Mon, Feb 2, 2026
[Archive]
Volume 6, Issue 1 (February 2019)
IJML 2019, 6(1): 43-50 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Zaker F, Sharifi M J, Nasiri N, Yaghmaie M, Namjoo S. Myeloid Cell Leukemia-1 Gene Expression and Clinicopathological Features in Myelodysplastic Syndrome. IJML 2019; 6 (1) :43-50
URL: http://ijml.ssu.ac.ir/article-1-279-en.html
URL: http://ijml.ssu.ac.ir/article-1-279-en.html
Myeloid Cell Leukemia-1 Gene Expression and Clinicopathological Features in Myelodysplastic Syndrome
Department of Medical Laboratory Sciences, Faculty of Paramedical Sciences, Shiraz University of Medical Sciences, Shiraz, Iran.
Full-Text [PDF 263 kb]
(831 Downloads)
| Abstract (HTML) (2371 Views)
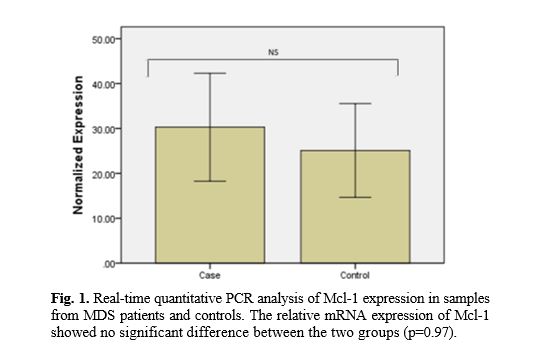

Table 2. Correlation between variables and cytogenetic risk category, WHO subgroups, IPSS and IPSS-R
*P-value
Discussion
References
Full-Text: (970 Views)
Introduction
Myeloid cell leukemia-1 (Mcl-1) is encoded by the Mcl-1 gene. Alternative splicing of the gene leads to isoform 1 (longer gene product) and isoform 2 (shorter gene product) [1]. Isoform 1, an important pro-survival protein of the Bcl-2 gene family, is of fundamental importance to the development, differentiation, proliferation and tumorgenesis [2]. The protein promotes cells survival by binding and sequestering their pro-apoptotic counterparts (Bak, Bim, and Bid) and inhibiting the release of cytochrome-c from mitochondria [3-6]. Following genotoxic stress, Mcl-1 is ubiquitinylated and rapidly degraded, therefore cells commit to apoptosis [7]. This protein plays a pivotal role in the survival of hematologic and solid tumors and is known as a substantial oncogene [8]. Overexpression of Mcl-1 has been observed in a broad range of human tumors including breast, prostate, ovarian and colorectal cancers, chronic myelogenous leukemia, multiple myeloma and others [9-13]. Studies have demonstrated that altered expression of Mcl-1 has been linked to malignancy development and poor prognosis [12, 14-18]. Myelodyslastic syndromes (MDS) are a type of hematological malignancy showing variable clinical courses from indolent to aggressive forms. There are few prognostic and predictive biological factors of outcomes in MDS and many investigations are ongoing to find such parameters. Regarding the correlations of Mcl-1 and clinical outcomes in other malignancies, we have studied the expression of Mcl-1 mRNA in MDS patients to determine its association with clinico-pathological factors, MDS subgroups as well as international prognostic scoring system (IPSS).
Materials and Methods
Patient selection and sample collection
Sixty MDS patients who had referred to Shariati and Firouzgar Hospitals (Tehran, Iran) were included in the study. The study was approved by the Ethics Committee of Iran University of Medical Sciences, Tehran, Iran. Informed consent was obtained from all the patients and normal controls. Clinical features of the patients are listed in table 1. According to World Health Organization criteria [19], at the time of sampling, 20, 6, 10, 4, 11 and 3 patients had RA, RT, RCMD, RAEB-1, RAEB-2 and 5q- syndrome respectively. According to IPSS [20], 34 patients were low risk, 7 patients intermediate-1, 6 patients intermediate-2, but 7 patients were at high risk.
Conventional cytogenetic analysis
Conventional cytogenetic investigation was carried out for 54 patients. Karyotypes were investigated according to the method previously described [21].
Gene expression analysis
The relative level of Mcl-1 was determined by real-time polymerase chain reaction (RT-PCR) and gene expression normalized to Glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Total RNA was derived by TriPure Isolation Reagent (Roche). 1µg RNA was used for cDNA synthesis using the QuantiTect Reverse Transcription Kit (Qiagen, Hilden, Germany). qPCR was conducted with the QuantiFast SYBR Green RT-PCR Kit (Qiagen, Hilden, Germany) on rotor gene 6000. Reaction mixes were prepared according to the manufacture's protocol. Forward and reverse primers for cDNA amplification used were Mcl-1, F: 5'CGGCGTAACAAACTGGGGCA-3', R: 5'-TCCACAAACCCATCCCAGCCT-3'(183 bp), GAPDH, F: 5'-CACCAGGGCTGCTTTTAACTCTGGA-3', R: 5 ' -CCTTGACGGTGCCATGGAATTTGC-3' (130 bp).
Thermal cycling conditions were compromised:
DNA polymerase activation at 95°C for 5 min, 40 cycles of denaturation at 95°C for 10 s and combined annealing/extension at 60°C for 30 s. Each measurement was down in triplicate. ∆∆Ct method was used to calculate the relative mRNA expression level of the samples compared to the amount of mRNA in the control samples.
Materials and Methods
Patient selection and sample collection
Sixty MDS patients who had referred to Shariati and Firouzgar Hospitals (Tehran, Iran) were included in the study. The study was approved by the Ethics Committee of Iran University of Medical Sciences, Tehran, Iran. Informed consent was obtained from all the patients and normal controls. Clinical features of the patients are listed in table 1. According to World Health Organization criteria [19], at the time of sampling, 20, 6, 10, 4, 11 and 3 patients had RA, RT, RCMD, RAEB-1, RAEB-2 and 5q- syndrome respectively. According to IPSS [20], 34 patients were low risk, 7 patients intermediate-1, 6 patients intermediate-2, but 7 patients were at high risk.
Conventional cytogenetic analysis
Conventional cytogenetic investigation was carried out for 54 patients. Karyotypes were investigated according to the method previously described [21].
Gene expression analysis
The relative level of Mcl-1 was determined by real-time polymerase chain reaction (RT-PCR) and gene expression normalized to Glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Total RNA was derived by TriPure Isolation Reagent (Roche). 1µg RNA was used for cDNA synthesis using the QuantiTect Reverse Transcription Kit (Qiagen, Hilden, Germany). qPCR was conducted with the QuantiFast SYBR Green RT-PCR Kit (Qiagen, Hilden, Germany) on rotor gene 6000. Reaction mixes were prepared according to the manufacture's protocol. Forward and reverse primers for cDNA amplification used were Mcl-1, F: 5'CGGCGTAACAAACTGGGGCA-3', R: 5'-TCCACAAACCCATCCCAGCCT-3'(183 bp), GAPDH, F: 5'-CACCAGGGCTGCTTTTAACTCTGGA-3', R: 5 ' -CCTTGACGGTGCCATGGAATTTGC-3' (130 bp).
Thermal cycling conditions were compromised:
DNA polymerase activation at 95°C for 5 min, 40 cycles of denaturation at 95°C for 10 s and combined annealing/extension at 60°C for 30 s. Each measurement was down in triplicate. ∆∆Ct method was used to calculate the relative mRNA expression level of the samples compared to the amount of mRNA in the control samples.
Table 1. Patient characteristics
| Characteristics | Median (Range) | N (%) |
| Age(Years) | 61.56 (23-90) | 54 (100) |
| Sex Male Female |
|
29 (53.7) 25 (46.3) |
| ANC(×109/L) | 2.69 (0.29-8.47) | |
| Hemoglobin (g/dL) | 9.5 (5.6-14.8) | |
| Platelets(×109/L) | 135 (7-752) | |
| BM Blast (%) <5% 5-9% 10-19% |
|
39 (72.2) 4 (7.4) 11 (20.4) |
| Cytogenetic prognostic subgroups (IPSS) Good Intermediate Poor |
40 (74.1) 7 (13) 7 (13 |
|
| Cytogenetic risk category (IPSS-R) Very good Good Intermediate Poor Very poor |
|
4 (7.4) 36 (66.7) 8 (14.8 3 (5.6) 3 (5.6) |
| Ferritin (ng/ml) | 386.35 (3-1600) | |
| LDH (U/L) | 410.17 (99-1886) | |
| IPSS Low Int-1 Int-2 High |
34 (63) 7 (13) 6 (11.1) 7 (13) |
|
| IPSS-R Very Low Low Intermediate High Very High |
20 (37) 13 (24.1) 8 (14.8) 4 (7.4) 9 (16.7) |
|
| WHO RA RT RCMD RAEB-1 RAEB-2 5q- |
20 (37) 6 (11.1) 10 (18.5) 4 (7.4) 11 (20.4) 3 (5.6) |
Statistical analysis
Statistical analysis was performed using the SPSS 23.0 software package. Mann–Whitney’s U-test, kruskal- wallis test, student’s t-test and chi square were utilized as required. For all analyses, a value of p≤0.05 was considered statistically significant.
Results
Patient characteristics
Demographic and hematologic findings are presented in table 1.
Relationship between expression of Mcl-1 mRNA and clinicopathological data
Levels of Mcl-1 mRNAs were investigated by RT-PCR in 54 MDS patients. Results indicated amplification of mRNA encoding Mcl-1 in 100% of the cases. A higher level of Mcl-1 existed in MDS patients compared with the healthy controls but there was no statistically difference between MDS subgroups (Fig.1).
Fold change in gene expression in cases with >60 years was higher compared with those of <60 years and the difference was statistically significant (31.23±26.59 vs. 17.4±14.01, p=0.046). In addition, the correlation between gene expression and cytogenetic prognostic subgroups (IPSS) was statistically significant (p=0.043) (Fig.2).
Fold change in gene expression was higher in the advanced stage MDS, high risk MDS, cases with >5% blast and lactic dehydrogenase (LDH) >400 to their corresponding groups. Difference in the LDH level (306.9±115.307 vs. 678.67±378.837 U/L, p=0.00) and ferritin (355.98±346.088 vs. 465.33±220.176 ng/mL, p=0.034) was statistically significant between advanced stage MDS and early stage MDS patients in this study. The LDH level (587.54± 199.176 vs. 353.93±273.727, p=0.00), ferritin (495.69±223.618 vs. 351.69±337.454, p=0.019) and age (68.077±8.84 vs. 59.48± 15.46, p=0.007) of the higher-risk group were significantly higher than those in the lower-risk group (Table.2).
Statistical analysis was performed using the SPSS 23.0 software package. Mann–Whitney’s U-test, kruskal- wallis test, student’s t-test and chi square were utilized as required. For all analyses, a value of p≤0.05 was considered statistically significant.
Results
Patient characteristics
Demographic and hematologic findings are presented in table 1.
Relationship between expression of Mcl-1 mRNA and clinicopathological data
Levels of Mcl-1 mRNAs were investigated by RT-PCR in 54 MDS patients. Results indicated amplification of mRNA encoding Mcl-1 in 100% of the cases. A higher level of Mcl-1 existed in MDS patients compared with the healthy controls but there was no statistically difference between MDS subgroups (Fig.1).
Fold change in gene expression in cases with >60 years was higher compared with those of <60 years and the difference was statistically significant (31.23±26.59 vs. 17.4±14.01, p=0.046). In addition, the correlation between gene expression and cytogenetic prognostic subgroups (IPSS) was statistically significant (p=0.043) (Fig.2).
Fold change in gene expression was higher in the advanced stage MDS, high risk MDS, cases with >5% blast and lactic dehydrogenase (LDH) >400 to their corresponding groups. Difference in the LDH level (306.9±115.307 vs. 678.67±378.837 U/L, p=0.00) and ferritin (355.98±346.088 vs. 465.33±220.176 ng/mL, p=0.034) was statistically significant between advanced stage MDS and early stage MDS patients in this study. The LDH level (587.54± 199.176 vs. 353.93±273.727, p=0.00), ferritin (495.69±223.618 vs. 351.69±337.454, p=0.019) and age (68.077±8.84 vs. 59.48± 15.46, p=0.007) of the higher-risk group were significantly higher than those in the lower-risk group (Table.2).


Table 2. Correlation between variables and cytogenetic risk category, WHO subgroups, IPSS and IPSS-R
| Mcl-1 mRNA | Ferritin | Lactic dehydrogenase | Hemoglobin | Platelets | ANC | Age | Groups |
| 0.043 | 0.258 | 0.027 | 0.781 | 0.233 | 0.017 | 0.282* | Cytogenetic risk category |
| 0.136 | 0.063 | 0.00 | 0.001 | 0.00 | 0.00 | 0.56 | WHO subgroups |
| 0.27 | 0.083 | 0.001 | 0.383 | 0.006 | 0.02 | 0.245 | IPSS |
| 0.177 | 0.004 | 0.00 | 0.017 | 0.116 | 0.1 | 0.004 | IPSS-R |
Discussion
MDS includes a heterogeneous group of clonal bone marrow disorders that are characterized by aberrant differentiation, cytopenias and leukemic transformation [22, 23]. In the present study, we aimed to clarify the correlation of Mcl-1 expression and clinicopathological findings in MDS. The results revealed significant differences in expression of Mcl-1 gene among cytogenetic prognostic subgroups of MDS. The higher risk-cytogenetic subgroup shows more expression of Mcl-1. Mcl-1 was initially characterized as an early response gene induced during the differentiation of human myeloid leukemia cells the increased expression of which is observed in various hematologic and solid tumors [24].
Mcl-1 has been associated with poor prognosis in advanced ovarian tumors, liver metastases from colorectal cancer and prostate cancer progression [9-11]. Mice expressing Mcl-1 as a transgene were found to develop lymphoma particularly diffuse large-cell lymphoma [25].
Mcl-1 plays a key role in the pathogenesis of myeloid neoplasms. Aichberger et al. demonstrated Mcl-1 detection in isolated primary chronic myeloid leukemia (CML) cells at the mRNA and protein level in all patients independent of phase of the disease and the levels of Mcl-1 being higher in patients compared to the normal samples. In addition, Mcl-1 expression was also detected in myeloid cell lines including K562 and the basophil cell line KU812. Moreover, they found that targeting of Mcl-1 is related to enhanced sensitivity of leukemic cells against the BCR/ABL tyrosine kinase inhibitor STI571. The researchers concluded that Mcl-1 is a BCR/ABL-dependent survival factor that can be used as a therapeutic target in CML [26]. In the present study, we not only showed that Mcl-1 is widely expressed in MDS samples, but also examined the relationship between Mcl-1 mRNA expression and MDS subgroups, IPSS, cytogenetic risk categories and other variables.
Evaluation of Mcl-1 expression in MDS specimens revealed several remarkable conclusions. First, the anti-apoptoticBcl-2 family member, Mcl-1, was detectable in all patient specimens. Not only were levels of Mcl-1 mRNA lower in normal controls but also, not detectable in some control specimens. Second, levels of this mRNA varied widely between different specimens. Third, the important aspect of our investigation was that Mcl-1 is expressed in MDS independent of the WHO and IPSS classification, but average of expression was higher in the advanced stage MDS and high risk MDS compared to the early stage MDS and low risk MDS. Thus, Mcl-1 may be up-regulated already in early stages of leukemogenesis. Fourth, there were a positive correlation between levels of Mcl-1 and increasing age, raising the possibility that increased levels of this anti-apoptotic polypeptide might be a factor that can contribute to the diminished response rate and poor outcomes generally observed in older patients. In addition, there was significant difference between Mcl-1 mRNA expression in cytogenetic risk categories. The highest expression rate was observed in the poor group.
Booy et al. indicated that Mcl-1 is a downstream target of epidermal growth factor receptor signaling. In addition, they suggested that overexpression of Mcl-1 in breast cancer cells results in resistance to drug-induced cell death [27].
Kaufmann et al. examined the expression of Bcl-2, Mcl-1, Bcl-xL, and Bax by immunoblotting in human acute leukemia cell lines and acute myeloid leukemia (including antecedent myelodysplastic syndrome and secondary leukemia) and acute lymphoblastic leukemia samples before therapy and at recurrence. They revealed that levels of Bcl-2, Mcl-1, Bcl-xL, and Bax varied over more than 10-fold range between pretreatment specimens. No association was found between pretreatment levels of these proteins and response to initial therapy. However, examination of 19 paired samples revealed about 2-fold increase in Mcl-1 levels in 10 out of 19 pairs at recurrence [28]. Although our observations are in line with Kaufmann's study, our results differ from a previous study reported in MDS patients. Bar et al. examined the gene expression patterns using microarray in MDS patients. They identified several genes related to promotion of apoptosis including Mcl-1, EPOR, and tumor necrosis factor anti-apoptotic modulator (TNFAIP3). Their data indicated that expression of both variants of Mcl-1 decreases significantly in low and high grade MDS compared to normal bone marrow and down regulation of Mcl-1 is consistent with the promotion of a pro-apoptotic state observed in MDS. They suggested that MDS and progression of MDS are related with not only a decrease in Mcl-1 level, but a shift in transcript in favor of a pro-apoptotic state [29]. With respect to the limited number of patients in our study, correlations which were found here should be examined in a larger study population.
Conclusion
In conclusion, our study revealed a correlation of Mcl-1 expression and high risk cytogenetic categories in MDS. The expression of Mcl-1 generally increased in all MDS subgroups which indicates a possible pathological role of this protein in MDS pathogenesis and progression.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
The work is supported by grants from Iran University of Medical Science (IUMS) and Iran National Science Foundation (INSF).
Mcl-1 has been associated with poor prognosis in advanced ovarian tumors, liver metastases from colorectal cancer and prostate cancer progression [9-11]. Mice expressing Mcl-1 as a transgene were found to develop lymphoma particularly diffuse large-cell lymphoma [25].
Mcl-1 plays a key role in the pathogenesis of myeloid neoplasms. Aichberger et al. demonstrated Mcl-1 detection in isolated primary chronic myeloid leukemia (CML) cells at the mRNA and protein level in all patients independent of phase of the disease and the levels of Mcl-1 being higher in patients compared to the normal samples. In addition, Mcl-1 expression was also detected in myeloid cell lines including K562 and the basophil cell line KU812. Moreover, they found that targeting of Mcl-1 is related to enhanced sensitivity of leukemic cells against the BCR/ABL tyrosine kinase inhibitor STI571. The researchers concluded that Mcl-1 is a BCR/ABL-dependent survival factor that can be used as a therapeutic target in CML [26]. In the present study, we not only showed that Mcl-1 is widely expressed in MDS samples, but also examined the relationship between Mcl-1 mRNA expression and MDS subgroups, IPSS, cytogenetic risk categories and other variables.
Evaluation of Mcl-1 expression in MDS specimens revealed several remarkable conclusions. First, the anti-apoptoticBcl-2 family member, Mcl-1, was detectable in all patient specimens. Not only were levels of Mcl-1 mRNA lower in normal controls but also, not detectable in some control specimens. Second, levels of this mRNA varied widely between different specimens. Third, the important aspect of our investigation was that Mcl-1 is expressed in MDS independent of the WHO and IPSS classification, but average of expression was higher in the advanced stage MDS and high risk MDS compared to the early stage MDS and low risk MDS. Thus, Mcl-1 may be up-regulated already in early stages of leukemogenesis. Fourth, there were a positive correlation between levels of Mcl-1 and increasing age, raising the possibility that increased levels of this anti-apoptotic polypeptide might be a factor that can contribute to the diminished response rate and poor outcomes generally observed in older patients. In addition, there was significant difference between Mcl-1 mRNA expression in cytogenetic risk categories. The highest expression rate was observed in the poor group.
Booy et al. indicated that Mcl-1 is a downstream target of epidermal growth factor receptor signaling. In addition, they suggested that overexpression of Mcl-1 in breast cancer cells results in resistance to drug-induced cell death [27].
Kaufmann et al. examined the expression of Bcl-2, Mcl-1, Bcl-xL, and Bax by immunoblotting in human acute leukemia cell lines and acute myeloid leukemia (including antecedent myelodysplastic syndrome and secondary leukemia) and acute lymphoblastic leukemia samples before therapy and at recurrence. They revealed that levels of Bcl-2, Mcl-1, Bcl-xL, and Bax varied over more than 10-fold range between pretreatment specimens. No association was found between pretreatment levels of these proteins and response to initial therapy. However, examination of 19 paired samples revealed about 2-fold increase in Mcl-1 levels in 10 out of 19 pairs at recurrence [28]. Although our observations are in line with Kaufmann's study, our results differ from a previous study reported in MDS patients. Bar et al. examined the gene expression patterns using microarray in MDS patients. They identified several genes related to promotion of apoptosis including Mcl-1, EPOR, and tumor necrosis factor anti-apoptotic modulator (TNFAIP3). Their data indicated that expression of both variants of Mcl-1 decreases significantly in low and high grade MDS compared to normal bone marrow and down regulation of Mcl-1 is consistent with the promotion of a pro-apoptotic state observed in MDS. They suggested that MDS and progression of MDS are related with not only a decrease in Mcl-1 level, but a shift in transcript in favor of a pro-apoptotic state [29]. With respect to the limited number of patients in our study, correlations which were found here should be examined in a larger study population.
Conclusion
In conclusion, our study revealed a correlation of Mcl-1 expression and high risk cytogenetic categories in MDS. The expression of Mcl-1 generally increased in all MDS subgroups which indicates a possible pathological role of this protein in MDS pathogenesis and progression.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
The work is supported by grants from Iran University of Medical Science (IUMS) and Iran National Science Foundation (INSF).
References
- Michels J, Johnson PW, Packham G. Mcl-1. Int J Biochem Cell Biol. 2005; 37(2): 267-71.
- Craig RW. MCL1 provides a window on the role of the BCL2 family in cell proliferation, differentiation and tumorigenesis. Leukemia 2002; 16(4): 444-54.
- Leverson JD, Zhang H, Chen J, Tahir SK, Phillips DC, Xue J, et al. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax). Cell Death Dis. 2015; 6(1): e1590.
- Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014; 15(1): 49-63.
- Chi X, Kale J, Leber B, Andrews DW. Regulating cell death at, on, and in membranes. Biochim Biophys Acta. 2014; 1843(9): 2100-113.
- Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family re::union::. Mol Cell. 2010; 37(3): 299-310.
- Schwickart M, Huang X, Lill JR, Liu J, Ferrando R, French DM, et al. Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature 2010; 463(7277): 103-107.
- Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, et al. The landscape of somatic copy-number alteration across human cancers. Nature 2010; 463(7283): 899-905.
- Krajewska M, Krajewski S, Epstein JI, Shabaik A, Sauvageot J, Song K, et al. Immunohisto-chemical analysis of bcl-2, bax, bcl-X, and mcl-1 expression in prostate cancers. Am J Pathol. 1996; 148(5): 1567-576.
- Backus HHJ, van Riel JMGH, van Groeningen CJ, Vos W, Dukers DF, Bloemena E, et al. Rb, mcl-1 and p53 expression correlate with clinical outcome in patients with liver metastases from colorectal cancer. Ann Oncol. 2001; 6(1): 779-85.
- Baekelandt M, Holm R, Nesland JM, Tropé CG, Kristensen GB. Expression of apoptosis-related proteins is an independent determinant of patient prognosis in advanced ovarian cancer. J Clin Oncol.2000; 18(22): 3775-781.
- O'Driscoll L, Cronin D, Kennedy SM, Purcell R, Linehan R, Glynn S, et al. Expression and Prognostic Relevance of Mcl-1 in Breast Cancer. Anticancer Res. 2004; 24(2A): 473-82.
- Le Gouill S, Podar K, Amiot M, Hideshima T, Chauhan D, Ishitsuka K, et al. VEGF induces Mcl-1 up-regulation and protects multiple myeloma cells against apoptosis. Blood. 2004; 104(9): 2886-892.
- Nagata M, Wada K, Nakajima A, Nakajima N, Kusayama M, Masuda T, et al. Role of myeloid cell leukemia-1 in cell growth of squamous cell carcinoma. J Pharmacol Sci. 2009; 110(3): 344-53.
- Shigemasa K, Katoh O, Shiroyama Y, Mihara S, Mukai K, Nagai N, et al. Increased MCL-1 expression is associated with poor prognosis in ovarian carcinomas. Jpn J Cancer Res. 2002; 93(5): 542-50.
- Zhang T, Zhao C, Luo L, Zhao H, Cheng J, Xu F. The expression of Mcl-1 in human cervical cancer and its clinical significance. Med Oncol. 2012; 29(3): 1985-991.
- Likui W, Qun L, Wanqing Z, Haifeng S, Fangqiu L, Xiaojun L. Prognostic role of myeloid cell leukemia-1 protein (Mcl-1) expression in human gastric cancer. J Surg Oncol. 2009; 100(5): 396-400.
- Akgul C. Mcl-1 is a potential therapeutic target in multiple types of cancer. Cell Mol Life Sci. 2009; 66(8): 1326-336.
- Vardiman J, Thiele J, Arber D, Brunning R, Borowitz M, Porwit A, et al. Revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009; 114 (5): 937-51.
- Greenberg P, Cox C, LeBeau M, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelo-dysplastic syndromes. Blood 1997; 89(6): 2079-2088.
- Zaker F, Amirizadeh N, Nasiri N, Razavi SM, Teimoori-Toolabi L, Yaghmaie M, et al. Gene expression and methylation pattern in HRK apoptotic gene in myelodysplastic syndrome. Int J Mol Cell Med. 2016; 5(2): 90-9.
- Valent P, Horny HP, Bennett JM, Fonatsch C, Germing U, Greenberg P, et al. Definitions and standards in the diagnosis and treatment of the myelodysplastic syndromes: Consensus statements and report from a working conference. Leuk Res. 2007; 31(6):727-36.
- Pfeilstöcker M, Karlic H, Nösslinger T, Sperr W, Stauder R, Krieger O, et al. Myelodysplastic syndromes, aging, and age: correlations, common mechanisms, and clinical implications. Leuk Lymphoma 2007; 48(10): 1900-909.
- Kozopas KM, Yang T, Buchan HL, Zhou P, Craig RW. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to bcl-2. Proc Natl Acad Sci U S A. 1993; 90(8): 3516-520.
- Zhou P, Levy NB, Xie H, Qian L, Lee CY, Gascoyne RD, et al. MCL1 transgenic mice exhibit a high incidence of B-cell lymphoma manifested as a spectrum of histologic subtypes. Blood 2001; 97(12): 3902-909.
- Aichberger KJ, Mayerhofer M, Krauth MT, Skvara H, Florian S, Sonneck K, et al. Identification of mcl-1 as a BCR/ABL-dependent target in chronic myeloid leukemia (CML): evidence for cooperative antileukemic effects of imatinib and mcl-1 antisense oligonucleotides. Blood 2005 ;105(8): 3303-311
- Booy EP, Henson ES, Gibson SB. Epidermal growth factor regulates Mcl-1 expression through the MAPK-Elk-1 signalling pathway contributing to cell survival in breast cancer. Oncogene 2011; 30(20): 2367-378.
- Kaufmann SH, Karp JE, Svingen PA, Krajewski S, Burke PJ, Gore SD, et al. Elevated expression of the apoptotic regulator Mcl-1 at the time of leukemic relapse. Blood 1998; 91(3): 991-1000.
- Bar M, Stirewalt D, Pogosova-Agadjanyan E, Wagner V, Gooley T, Abbasi N, et al. Gene expression patterns in myelodyplasia underline the role of apoptosis and differentiation in disease initiation and progression. Transl Oncogenomics. 2008; 3(1): 137-149.
Type of Study: Research |
Subject:
Hematology & Blood Banking
Received: 2018/12/1 | Accepted: 2019/01/14 | Published: 2019/03/10
Received: 2018/12/1 | Accepted: 2019/01/14 | Published: 2019/03/10
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |