Sun, Feb 22, 2026
[Archive]
Volume 5, Issue 3 (August 2018)
IJML 2018, 5(3): 173-181 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Alaei Sheini F, Tabnak M, Hasanzadeh Bezvan M, Mahdiannasser M, Musavi H, Choobineh H et al . A Systematic Review of the Evidence on the Effects of Cytomegalovirus on Abortion. IJML 2018; 5 (3) :173-181
URL: http://ijml.ssu.ac.ir/article-1-244-en.html
URL: http://ijml.ssu.ac.ir/article-1-244-en.html
Farzaneh Alaei Sheini 
 , Malihe Tabnak
, Malihe Tabnak 
 , Maryam Hasanzadeh Bezvan
, Maryam Hasanzadeh Bezvan 
 , Mojdeh Mahdiannasser
, Mojdeh Mahdiannasser 
 , Hadis Musavi
, Hadis Musavi 
 , Hamid Choobineh
, Hamid Choobineh 
 , Mojtaba Abbasi *
, Mojtaba Abbasi * 


 , Malihe Tabnak
, Malihe Tabnak 
 , Maryam Hasanzadeh Bezvan
, Maryam Hasanzadeh Bezvan 
 , Mojdeh Mahdiannasser
, Mojdeh Mahdiannasser 
 , Hadis Musavi
, Hadis Musavi 
 , Hamid Choobineh
, Hamid Choobineh 
 , Mojtaba Abbasi *
, Mojtaba Abbasi * 

Veterinary Medicine, Faculty of Veterinary Medicine, Shahrekord Branch, Islamic Azad University, Shahrekord, Iran.
Full-Text [PDF 512 kb]
(1107 Downloads)
| Abstract (HTML) (2774 Views)
Table 2. Quality of studies using NIH’s quality assessment for Case-Control studies
Table 3. General information of included studies
Full-Text: (1531 Views)
Introduction
Cytomegalovirus (CMV) is a species of the Herpesviridae family [1-3]. The virus is found all over the world. However, it is more prevalent in the developing countries and people of lower socioeconomic status [4, 5]. The overall incidence of CMV is reported to be 40-80% [1, 6-8]. Clinical symptoms are not commonly seen in CMV-infected individuals while they can transmit the virus through saliva, body fluids, blood, cervical secretions, sperm, and urine. The virus can also be transmitted through sexual intercourse, blood transfusion, organ transplant, and breast-feeding thus being life-threatening for the people and infants with a weakened immune system [9-11].
According to various studies, primary CMV infection has been reported in 0.15% - 2.0% of pregnancies which may transfer infections to the fetus in 40% of cases. In such conditions, 10% of the cases are symptomatic and 10-15% are asymptomatic newborns. In addition, some of them show growth disorders over time. It has also been reported that 40000 children yearly in the United States suffer from congenital CMV, resulting in the death of 400 children and permanent disabilities such as deafness, blindness, and mental retardation in 8000 cases. CMV contamination may occur at any time during pregnancy. However, the probability of infection in the first trimester is higher than in the third trimester. Some studies have reported that CMV can lead to abortion [1, 12, 13].
In pregnant mothers, CMV is largely asymptomatic and therefore difficult to diagnose. Symptoms are usually non-specific and may be similar to that of the flu. Other symptoms such as fever, cervical lymphadenopathy, sore throat, fatigue, and muscle soreness may be observed as well. Laboratory findings include lymphocytosis and increased levels of liver enzymes [14, 15]. Laboratory diagnosis is more likely to be seen in women with primary infection than women with chronic infections [16, 17].
Whether primary infection or recurrent infections in the mother can lead to abortions is controversial and its mechanisms are unknown. In some studies, CMV antigen has been reported in abortion leftovers. However, the role of CMV in recurrent pregnancy loss (RPL) is unknown [9, 18]. RPL is defined as two or more consecutive abortions and is one of the most disappointing and difficult approaches in the treatment of infertility. Studies related to RPL and CMV have reported controversial results as some studies have identified an increase of antibody in recurrent abortions while others have demonstrated a decrease in its level [1, 9, 19, 20]. Despite its importance, few studies have been conducted in this regard. Considering the contradictory outcomes, the present study presents a systematic review based on the evidence of CMV effects on abortion.
Materials and Methods
This cross-sectional study was carried out on the basis of PRISMA protocol [21].
Data Source
Findings of this research were based on the studies carried out on the effects of CMV on abortion written in English regardless of the publication time. In order to collect data, keywords including abortion, recurrent abortion, B19, Cytomegalovirus, spontaneous abortion, and placenta were looked up using databases such as Web of Science (ISI), PubMed, Scopus, Ovid, and EMBASE databases. To gain access to the maximum of useful information, both electronic and manual searching was performed to obtain papers published by May 2018.
Inclusion criteria
Women with a history of abortion and RPL; abortion prior to 22 weeks of gestation; reports of IgG in abortion leftovers
Exclusion criteria
No reports of IgG in abortion leftovers; reports on IgG against herpes virus, HIV, etc; non-specified sample size
Quality Tests
At this stage, all the articles were independently reviewed and evaluated by two authors. The National Institutes of Health's Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies was employed for quality assessment of descriptive-analytic studies, and the National Institutes of Health Quality Assessment Tool for Case-Control Studies was applied for quality assessment of case-control studies (https://www.nhlbi.nih.gov/ health-topics/study-quality-assessment-tools).
Excavating information
The information was extracted by two authors and, in the case of any contradiction, the problem was resolved with the help of the third author. At this stage, the authors reviewed first the abstract and then the full-text articles. Subsequently, the information was collected in the form of a checklist containing the name of the author, the year, the sample size, the place where the research was carried out, the level of IgG in IgG-positive cases, the type of the test, and the side effects.
Results
Initially, 1135 articles were identified and then according to the inclusion and exclusion criteria, repetitive and non-related articles were eliminated and finally a total of 15 papers published from 1993 to 2018 were reviewed including 11 descriptive-analytic and 6 case-control studies (Fig. 1). In the case-control studies, the control groups comprised healthy pregnant women with no history of abortion. The case groups comprised samples of abortion and RPL. Quality assessment of studies has shown that most of the case-control studies are in a low risk of bias. However, analysis status of cases at the beginning was not mentioned in any of the studies. Moreover, in most studies, it was neither clear whether the researcher was blind to the case and control samples, nor was it defined if sample distribution was random (Table 1). Additionally, quality assessment of the descriptive-analytic studies revealed that most of them were moderately biased. Most studies did not mention whether samples were collected from the same population nor were the inclusion and exclusion criteria described. In addition, sample analysis was either unspecified or not carried out at the beginning of the study. The number of the replicates was not specified as well (Table 2).
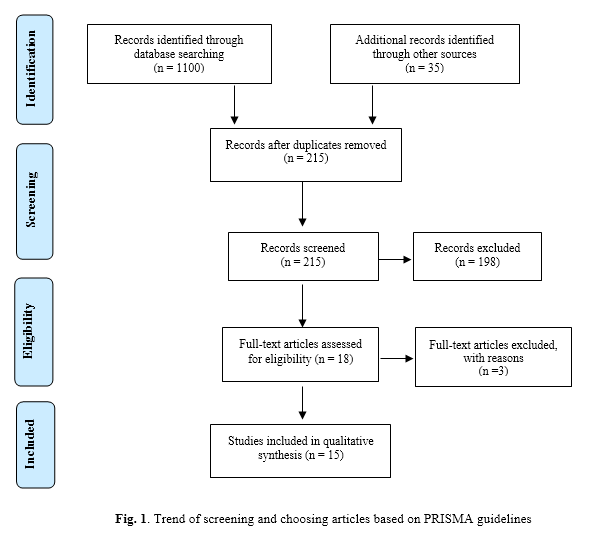
Table 1. Quality of studies using NIH’s quality assessment for cohort and cross-sectional studies
Cytomegalovirus (CMV) is a species of the Herpesviridae family [1-3]. The virus is found all over the world. However, it is more prevalent in the developing countries and people of lower socioeconomic status [4, 5]. The overall incidence of CMV is reported to be 40-80% [1, 6-8]. Clinical symptoms are not commonly seen in CMV-infected individuals while they can transmit the virus through saliva, body fluids, blood, cervical secretions, sperm, and urine. The virus can also be transmitted through sexual intercourse, blood transfusion, organ transplant, and breast-feeding thus being life-threatening for the people and infants with a weakened immune system [9-11].
According to various studies, primary CMV infection has been reported in 0.15% - 2.0% of pregnancies which may transfer infections to the fetus in 40% of cases. In such conditions, 10% of the cases are symptomatic and 10-15% are asymptomatic newborns. In addition, some of them show growth disorders over time. It has also been reported that 40000 children yearly in the United States suffer from congenital CMV, resulting in the death of 400 children and permanent disabilities such as deafness, blindness, and mental retardation in 8000 cases. CMV contamination may occur at any time during pregnancy. However, the probability of infection in the first trimester is higher than in the third trimester. Some studies have reported that CMV can lead to abortion [1, 12, 13].
In pregnant mothers, CMV is largely asymptomatic and therefore difficult to diagnose. Symptoms are usually non-specific and may be similar to that of the flu. Other symptoms such as fever, cervical lymphadenopathy, sore throat, fatigue, and muscle soreness may be observed as well. Laboratory findings include lymphocytosis and increased levels of liver enzymes [14, 15]. Laboratory diagnosis is more likely to be seen in women with primary infection than women with chronic infections [16, 17].
Whether primary infection or recurrent infections in the mother can lead to abortions is controversial and its mechanisms are unknown. In some studies, CMV antigen has been reported in abortion leftovers. However, the role of CMV in recurrent pregnancy loss (RPL) is unknown [9, 18]. RPL is defined as two or more consecutive abortions and is one of the most disappointing and difficult approaches in the treatment of infertility. Studies related to RPL and CMV have reported controversial results as some studies have identified an increase of antibody in recurrent abortions while others have demonstrated a decrease in its level [1, 9, 19, 20]. Despite its importance, few studies have been conducted in this regard. Considering the contradictory outcomes, the present study presents a systematic review based on the evidence of CMV effects on abortion.
Materials and Methods
This cross-sectional study was carried out on the basis of PRISMA protocol [21].
Data Source
Findings of this research were based on the studies carried out on the effects of CMV on abortion written in English regardless of the publication time. In order to collect data, keywords including abortion, recurrent abortion, B19, Cytomegalovirus, spontaneous abortion, and placenta were looked up using databases such as Web of Science (ISI), PubMed, Scopus, Ovid, and EMBASE databases. To gain access to the maximum of useful information, both electronic and manual searching was performed to obtain papers published by May 2018.
Inclusion criteria
Women with a history of abortion and RPL; abortion prior to 22 weeks of gestation; reports of IgG in abortion leftovers
Exclusion criteria
No reports of IgG in abortion leftovers; reports on IgG against herpes virus, HIV, etc; non-specified sample size
Quality Tests
At this stage, all the articles were independently reviewed and evaluated by two authors. The National Institutes of Health's Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies was employed for quality assessment of descriptive-analytic studies, and the National Institutes of Health Quality Assessment Tool for Case-Control Studies was applied for quality assessment of case-control studies (https://www.nhlbi.nih.gov/ health-topics/study-quality-assessment-tools).
Excavating information
The information was extracted by two authors and, in the case of any contradiction, the problem was resolved with the help of the third author. At this stage, the authors reviewed first the abstract and then the full-text articles. Subsequently, the information was collected in the form of a checklist containing the name of the author, the year, the sample size, the place where the research was carried out, the level of IgG in IgG-positive cases, the type of the test, and the side effects.
Results
Initially, 1135 articles were identified and then according to the inclusion and exclusion criteria, repetitive and non-related articles were eliminated and finally a total of 15 papers published from 1993 to 2018 were reviewed including 11 descriptive-analytic and 6 case-control studies (Fig. 1). In the case-control studies, the control groups comprised healthy pregnant women with no history of abortion. The case groups comprised samples of abortion and RPL. Quality assessment of studies has shown that most of the case-control studies are in a low risk of bias. However, analysis status of cases at the beginning was not mentioned in any of the studies. Moreover, in most studies, it was neither clear whether the researcher was blind to the case and control samples, nor was it defined if sample distribution was random (Table 1). Additionally, quality assessment of the descriptive-analytic studies revealed that most of them were moderately biased. Most studies did not mention whether samples were collected from the same population nor were the inclusion and exclusion criteria described. In addition, sample analysis was either unspecified or not carried out at the beginning of the study. The number of the replicates was not specified as well (Table 2).

Table 1. Quality of studies using NIH’s quality assessment for cohort and cross-sectional studies
Cross- sectional |
Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 |
| Gao et al. [22] | ü | ü | ü | × | ü | ? | ü | × | ü | ? | ü | × | ü | ü |
| Bagheri et al. [1] | ü | × | × | × | × | ? | × | ü | ü | ? | ü | × | ü | ü |
| Cook et al. [19] | ü | ü | × | × | × | ? | × | ü | ü | ? | ü | × | ü | ü |
| Sifakisa et al. [12] | ü | ü | ü | × | ü | ? | ü | ü | ü | ? | ü | × | ü | ü |
| Kakru et al. [6] | ü | ü | ü | × | ü | ? | ü | ü | ü | ? | ü | × | ü | × |
| Zhou et al. [26] | ü | ü | ü | × | ü | ? | ü | × | ü | ? | ü | × | ü | ü |
| Oliveira et al. [28] | ü | ü | ü | ü | ü | ? | ü | × | ü | ? | ü | × | ü | ü |
| Saraswathy et al. [29] | ü | ü | × | ü | × | ? | × | ü | ü | ? | ü | × | ü | ü |
| Cheshik et al. [30] | ü | ü | ü | × | ü | ? | ü | ü | ü | ? | ü | × | ü | ? |
Table 2. Quality of studies using NIH’s quality assessment for Case-Control studies
| Case control study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 |
| Tarokhian et al. [31] | ü | ü | ü | ü | ü | ü | ? | ü | × | ü | ? | ü |
| Sherkat et al. [9] | ü | ü | ü | ü | ü | ü | × | ü | × | ü | ? | ü |
| Al-Saeed et al. [10] | ü | ü | ü | ü | ü | ü | ü | ü | × | ü | ? | ü |
| Spano et al. [24] | ü | ü | ü | ü | ü | ü | ? | ü | × | ü | ? | ü |
| Fatima et al. [25] | ü | ü | ü | ü | ü | ü | ? | ü | × | ü | ? | ü |
| Rasti et al. [27] | ü | ü | ü | ü | ü | ü | ? | ü | × | ü | ? | ü |
Table 3. General information of included studies
| Year | Abortion | CMV-Ig+ | Place | Type of study | Method | Reference |
| 2018 | 100 | 95% | Chinese | Descriptive study | PCR | [22] |
| 2013 | 44 | 97.73% | Iran | Case control study | ELISA | [23] |
| 2012 | - | 72.1% | Iran | Descriptive study | ELISA | [1] |
| 2014 | 43 | 90.6% | Iran | Case-control | ELISA | [9] |
| 1993 | 21 | 85.71% | USA | Descriptive study | DNA CMV | [19] |
| 1998 | 102 | 86.27% | Island | Descriptive study | PCR | [12] |
| 2004 | 779 | 16.5% | India | Descriptive study | ELISA | [6] |
| 2008 | 44 | 79.54% | Iraq | Case control study | ELISA | [10] |
| 2002 | 95 | 97.3% | Brazil | A Case control study | PCR | [24] |
| 2017 | 122 | 44.26% | India | Case-control | PCR | [25] |
| 2015 | 290 | 79.31 | USA | Descriptive study | PCR | [26] |
| 2016 | 81 | 24.69% | Iran | Case control study | ELISA | [27] |
| 2017 | 70 | 0.04 | Brazil | Cross-sectional study | ELISA | [28] |
| 2011 | 17 | 100% | Malaysia | cross-sectional study | ELISA | [29] |
| 2016 | 116 | 94% | Russian | descriptive study | ELISA | [30] |
PCR= Polymerase chain reaction ; ELISA= Enzyme-linked immunosorbent assay
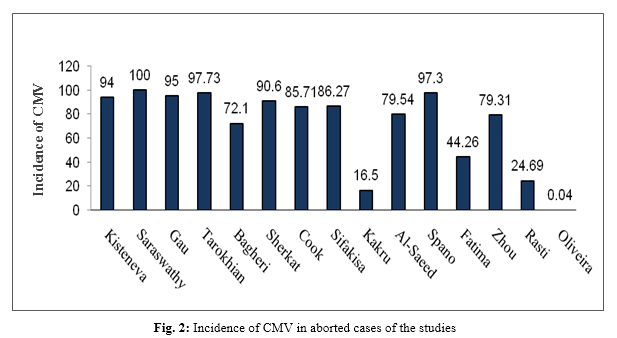
In the present study, abortion leftovers were considered as the sample size. The largest sample size was 779 and the smallest included 17 cases of abortion. In one study, the sample size was not indicated. Serological testing was performed in 9 Enzyme-linked immunosorbent assay (ELISA) and 6 Polymerase chain reaction (PCR) studies. The higher number of studies had been carried out in Iran with 4 papers. India, the United States, and Brazil each had two studies, and Russia, Malaysia, Iceland, Iraq, and China each reported one study. Three studies had looked up the side effects of CMV infection (Table 3). The highest incidence of CMV infection in abortion was 100% reported by Saraswathy and 97.73% reported by Tarokhian. The lowest incidence was 0.04% and 16% observed by Oliveira and Kakru, respectively (Fig. 2).
CMV antibody was positive in 95% of abortion cases. Totally, 97.73% of abortion cases were IgG-positive. There was a significant correlation between cases with a history of abortion and those indicated as IgG-positive (OR=2.72). IgG titers were significantly higher in the case group compared to the controls as 90.6% of cases were affected with CMV. CMV DNA was found in 18 out of 21 cases, indicating the association between CMV infection and recurrent abortion. On the whole, 88 out of 102 abortions were affected with CMV, 117 out of 779 cases were affected with CMV, and 35 out of 44 were affected with CMV. There was a significant correlation between abortion and CMV; 54 out of 122 abortions were affected with CMV. Moreover, 230 out of 290 cases with spontaneous abortion were affected with CMV; 20 out of 81 women with spontaneous abortion who were affected with CMV had an odds ratio (OR) of only 0.86 and there was no significant correlation (p=0.6). Out of 70 leftovers examined, only three demonstrated influence of CMV and the correlation was significant.
On the whole, 109 women with spontaneous abortion were affected with CMV and showed a significant correlation.
Discussion
The present study was a systematic review of the evidence on the effect of CMV infection on abortion. In this study, the term "abortion" is defined as the termination of pregnancy prior to 22 weeks of gestation and RPL is defined as two or more consecutive spontaneous pregnancy losses prior to 22 weeks of gestation. Infections during pregnancy can lead to premature rupture of the membranes, pre-term labor, and spontaneous abortion in the first trimester, intrauterine growth restriction, low birth weight, and intrauterine fetal death, as well as irreversible neonatal complications [31]. The production of toxic metabolites, fetal infections, chronic endometrial infections, and chorioamnionitis is among the possible mechanisms for abortion due to maternal infection [32]. In the present study, 15 papers were reviewed published from 1993 to 2018. The largest incidence of CMV infection was reported by Saraswathy in 2011. In the study, the sample size included 17 cases of abortion, all reported to be CMV-infected and IgG-positive by ELISA [29]. The small sample size seems to bring about serious bias to the results thus the results does not seem to be reliable. In studies of Gao, Tarokhian, Sherkat, Sifakis, Spano et al., 97.7%, 97.3%, 95%, 94% and 90.4% of CMV cases were IgG-positive in abortion cases [9, 22, 24, 30]. In these studies, CMV was clearly evident in abortion samples. Due to the fact that CMV infection exists in body fluids, it can be transfused through the blood to the trophoblast cells, and also affect the amniotic fluid and involve the uterus and pregnancy products. Endotoxins can stimulate amniotic cells to secrete cytokines that enter the amniotic fluid to give rise to immune responses. In case the infection is chronic, the endometrium of the womb results in chronic endometrium through an inflammatory response which is a strong cause of abortion. Immunocompromised women are more susceptible to CMV infection and since the immune system is weakened in the first trimester of pregnancy to facilitate the implantation of the fetus, the probability of transferring CMV to the fetus will increase [9, 23, 32]. CMV DNA testing is another method with remarkable sensitivity. In a study by Cook, CMV DNA was found in the tissue leftover, indicating the transfer of CMV to gestational products [19]. The results of this study can be considered as strong evidence of the presence of CMV in the leftovers of abortion. The study by Al-Saeed et al. indicated that levels of CMV antibodies in abortion leftovers are higher than those in controls. This study reported 79.54% of IgG against CMV in abortive cases [10]. The results of these studies depend on the pregnancy grade as well since naturally-acquired immunity during pregnancy reduces the risk of congenital CMV infections in subsequent pregnancies by 70%. However, maternal immunity does not prevent recurrences and maternal antibodies fail to prevent the onset of fetal infections [32]. Therefore, based on current evidence, 11 studies have shown a strong association between abortion and CMV so that the level of IgG against CMV is higher than 79% in all the samples studied. In spite of this, contradicting results have appeared in some investigations. CMV is a virus that has not been clearly proved to be related with abortion and this part of the story remains to be controversial. The study of Fatima et al. in India on 122 abortion cases using PCR demonstrated IgG-positive state of CMV (44.26%) [25]. However, the results differed significantly from those of other studies. In the study of Rasti et al. on 81 abortion cases, 24.69% of cases were IgG-positive [27]. However, the study of Kakru et al. with the highest sample size (n=779) reported CMV IgG in 16.5% of the samples [6]. It should be noted that the prevalence of CMV varies among different geographical regions. It has also been observed that in societies with low socioeconomic status, the incidence of infections, as well as CMV infection is more prevalent, while in societies of high socioeconomic status, the rate of infection is lower. It seems that the prevalence of CMV infection is low in some countries due to increased primary health care. Additionally, a small sample size or laboratory method is effective in revealing such results. Two studies in India have shown a weak link between abortion and CMV [6, 25]. Another cause of this disparity is the low prevalence of CMV infection in that region. The study of Oliveira et al. with a sample size of 70 reported CMV IgG in only 0.04% of positive samples, which is not consistent with the results of other studies [28]. As mentioned above, the difference in the results of studies can be due to the difference in sample size as well as the experimental method. Distribution of the patients' age may be another reason for contradictions in the results; CMV is more prevalent in younger women. Religious beliefs and high-risk behaviors are also effective in the spread of the infection so that the more there is high-risk sexual behaviors in a society, the greater will be the prevalence of CMV.
Conclusions
The results of most studies indicate that CMV infection can lead to abortion by transmission through body fluids, activation of the inflammatory responses of the uterus and the immune response, and transmission to fetal tissues. Therefore, analysis and prevention are recommended for all women at the reproductive age. It is also suggested that countries with low socioeconomic and health status, as well as those with greater rate of high-risk sexual behaviors consider CMV check-up in the country protocol for women prior to their gestational age.
Conflict of Interest
We have no conflict of interest to declare.
Acknowledgements
Thanks to Dr. Mehdi Norouzi and Dr. Babak Shahbaz for their constructive comments and help with preparing the manuscript and formatting the whole article.
References
In the present study, abortion leftovers were considered as the sample size. The largest sample size was 779 and the smallest included 17 cases of abortion. In one study, the sample size was not indicated. Serological testing was performed in 9 Enzyme-linked immunosorbent assay (ELISA) and 6 Polymerase chain reaction (PCR) studies. The higher number of studies had been carried out in Iran with 4 papers. India, the United States, and Brazil each had two studies, and Russia, Malaysia, Iceland, Iraq, and China each reported one study. Three studies had looked up the side effects of CMV infection (Table 3). The highest incidence of CMV infection in abortion was 100% reported by Saraswathy and 97.73% reported by Tarokhian. The lowest incidence was 0.04% and 16% observed by Oliveira and Kakru, respectively (Fig. 2).

CMV antibody was positive in 95% of abortion cases. Totally, 97.73% of abortion cases were IgG-positive. There was a significant correlation between cases with a history of abortion and those indicated as IgG-positive (OR=2.72). IgG titers were significantly higher in the case group compared to the controls as 90.6% of cases were affected with CMV. CMV DNA was found in 18 out of 21 cases, indicating the association between CMV infection and recurrent abortion. On the whole, 88 out of 102 abortions were affected with CMV, 117 out of 779 cases were affected with CMV, and 35 out of 44 were affected with CMV. There was a significant correlation between abortion and CMV; 54 out of 122 abortions were affected with CMV. Moreover, 230 out of 290 cases with spontaneous abortion were affected with CMV; 20 out of 81 women with spontaneous abortion who were affected with CMV had an odds ratio (OR) of only 0.86 and there was no significant correlation (p=0.6). Out of 70 leftovers examined, only three demonstrated influence of CMV and the correlation was significant.
On the whole, 109 women with spontaneous abortion were affected with CMV and showed a significant correlation.
Discussion
The present study was a systematic review of the evidence on the effect of CMV infection on abortion. In this study, the term "abortion" is defined as the termination of pregnancy prior to 22 weeks of gestation and RPL is defined as two or more consecutive spontaneous pregnancy losses prior to 22 weeks of gestation. Infections during pregnancy can lead to premature rupture of the membranes, pre-term labor, and spontaneous abortion in the first trimester, intrauterine growth restriction, low birth weight, and intrauterine fetal death, as well as irreversible neonatal complications [31]. The production of toxic metabolites, fetal infections, chronic endometrial infections, and chorioamnionitis is among the possible mechanisms for abortion due to maternal infection [32]. In the present study, 15 papers were reviewed published from 1993 to 2018. The largest incidence of CMV infection was reported by Saraswathy in 2011. In the study, the sample size included 17 cases of abortion, all reported to be CMV-infected and IgG-positive by ELISA [29]. The small sample size seems to bring about serious bias to the results thus the results does not seem to be reliable. In studies of Gao, Tarokhian, Sherkat, Sifakis, Spano et al., 97.7%, 97.3%, 95%, 94% and 90.4% of CMV cases were IgG-positive in abortion cases [9, 22, 24, 30]. In these studies, CMV was clearly evident in abortion samples. Due to the fact that CMV infection exists in body fluids, it can be transfused through the blood to the trophoblast cells, and also affect the amniotic fluid and involve the uterus and pregnancy products. Endotoxins can stimulate amniotic cells to secrete cytokines that enter the amniotic fluid to give rise to immune responses. In case the infection is chronic, the endometrium of the womb results in chronic endometrium through an inflammatory response which is a strong cause of abortion. Immunocompromised women are more susceptible to CMV infection and since the immune system is weakened in the first trimester of pregnancy to facilitate the implantation of the fetus, the probability of transferring CMV to the fetus will increase [9, 23, 32]. CMV DNA testing is another method with remarkable sensitivity. In a study by Cook, CMV DNA was found in the tissue leftover, indicating the transfer of CMV to gestational products [19]. The results of this study can be considered as strong evidence of the presence of CMV in the leftovers of abortion. The study by Al-Saeed et al. indicated that levels of CMV antibodies in abortion leftovers are higher than those in controls. This study reported 79.54% of IgG against CMV in abortive cases [10]. The results of these studies depend on the pregnancy grade as well since naturally-acquired immunity during pregnancy reduces the risk of congenital CMV infections in subsequent pregnancies by 70%. However, maternal immunity does not prevent recurrences and maternal antibodies fail to prevent the onset of fetal infections [32]. Therefore, based on current evidence, 11 studies have shown a strong association between abortion and CMV so that the level of IgG against CMV is higher than 79% in all the samples studied. In spite of this, contradicting results have appeared in some investigations. CMV is a virus that has not been clearly proved to be related with abortion and this part of the story remains to be controversial. The study of Fatima et al. in India on 122 abortion cases using PCR demonstrated IgG-positive state of CMV (44.26%) [25]. However, the results differed significantly from those of other studies. In the study of Rasti et al. on 81 abortion cases, 24.69% of cases were IgG-positive [27]. However, the study of Kakru et al. with the highest sample size (n=779) reported CMV IgG in 16.5% of the samples [6]. It should be noted that the prevalence of CMV varies among different geographical regions. It has also been observed that in societies with low socioeconomic status, the incidence of infections, as well as CMV infection is more prevalent, while in societies of high socioeconomic status, the rate of infection is lower. It seems that the prevalence of CMV infection is low in some countries due to increased primary health care. Additionally, a small sample size or laboratory method is effective in revealing such results. Two studies in India have shown a weak link between abortion and CMV [6, 25]. Another cause of this disparity is the low prevalence of CMV infection in that region. The study of Oliveira et al. with a sample size of 70 reported CMV IgG in only 0.04% of positive samples, which is not consistent with the results of other studies [28]. As mentioned above, the difference in the results of studies can be due to the difference in sample size as well as the experimental method. Distribution of the patients' age may be another reason for contradictions in the results; CMV is more prevalent in younger women. Religious beliefs and high-risk behaviors are also effective in the spread of the infection so that the more there is high-risk sexual behaviors in a society, the greater will be the prevalence of CMV.
Conclusions
The results of most studies indicate that CMV infection can lead to abortion by transmission through body fluids, activation of the inflammatory responses of the uterus and the immune response, and transmission to fetal tissues. Therefore, analysis and prevention are recommended for all women at the reproductive age. It is also suggested that countries with low socioeconomic and health status, as well as those with greater rate of high-risk sexual behaviors consider CMV check-up in the country protocol for women prior to their gestational age.
Conflict of Interest
We have no conflict of interest to declare.
Acknowledgements
Thanks to Dr. Mehdi Norouzi and Dr. Babak Shahbaz for their constructive comments and help with preparing the manuscript and formatting the whole article.
References
- Bagheri L, Mokhtarian H, Sarshar N, Ghahramani M. Seroepidemiology of cyto-megalovirus infection during pregnancy in Gonabad, east of Iran: a cross-sectional study. J Res Health Sc. 2012; 12(1): 38-44.
- Choobineh H, Alizadeh S, Yazdi MS, Vaezzadeh F, Dargahi H, Pourfatholah A. Serological Evaluation of major beta thalassemia patients below15 for cytomegalovirus infection in Iran. Res J Biologic Sci. 2009; 2: 584-89.
- Choobineh H, Alizadeh S, Sharifi yazdi M, Vaezzadeh F, Dargahi H, Pourfatolah A. The effect of repeated transfusions on active cytomegalovirus infection, in the presence of IgM, in patients with thalassemia major in Iran. Payavard Salamat. 2007; 1(1): 8-16.
- Marin LJ, Santos de Carvalho Cardoso E, Bispo Sousa SM, Debortoli de Carvalho L, Marques Filho MF, Raiol MR, et al. Prevalence and clinical aspects of CMV congenital infection in a low-income population. Virol J. 2016; 13(1): 148.
- Lanzieri TM, Dollard SC, Bialek SR, Grosse SD. Systematic review of the birth prevalence of congenital cytomegalovirus infection in developing countries. Int J Infect Dis. 2014; 22: 44-8.
- Kakru M, Shaheen R, Nazir A. Seroprevalence of cytomegalovirus (CMV) in Kashmir valley-a preliminary study. JK Pract. 2004; 11(4): 261-62.
- Staras SAS, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clin Infect Dis. 2006; 43(9): 1143-151.
- Stockdale L, Nash S, Nalwoga A, Painter H, Asiki G, Fletcher H, et al. Human cyto-megalovirus epidemiology and relationship to tuberculosis and cardiovascular disease risk factors in a rural Ugandan cohort. PloS one 2018; 13(2): e0192086.
- Sherkat R, Meidani M, Zarabian H, Rezaei A, Gholamrezaei A. Seropositivity of cytomegalo-virus in patients with recurrent pregnancy loss. J Res Med Sci. 2014; 19(S1): S22.
- Al-Saeed MS, Muhsin MA, AL-Juburi GJ. Study the role of Toxoplasma gondii, cyto-megalovirus and anti-phospholipids antibodies in cases of abortion among women in Hilla city. Al-Qadisiah Med J. 2017; 4(6): 27-34.
- Karimi Zarchi M, Heydari E, Tabatabaie A, Moghimi M, Kooti W. Diagnostic value of the careTM HPV test in screening for cervical intraepithelial neoplasia grade 2 or worse. Asian Pac J Cancer Prev. 2017; 18(3): 687-93.
- Sifakis S, Ergazaki M, Sourvinos G, Koffa M, Koumantakis E, Spandidos D. Evaluation of Parvo B19, CMV and HPV viruses in human aborted material using the polymerase chain reaction technique. Eur J Obstet Gynecol Reprod Biol. 1998; 76(2): 169-73.
- Mirambo M, Chibwe E, Mushi M, Majigo M, Mshana S. Cytomegalovirus, parvovirus B19 and rubella co-infection among pregnant women attending antenatal clinics in Mwanza City: The need to be considered in Tanzanian Antenatal Care Package. Epidemiology 2016; 6(230): 2161-165.
- Nigro G, Anceschi MM, Cosmi EV. Clinical manifestations and abnormal laboratory findings in pregnant women with primary cytomegal-ovirus infection. BJOG. 2003; 110(6): 572-77.
- Habibi M, Bahrami A, Morteza A, Sadighi Gilani MA, Hassanzadeh G, Ghadami M, et al. Study of cytomegalovirus infection in idiopathic infertility men referred to Shariati hospital, Tehran, Iran. Iran J Reproduct Med. 2014; 12(2): 151-54.
- Revello MG, Gerna G. Diagnosis and manage-ment of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clin Microbiol Rev. 2002; 15(4): 680-715.
- Choobineh H, Gilani MAS, Pasalar P, Jahanzad I, Ghorbani R, Hassanzadeh G. The effects of testosterone on oxidative stress markers in mice with spinal cord injuries. Int J Fertil Steril. 2016; 10(1): 87.
- Nigro G, Mazzocco M, Mattia E, Di Renzo GC, Carta G, Anceschi MM. Role of the infections in recurrent spontaneous abortion. J Matern Fetal Neonatal Med. 2011; 24(8): 983-89.
- Cook SM, Himebaugh KS, Frank TS. Absence of cytomegalovirus in gestational tissue in recurrent spontaneous abortion. Diagn Mol Pathol. 1993; 2(2): 116-19.
- Ghafourian M, Ayandeband N, Fardipour A, Kooti W, Foroutanrad M, Badiee M. The role of CD16+, CD56+, NK (CD16+/CD56+) and B CD20+ cells in the outcome of pregnancy in women with recurrent spontaneous abortion. Int J Wom Health Reprod Sci. 2015; 3: 61-6.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4): 264-69.
- Gao YL, Gao Z, He M, Liao P. Infection status of human parvovirus B19, Cytomegalovirus and herpes simplex Virus-1/2 in women with first-trimester spontaneous abortions in Chongqing, China. Virol J. 2018; 15(1): 74.
- Burkman RT. Berek and Novak’s gynecology. JAMA. 2007; 297(14): 1601-604.
- Spano L, Vargas P, Ribeiro F, Leite J, Nascimento J. Cytomegalovirus in human abortion in Espı́rito Santo, Brazil. J Clin Virol. 2002; 25(S2): 173-78.
- Fatima T, Siddiqui H, Ghildiyal S, Baluni M, Singh DV, Zia A, et al. Cytomegalovirus infection in pregnant women and its association with bad obstetric outcomes in Northern India. Microb Pathog. 2017; 113: 282-85.
- Zhou Y, Bian G, Zhou Q, Gao Z, Liao P, Liu Y, et al. Detection of Cytomegalovirus, human parvovirus B19, and herpes simplex virus‐1/2 in women with first‐trimester spontaneous abortions. J Med Virol. 2015; 87(10): 1749-753.
- Rasti S, Ghasemi FS, Abdoli A, Piroozmand A, Mousavi SGA, Fakhrie‐Kashan Z. ToRCH “co‐infections” are associated with increased risk of abortion in pregnant women. Congenit Anom. 2016; 56(2): 73-8.
- Oliveira GM, Pascoal-Xavier MA, Moreira DR, Guimarães VS, Aguiar RALP, Miranda DM, et al. Detection of cytomegalovirus, herpes virus simplex, and parvovirus b19 in spontaneous abortion placentas. J Matern Fetal Neonatal Med. 2017; 7: 1-8.
- Saraswathy T, Az-Ulhusna A, Asshikin RN, Suriani S, Zainah S. Seroprevalence of cytomegalovirus infection in pregnant women and associated role in obstetric complications: a preliminary study. Southeast Asian J Trop Med Public Health. 2011; 42(2): 320-22.
- Cheshik S, Kisteneva L. Human cytomegalo-virus infection and spontaneous abortion in pregnant women of I and II trimester. Vopr Virusol. 2016; 61(2): 74-8.
- Tarokhian B, Sherkat R, Esfahani MHN, Adib M, Esfahani AK, Ataei B. CD107a Expression and IFN-γ Production as Markers for Evaluation of Cytotoxic CD3+ CD8+ T Cell Response to CMV Antigen in Women with Recurrent Spontaneous Abortion. Int J Fertil Steril. 2014; 7(4): 323-30.
- Cunningham F, Leveno K, Bloom S, Spong CY, Dashe J. Williams obstetrics, 24th ed, New York: Mcgraw-hill; 2014.
Type of Study: Research |
Subject:
Virology
Received: 2018/06/28 | Accepted: 2018/08/20 | Published: 2018/08/15
Received: 2018/06/28 | Accepted: 2018/08/20 | Published: 2018/08/15
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



