following bacterial lipopolysaccharide treatment. Sys Biol Reprod Med. 2014; 60(4): 217-26.
- Yousef MI, Kamel KI, Hassan MS, El-Morsy AM. Protective role of propolis against reproductive toxicity of triphenyltin in male rabbits. Food Chem Toxicol. 2010; 48(7): 1846-852.
- ElMazoudy RH, Attia AA, El-Shenawy NS. Protective role of propolis against reproductive toxicity of chlorpyrifos in male rats. Pesticide Biochem Physiol. 2011; 101(3): 175-81.
- Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012; 9(12): e1001356.
- Jungwirth A, Giwercman A, Tournaye H, Diemer T, Kopa Z, Dohle G, et al. European Association of Urology guidelines on Male Infertility: the 2012 update. Euro Urol. 2012; 62(2): 324-32.
- Künzle R, Mueller MD, Hänggi W, Birkhäuser MH, Drescher H, Bersinger NA. Semen quality of male smokers and nonsmokers in infertile couples. Fertil Steril. 2003; 79(2): 287-91.
- Jensen TK, Gottschau M, Madsen JOB, Andersson AM, Lassen TH, Skakkebæk NE et al. Habitual alcohol consumption associated with reduced semen quality and changes in reproductive hormones; a cross-sectional study among 1221 young Danish men. BMJ Open. 2014; 4(9): e005462.
- Khosrowbeygi A, Zarghami N. Levels of oxidative stress biomarkers in seminal plasma and their relationship with seminal parameters. BMC Clinic Pathol. 2007; 7(1): 1-6.
- Haghighian HK, Haidari F, Mohammadi-asl J, Dadfar M. Randomized, triple-blind, placebo-controlled clinical trial examining the effects of alpha-lipoic acid supplement on the spermato-gram and seminal oxidative stress in infertile men. Fertil Steril. 2015; 104(2): 318-24.
- Capucho C, Sette R, de Souza Predes F, de Castro Monteiro J, Pigoso AA, Barbieri R, et al. Green Brazilian propolis effects on sperm count and epididymis morphology and oxidative stress. Food Chem Toxicol. 2012; 50(11): 3956-962.
- Rizk SM, Zaki HF, Mina MA. Propolis attenuates doxorubicin-induced testicular toxicity in rats. Food Chem Toxicol. 2014; 67: 176-86.
- Bansal AK, Bilaspuri G. Impacts of oxidative stress and antioxidants on semen functions. Vet Med Int. 2010; 2010(686137): 7.
- Newairy AS, Salama AF, Hussien HM, Yousef MI. Propolis alleviates aluminium-induced lipid peroxidation and biochemical parameters in male rats. Food Chem Toxicol. 2009; 47(6): 1093-1098.
- Heikal TM, Mossa ATH, Ibrahim AW, Abdel-Hamid HF. Oxidative damage and reproductive toxicity associated with cyromazine and chlorpyrifos in male rats: the protective effects of green tea extract. Res J Environ Toxicol. 2014; 8(2): 53-67.
- Walczak-Jedrzejowska R, Wolski JK, Slowikowska-Hilczer J. The role of oxidative stress and antioxidants in male fertility. Cent European J Urol. 2013; 66(1): 60-67.
- García-Vázquez FA, Gadea J, Matás C, Holt WV. Importance of sperm morphology during sperm transport and fertilization in mammals. Asian J Androl. 2016; 18(6): 844-50.
- Alavi SMH, Cosson J, Karami M, Amiri BM, Akhoundzadeh MA. Spermatozoa motility in the Persian sturgeon, Acipenser persicus: effects of pH, dilution rate, ions and osmolality. Reprod. 2004; 128(6): 819-28.
- Kanbur M, Eraslan G, Silici S. Antioxidant effect of propolis against exposure to prope-tamphos in rats. Ecotoxicol Environ Safety 2009; 72(3): 909-15.
- Russo A, Troncoso N, Sanchez F, Garbarino J, Vanella A. Propolis protects human sperma-tozoa from DNA damage caused by benzo [a] pyrene and exogenous reactive oxygen species. Life Sci. 2006; 78(13): 1401-406.
- Syazana NS, Hashida NH, Majid AM, Durriyah HA, Kamaruddin MY. Effects of Gelam Honey on sperm quality and testis of rat. Sains Malaysiana 2011; 40(11): 1243-246.
- de Moraes GV, Mataveli M, de Moura LPP, Scapinello C, Mora F, Osmari MP. Inclusion of propolis in rabbit diets and semen characteristics. Arquivos de Ciências Veterinárias e Zoologia da UNIPAR. 2014; 17(4): 227-31.
- Garrido N, Meseguer M, Simon C, Pellicer A, Remohi J. Pro-oxidative and anti-oxidative imbalance in human semen and its relation with male fertility. Asian J Androl. 2004; 6(1): 59-66.
- Contri A, De Amicis I, Molinari A, Faustini M, Gramenzi A, Robbe D, et al. Effect of dietary antioxidant supplementation on fresh semen quality in stallion. Theriogenol. 2011; 75(7): 1319-326.
- Michael A, Alexopoulos C, Pontiki E, Hadjipavlou-Litina D, Saratsis P, Boscos C. Effect of antioxidant supplementation on semen quality and reactive oxygen species of frozen-thawed canine spermatozoa. Theriogenol. 2007; 68(2): 204-12.
- Sforcin JM, Bankova V. Propolis: is there a potential for the development of new drugs? J Ethnopharmacol. 2011; 133(2): 253-60.
- Viuda‐Martos M, Ruiz‐Navajas Y, Fernández López J, Pérez Álvarez J. Functional properties of honey, propolis, and royal jelly. J Food Sci. 2008; 73(9): 117-24.
- Wang K, Zhang J, Ping S, Ma Q, Chen X, Xuan H, et al. Anti-inflammatory effects of ethanol extracts of Chinese propolis and buds from poplar (Populus× canadensis). J Ethno-pharmacol. 2014; 155(1): 300-11.
- Mylonas CC, Duncan NJ, Asturiano JF. Hormonal manipulations for the enhancement of sperm production in cultured fish and evaluation of sperm quality. Aquaculture 2017; 472(1): 21-44.
- Yousef MI, Salama AF. Propolis protection from reproductive toxicity caused by aluminium chloride in male rats. Food Chem Toxicol. 2009; 47(6): 1168-175.
- Guo CH, Lu YF, Hsu GSW. The influence of aluminum exposure on male reproduction and offspring in mice. Environ Toxicol Pharmacol. 2005; 20(1): 135-41.
- Dobashi M, Fujisawa M, Yamazaki T, Okuda Y, Kanzaki M, Tatsumi N, et al. Inhibition of steroidogenesis in Leydig cells by exogenous nitric oxide occurs independently of steroidogenic acute regulatory protein (star) mRNA. Archiv Androl. 2001; 47(3): 203-209.
- Reddy MM, Mahipal SV, Subhashini J, Reddy MC, Roy KR, Reddy GV, et al. Bacterial lipo-polysaccharide-induced oxidative stress in the impairment of steroidogenesis and spermato-genesis in rats. Reprod Toxicol. 2006; 22(3): 493-500.
Type of Study: Research |
Subject:
Pathology
Received: 2018/04/8 | Accepted: 2018/12/3 | Published: 2019/03/10
Received: 2018/04/8 | Accepted: 2018/12/3 | Published: 2019/03/10
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




