Tue, Feb 17, 2026
[Archive]
Volume 7, Issue 1 (February 2020)
IJML 2020, 7(1): 41-48 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ghorban K, Dadmanesh M, Sheikhfathollahi M, Rezai Z, Ezati P, Salmani M H, et al . IL-8 May Be the Target of Arsenic in Human Breast Milk. IJML 2020; 7 (1) :41-48
URL: http://ijml.ssu.ac.ir/article-1-300-en.html
URL: http://ijml.ssu.ac.ir/article-1-300-en.html
Khodayar Ghorban 

 , Maryam Dadmanesh
, Maryam Dadmanesh 

 , Mahmood Sheikhfathollahi
, Mahmood Sheikhfathollahi 

 , Zeynab Rezai
, Zeynab Rezai 

 , Paria Ezati
, Paria Ezati 

 , Mohammad Hossein Salmani
, Mohammad Hossein Salmani 

 , Fatemeh Akrami Mohajeri *
, Fatemeh Akrami Mohajeri * 

 , Hasan Yousefi-Daredor
, Hasan Yousefi-Daredor 

 , Hasan Ebrahimi
, Hasan Ebrahimi 

 , Derek Kennedy
, Derek Kennedy 

 , Mohammad Kazemi Arababadi
, Mohammad Kazemi Arababadi 




 , Maryam Dadmanesh
, Maryam Dadmanesh 

 , Mahmood Sheikhfathollahi
, Mahmood Sheikhfathollahi 

 , Zeynab Rezai
, Zeynab Rezai 

 , Paria Ezati
, Paria Ezati 

 , Mohammad Hossein Salmani
, Mohammad Hossein Salmani 

 , Fatemeh Akrami Mohajeri *
, Fatemeh Akrami Mohajeri * 

 , Hasan Yousefi-Daredor
, Hasan Yousefi-Daredor 

 , Hasan Ebrahimi
, Hasan Ebrahimi 

 , Derek Kennedy
, Derek Kennedy 

 , Mohammad Kazemi Arababadi
, Mohammad Kazemi Arababadi 


5Department of Food Hygiene and Safety, Faculty of Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Full-Text [PDF 326 kb]
(855 Downloads)
| Abstract (HTML) (2044 Views)
Introduction
Breastfeeding is a common recommendation by the World Health Organization (WHO) [1] and it is well documented that the best method for providing an infant's nutrition is through breastfeeding. The mother's milk consists of several factors which are essential for a normal newborn and for infant growth including α-lactalbumin, lactoferrin, lysozyme and cadmium. Breast milk also includes several factors, such as maternal IgG, which helps the new born to have a good immune response against infections [2]. Combined, these factors lead to the development of the immune system, intestinal microflora and the functional maturity of mucous membranes [3]. However, contamination of maternal milk with environmental toxins such as aflatoxins and heavy metals including Nickel and Arsenic, can be harmful for the newborns and infants, especially in the first months of life [4].
Cytokines are the main immunological factors which are produced during infection and inflammation [5, 6] and Interlukine (IL)-6 and IL-8 are the main innate immune cytokines and can induce inflammation and chemotaxis in immune cells such as neutrophils [7]. These are members of the pro-inflammatory cytokines and their chronic expression may be harmful for mucosal structure and immunity. Cytokines may be produced in response to contamination with heavy metals and biological toxins, and it may be hypothesized that production of IL-6 and IL-8 is one of the mechanisms which results in mucosal inflammation in infants. Breastfeeding has become more popular in Iran over the last decade, hence, its safety on new born babies needs to be evaluated, especially in light of increased contamination of milk with heavy metals and toxins, which are the main inducers of inflammatory responses in the infants [8].
Our previous investigation has revealed that in more than 86 % of the cases the maternal milk of the centered part of Iranian population is contaminated with aflatoxin M1 (AFM1) and heavy metals [9]. Thus, the main aim of this study was to evaluate the effects of the contamination of breast milk with Nickel, Arsenic and AFM1 on the milk levels of IL-6 and IL-8.
Materials and Methods
Subjects
In this cross-sectional study, breast-milk was collected from 76 lactating mothers, with the mean age 27.40±4.66 years, 147±4.66 cm high, 27.81±19.98 BMI (kg/m2) and 68.26±10.56 weight (kg), during regular feeding of their infants when they had referred to health centers in the Yazd city from June 2013 to June 2014. Mothers with pre-term infants, smoking, addiction, alcohol drinking, immunosuppressive drug administration and mental, autoimmune or allergic disorders were excluded from the study. The breast milk was collected on the 30th day post-parturition, and evaluated for of levels of AFM1, Nickel, Arsenic, IL-6 and IL-8. Accordingly, based on the detection of AFM1, Nickel and Arsenic in the milk samples, they were divided into three groups including Nickel and Arsenic contaminated group (being contaminated with Nickel and Arsenic but not AFM1), AFM1 contaminated group (being contaminated with AFM1 but not Nickel and Arsenic), and a control group, not contaminated with Nickel, Arsenic and AFM1. The Ethical Committee of Shahid Sedoughi University of Medical Sciences, Yazd, Iran approved the protocol of the study and all the participants filled out the written informed consent.
Evaluation of Nickel concentrations
In order to evaluate the concentration of Nickel, 1 mL of acetic acid (1 M) was added to 3 mL of each sample, mixed and centrifuged at 3200 RPM for 15 minutes. The lower liquid was extracted and milk fat was isolated. The samples were centrifuged again at 3200 RPM for 15 minutes to obtain a completely clear solution. The nickel concentration was measured using an atomic absorption spectrophotometer model 20AA (Varian) at a wavelength of 232 nm. The atomic absorption was read three times and the absorption mean was calculated [10]. The limitation rate for evaluation of Nickel using this method was 2-80 ppm.
Evaluation of Arsenic concentrations
Breast milk samples (1 mL) were transferred and 10 mL of toluene and 15 mL of a mixture of nitric acid and sulfuric acid were added to the sample, mixed for 10 minutes and subsequently two phases were separated and the lower phase was used to measure Arsenic concentrations employing an atomic absorption spectro-photometer model 20AA (Varian) at 197.3 nm wavelength, and 3 samples were measured after which the absorption mean was calculated [11]. The limitation rate for evaluation of Arsenic using this method was 0.15-3.73 ppm.
Measurement of aflatoxin M1
Aflatoxins are water soluble [12] and this property was exploited; the samples were centrifuged for 15 minutes at 15000 RPM, the upper creamy layers were completely discarded and the lower phases were used for evaluation of AFM1 employing a competitive enzyme-linked immunosorbent assay (ELISA) technique using a commercial kit (R-BiopharmGmbh-Rocket international Company) according to the manufacture’s guidelines. Based on using 6 standards which cover a range from 0 to 80 ppt, the limitation rate for evaluation of AFM1 using this method was 0-80 ppt.
Evaluation of IL-6 and IL-8 concentrations
Milk levels of IL-6 and IL-8 were measured by ELISA using a commercial kit from the Karmania Pars Gene, Iran, according to the manufacturer's guidelines. The limitation rate for evaluation of IL-6 was from 2 to 2000 pg/mL, while for IL-8 it was 2 to 2500 pg/mL.
Statistical analysis
Results are presented as the median (1st quartile and 3rd quartile). A non-parametric Kolmogorov-Smirnov test was used to evaluate the normal distribution of IL-6 and IL-8 in each of the study groups (AFM1 contaminated, high levels of Nickel and Arsenic, and the controls), but the presumption of normality was not met (p<0.05). Levene's test was applied to assess the homogeneity of variances of IL-6 and IL-8 across the three groups, but the presumption of homogeneity of variances was not also met (p<0.05). As the presumptions of one-way analysis of variance (ANOVA) were not met, the alternative non-parametric Kruskal-Wallis H test was utilized to compare medians of IL-6 and IL-8 across the three groups and Spearman`s ratio for correlation between Nickel and Arsenic levels with milk levels of IL-6 and 8. For statistical analysis, the statistical software SPSS version 15.0 for windows (SPSS Inc., Chicago, IL) was used. P-values of 0.05 or less were considered statistically significant.
Results
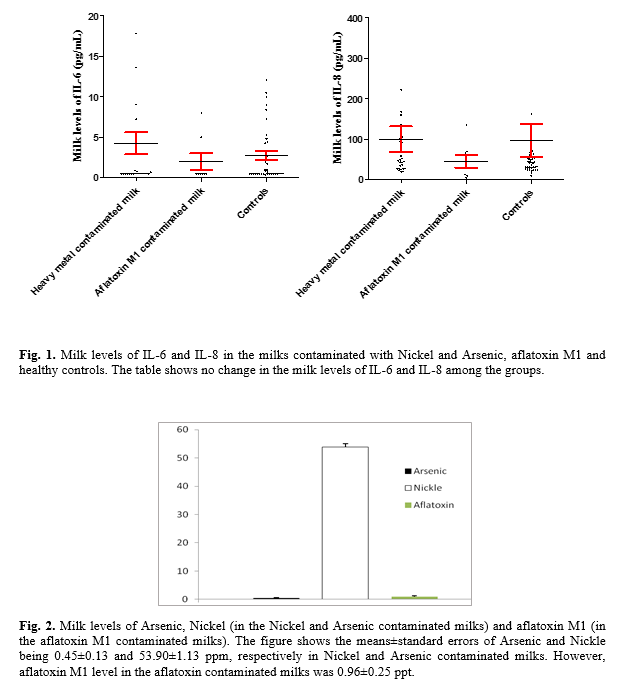
The results demonstrated 8 samples being contaminated with AFM1 (AFM1 contaminated group) while 29 samples were contaminated with both Nickel and Arsenic (Nickel and Arsenic contaminated group, table 1). The results revealed 39 milk samples being free of AFM1, Nickel and Arsenic (Control group). Evaluation of milk levels of IL-6 and IL-8 revealed milk levels of IL-6 (p=0.090) and IL-8 (p=0.413) failing to be different in AFM1, Nickel and Arsenic contaminated milk when compared to normal controls (Table 1 and Fig. 1).
Evaluation of milk levels of Arsenic and Nickle revealed the means±standard errors (SE) of Arsenic and Nickle being 0.45±0.13 and 53.90±1.13 ppb, respectively in Nickel and Arsenic contaminated milks. However, AFM1 levels in the aflatoxin contaminated milks were 0.96±0.25 ppt (Fig. 2). The concentration of AFM1, Nickel and Arsenic was undetectable in the control group. The concentration of AFM1 in the Nickel and Arsenic contaminated group and Nickel and Arsenic in the AFM1 contaminated group were undetectable too. Statistical analysis also revealed that milk levels of IL-8 have a moderate correlation with Arsenic in Nickel and Arsenic contaminated group (r=0.314, p<0.05, Fig. 3). Milk levels of IL-6 have also a good positive correlation with IL-8 (r=0.720, p>0.001, Fig. 3).
Discussion
Cytokines are small glycoproteins which play significant roles in the pathogenesis of inflammatory based disorders [5, 6]. Our previous investigation also demonstrated that metal contamination is prevalent among Iranian breast milk [9]. However, recent results demonstrated that AFM1, Nickel and Arsenic contamination being low among the Iranian breast milk and it may be related to the increased quality of national heath programs. The results demonstrated that milk levels of IL-6 and IL-8 did not differ among AFM1, Nickel and Arsenic contaminated and non-contaminated breast milk while, there was a positive moderate correlation between IL-8 and milk levels of Arsenic.
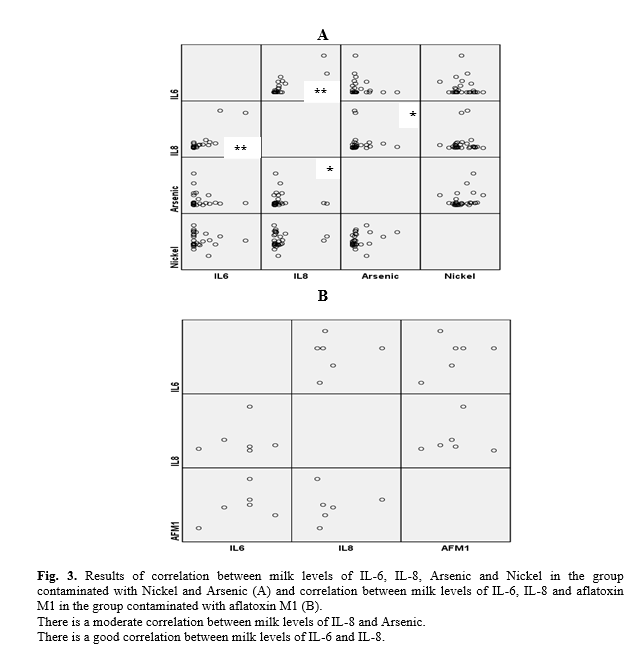
AFM1, Nickel and Arsenic contamination can induce inflammation in the affected individuals [13, 14]; however, according to the results presented here, it seems that inflammation following AFM1, Nickel and Arsenic contamination is not related to IL-6 in the breast milk while, based on the results it may be hypothesized that Arsenic may induce an inflammation in the milk in IL-8 dependent manner. It is worthy to note that, although milk levels of the cytokines do not differ among the groups, contamination of the milks with AFM1, Nickel and Arsenic may induce inflammation in the infants as well [15] which needs more follow-up investigations.
IL-8 is a main factor which participates in the recruitment of neutrophils; hence, it may be concluded that neutrophilic inflammation is the complication observed in the infants after being fed on milk contaminated with Arsenic. Additionally, Asakawa et al., showed that Nickel decreases IL-6 mRNA stability and subsequently suppresses lipopolysaccharide-induced IL-6 production [16]. Moreover, it has been revealed that Nickel induces dendritic cells maturation [17]. Dendritic cells are the main sources of tumor necrosis factor-α and IL-12 which are important pro-inflammatory cytokines. As a result, it appears that the production of cytokines other than IL-6 may be responsible for inflammation-related issues after consumption of breast milk contaminated with Nickel and Arsenic. Interestingly, an in vitro study reported that AFM1 does not stimulate T lymphocytes to produce IL-8 [18]. Based on our results, it seems that AFM1 is also unable to induce IL-8 production in the in vivo condition. There are several studies which explain the role of aflatoxin B1 in the production of pro-inflammatory cytokines such as tumor necrosis factor-α and IL-12 [19], but to the best of our knowledge, there is no investigation regarding the role of AFM1 on the production of cytokines in vivo. Thus, it may be hypothesized that other pro-inflammatory cytokines may be produced after contamin-ation with AFM1. Further studies regarding the role of AFM1, and Nickel and Arsenic on the production of pro-inflammatory cytokines, other than IL-6 and IL-8, in breast-milk need to be performed to improve our knowledge regarding inflammatory complications correlated to AFM1, Nickel and Arsenic contamination in the milk of breast-fed infants. Additionally, it is proposed to investigate pro-inflammatory cytokines in the serum of breast-fed infants that have been fed on milk contaminated with AFM1, Nickel and Arsenic.
The results also revealed that a positive relation between milk levels of IL-6 and IL-8. Therefore, it appears that IL-8 and IL-6 have positive feedback effects on the production of each other by immune cells. Based on the fact that cytokines play their role in a network manner, it can be proposed that other cytokines, including pro-inflammatory and anti-inflammatory cytokines, may participate in responses to human milk AFM1, Nickel and Arsenic contaminations.
Conclusion
Based on the results, it may be concluded that AFM1, Nickel and Arsenic are unable to induce secretion of IL-6 into breast milk while it appears that Arsenic can be considered as IL-8 inducer in human milk therefore, the inflammation-related disorders following contamination with Arsenic may be associated with IL-8.
Conflict of Interest
None.
Acknowledgments
We thank all the participants which openly contributed to this study supported by Shahid Sadoughi University of Medical Sciences and NAJA University of Medical Sciences.
References
Full-Text: (745 Views)
Introduction
Breastfeeding is a common recommendation by the World Health Organization (WHO) [1] and it is well documented that the best method for providing an infant's nutrition is through breastfeeding. The mother's milk consists of several factors which are essential for a normal newborn and for infant growth including α-lactalbumin, lactoferrin, lysozyme and cadmium. Breast milk also includes several factors, such as maternal IgG, which helps the new born to have a good immune response against infections [2]. Combined, these factors lead to the development of the immune system, intestinal microflora and the functional maturity of mucous membranes [3]. However, contamination of maternal milk with environmental toxins such as aflatoxins and heavy metals including Nickel and Arsenic, can be harmful for the newborns and infants, especially in the first months of life [4].
Cytokines are the main immunological factors which are produced during infection and inflammation [5, 6] and Interlukine (IL)-6 and IL-8 are the main innate immune cytokines and can induce inflammation and chemotaxis in immune cells such as neutrophils [7]. These are members of the pro-inflammatory cytokines and their chronic expression may be harmful for mucosal structure and immunity. Cytokines may be produced in response to contamination with heavy metals and biological toxins, and it may be hypothesized that production of IL-6 and IL-8 is one of the mechanisms which results in mucosal inflammation in infants. Breastfeeding has become more popular in Iran over the last decade, hence, its safety on new born babies needs to be evaluated, especially in light of increased contamination of milk with heavy metals and toxins, which are the main inducers of inflammatory responses in the infants [8].
Our previous investigation has revealed that in more than 86 % of the cases the maternal milk of the centered part of Iranian population is contaminated with aflatoxin M1 (AFM1) and heavy metals [9]. Thus, the main aim of this study was to evaluate the effects of the contamination of breast milk with Nickel, Arsenic and AFM1 on the milk levels of IL-6 and IL-8.
Materials and Methods
Subjects
In this cross-sectional study, breast-milk was collected from 76 lactating mothers, with the mean age 27.40±4.66 years, 147±4.66 cm high, 27.81±19.98 BMI (kg/m2) and 68.26±10.56 weight (kg), during regular feeding of their infants when they had referred to health centers in the Yazd city from June 2013 to June 2014. Mothers with pre-term infants, smoking, addiction, alcohol drinking, immunosuppressive drug administration and mental, autoimmune or allergic disorders were excluded from the study. The breast milk was collected on the 30th day post-parturition, and evaluated for of levels of AFM1, Nickel, Arsenic, IL-6 and IL-8. Accordingly, based on the detection of AFM1, Nickel and Arsenic in the milk samples, they were divided into three groups including Nickel and Arsenic contaminated group (being contaminated with Nickel and Arsenic but not AFM1), AFM1 contaminated group (being contaminated with AFM1 but not Nickel and Arsenic), and a control group, not contaminated with Nickel, Arsenic and AFM1. The Ethical Committee of Shahid Sedoughi University of Medical Sciences, Yazd, Iran approved the protocol of the study and all the participants filled out the written informed consent.
Evaluation of Nickel concentrations
In order to evaluate the concentration of Nickel, 1 mL of acetic acid (1 M) was added to 3 mL of each sample, mixed and centrifuged at 3200 RPM for 15 minutes. The lower liquid was extracted and milk fat was isolated. The samples were centrifuged again at 3200 RPM for 15 minutes to obtain a completely clear solution. The nickel concentration was measured using an atomic absorption spectrophotometer model 20AA (Varian) at a wavelength of 232 nm. The atomic absorption was read three times and the absorption mean was calculated [10]. The limitation rate for evaluation of Nickel using this method was 2-80 ppm.
Evaluation of Arsenic concentrations
Breast milk samples (1 mL) were transferred and 10 mL of toluene and 15 mL of a mixture of nitric acid and sulfuric acid were added to the sample, mixed for 10 minutes and subsequently two phases were separated and the lower phase was used to measure Arsenic concentrations employing an atomic absorption spectro-photometer model 20AA (Varian) at 197.3 nm wavelength, and 3 samples were measured after which the absorption mean was calculated [11]. The limitation rate for evaluation of Arsenic using this method was 0.15-3.73 ppm.
Measurement of aflatoxin M1
Aflatoxins are water soluble [12] and this property was exploited; the samples were centrifuged for 15 minutes at 15000 RPM, the upper creamy layers were completely discarded and the lower phases were used for evaluation of AFM1 employing a competitive enzyme-linked immunosorbent assay (ELISA) technique using a commercial kit (R-BiopharmGmbh-Rocket international Company) according to the manufacture’s guidelines. Based on using 6 standards which cover a range from 0 to 80 ppt, the limitation rate for evaluation of AFM1 using this method was 0-80 ppt.
Evaluation of IL-6 and IL-8 concentrations
Milk levels of IL-6 and IL-8 were measured by ELISA using a commercial kit from the Karmania Pars Gene, Iran, according to the manufacturer's guidelines. The limitation rate for evaluation of IL-6 was from 2 to 2000 pg/mL, while for IL-8 it was 2 to 2500 pg/mL.
Statistical analysis
Results are presented as the median (1st quartile and 3rd quartile). A non-parametric Kolmogorov-Smirnov test was used to evaluate the normal distribution of IL-6 and IL-8 in each of the study groups (AFM1 contaminated, high levels of Nickel and Arsenic, and the controls), but the presumption of normality was not met (p<0.05). Levene's test was applied to assess the homogeneity of variances of IL-6 and IL-8 across the three groups, but the presumption of homogeneity of variances was not also met (p<0.05). As the presumptions of one-way analysis of variance (ANOVA) were not met, the alternative non-parametric Kruskal-Wallis H test was utilized to compare medians of IL-6 and IL-8 across the three groups and Spearman`s ratio for correlation between Nickel and Arsenic levels with milk levels of IL-6 and 8. For statistical analysis, the statistical software SPSS version 15.0 for windows (SPSS Inc., Chicago, IL) was used. P-values of 0.05 or less were considered statistically significant.
Results
The results demonstrated 8 samples being contaminated with AFM1 (AFM1 contaminated group) while 29 samples were contaminated with both Nickel and Arsenic (Nickel and Arsenic contaminated group, table 1). The results revealed 39 milk samples being free of AFM1, Nickel and Arsenic (Control group). Evaluation of milk levels of IL-6 and IL-8 revealed milk levels of IL-6 (p=0.090) and IL-8 (p=0.413) failing to be different in AFM1, Nickel and Arsenic contaminated milk when compared to normal controls (Table 1 and Fig. 1).
Evaluation of milk levels of Arsenic and Nickle revealed the means±standard errors (SE) of Arsenic and Nickle being 0.45±0.13 and 53.90±1.13 ppb, respectively in Nickel and Arsenic contaminated milks. However, AFM1 levels in the aflatoxin contaminated milks were 0.96±0.25 ppt (Fig. 2). The concentration of AFM1, Nickel and Arsenic was undetectable in the control group. The concentration of AFM1 in the Nickel and Arsenic contaminated group and Nickel and Arsenic in the AFM1 contaminated group were undetectable too. Statistical analysis also revealed that milk levels of IL-8 have a moderate correlation with Arsenic in Nickel and Arsenic contaminated group (r=0.314, p<0.05, Fig. 3). Milk levels of IL-6 have also a good positive correlation with IL-8 (r=0.720, p>0.001, Fig. 3).
Discussion
Cytokines are small glycoproteins which play significant roles in the pathogenesis of inflammatory based disorders [5, 6]. Our previous investigation also demonstrated that metal contamination is prevalent among Iranian breast milk [9]. However, recent results demonstrated that AFM1, Nickel and Arsenic contamination being low among the Iranian breast milk and it may be related to the increased quality of national heath programs. The results demonstrated that milk levels of IL-6 and IL-8 did not differ among AFM1, Nickel and Arsenic contaminated and non-contaminated breast milk while, there was a positive moderate correlation between IL-8 and milk levels of Arsenic.
Table1. Results of median comparison of variables across studied groups
| Groups | ||||
| P-value | Control (N=39) |
Heavy Metal (N=29) |
Aflatoxin M1 (N=8) |
Variable |
| 0.090 | 0.50 (0.50-4.25) | 0.50 (0.50-11.35) | 0.50 (0.50-0.50) | IL-6 (pg/mL) |
| 0.413 | 42.37 (29.90-63.71) | 48.98 (27.60-118.04) | 39.25 (31.46-48.21) | IL-8 (pg/mL) |
Data are presented as median (1st quartile-3rd quartile).
Medians of IL-6 and IL-8 are compared using non-parametric Kruskal-Wallis H test among the three groups.
Medians of IL-6 and IL-8 are compared using non-parametric Kruskal-Wallis H test among the three groups.


AFM1, Nickel and Arsenic contamination can induce inflammation in the affected individuals [13, 14]; however, according to the results presented here, it seems that inflammation following AFM1, Nickel and Arsenic contamination is not related to IL-6 in the breast milk while, based on the results it may be hypothesized that Arsenic may induce an inflammation in the milk in IL-8 dependent manner. It is worthy to note that, although milk levels of the cytokines do not differ among the groups, contamination of the milks with AFM1, Nickel and Arsenic may induce inflammation in the infants as well [15] which needs more follow-up investigations.
IL-8 is a main factor which participates in the recruitment of neutrophils; hence, it may be concluded that neutrophilic inflammation is the complication observed in the infants after being fed on milk contaminated with Arsenic. Additionally, Asakawa et al., showed that Nickel decreases IL-6 mRNA stability and subsequently suppresses lipopolysaccharide-induced IL-6 production [16]. Moreover, it has been revealed that Nickel induces dendritic cells maturation [17]. Dendritic cells are the main sources of tumor necrosis factor-α and IL-12 which are important pro-inflammatory cytokines. As a result, it appears that the production of cytokines other than IL-6 may be responsible for inflammation-related issues after consumption of breast milk contaminated with Nickel and Arsenic. Interestingly, an in vitro study reported that AFM1 does not stimulate T lymphocytes to produce IL-8 [18]. Based on our results, it seems that AFM1 is also unable to induce IL-8 production in the in vivo condition. There are several studies which explain the role of aflatoxin B1 in the production of pro-inflammatory cytokines such as tumor necrosis factor-α and IL-12 [19], but to the best of our knowledge, there is no investigation regarding the role of AFM1 on the production of cytokines in vivo. Thus, it may be hypothesized that other pro-inflammatory cytokines may be produced after contamin-ation with AFM1. Further studies regarding the role of AFM1, and Nickel and Arsenic on the production of pro-inflammatory cytokines, other than IL-6 and IL-8, in breast-milk need to be performed to improve our knowledge regarding inflammatory complications correlated to AFM1, Nickel and Arsenic contamination in the milk of breast-fed infants. Additionally, it is proposed to investigate pro-inflammatory cytokines in the serum of breast-fed infants that have been fed on milk contaminated with AFM1, Nickel and Arsenic.
The results also revealed that a positive relation between milk levels of IL-6 and IL-8. Therefore, it appears that IL-8 and IL-6 have positive feedback effects on the production of each other by immune cells. Based on the fact that cytokines play their role in a network manner, it can be proposed that other cytokines, including pro-inflammatory and anti-inflammatory cytokines, may participate in responses to human milk AFM1, Nickel and Arsenic contaminations.
Conclusion
Based on the results, it may be concluded that AFM1, Nickel and Arsenic are unable to induce secretion of IL-6 into breast milk while it appears that Arsenic can be considered as IL-8 inducer in human milk therefore, the inflammation-related disorders following contamination with Arsenic may be associated with IL-8.
Conflict of Interest
None.
Acknowledgments
We thank all the participants which openly contributed to this study supported by Shahid Sadoughi University of Medical Sciences and NAJA University of Medical Sciences.
References
- Eidelman AI, Schanler RJ. Breastfeeding and the use of human milk. Pediatrics. 2012; 129(3): 827-41.
- Lönnerdal B. Nutritional and physiologic significance of human milk proteins. The American journal of clinical nutrition. 2003; 77(6): 1537-543.
- Andreas NJ, Kampmann B, Mehring Le-Doare K. Human breast milk: A review on its composition and bioactivity. Early Hum Dev. 2015; 91(11): 629-35.
- Das A, Bhattacharya S, Palaniswamy M, Angayarkanni J. Biodegradation of aflatoxin B1 in contaminated rice straw by Pleurotus ostreatus MTCC 142 and Pleurotus ostreatus GHBBF10 in the presence of metal salts and surfactants. World Journal of Microbiology and Biotechnology. 2014; 30(8): 2315-324.
- Arababadi MK, Aminzadeh F, Hassanshahi G, Khoramdelazad H, Karimabad MN, zarandi ER, et al. Cytokines in preterm delivery. LabMedicine. 2012; 43(4): 131-34.
- Arababadi MK, Mosavi R, Khorramdelazad H, Yaghini N, Zarandi ER, Araste M, et al. Cytokine patterns after therapy with Avonex(R), Rebif(R), Betaferon(R) and CinnoVex in relapsing-remitting multiple sclerosis in Iranian patients. Biomark Med. 2010; 4(5): 755-59.
- Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. 1994; 56(5): 559-64.
- Bahadoran P, Alijanpoor M, Usefy A. Relationship between infants’ feeding pattern and mothers’ physical and psychological health among the mothers covered by the health centers of Isfahan in 2013. Iran J Nurs Midwifery Res. 2015; 20(2): 216.
- Salmani MH, Mozaffari-Khosravi H, Rezaei Z. The nickel concentration in breast milk during the first month of lactation in Yazd, Center of Iran. Biologic Trace Element Res. 2016; 174(1): 65-70.
- Amanna EN, Bhat SS, Hegde SK. An in vitro evaluation of nickel and chromium release from different commercially available stainless steel crowns. J Indian Soc Pedod Prev Dent. 2019; 37(1): 31-8.
- Pandey N, Bhatt R. Arsenic resistance and accumulation by two bacteria isolated from a natural arsenic contaminated site. J Basic Microbiol. 2015; 55(11): 1275-286.
- Shashidhar J, Sashidhar R, Deshpande V. Role of mycoferritin from Aspergillus parasiticus (255) in secondary metabolism (aflatoxin production). FEMS microbiology Letters 2005; 251(1): 113-17.
- Liu C, Shen H, Yi L, Shao P, Soulika AM, Meng X, et al. Oral administration of aflatoxin G 1 induces chronic alveolar inflammation associated with lung tumorigenesis. Toxicol Lett. 2015; 232(3): 547-56.
- Iannitti T, Capone S, Gatti A, Capitani F, Cetta F, Palmieri B. Intracellular heavy metal nanoparticle storage: progressive accumulation within lymph nodes with transformation from chronic inflammation to malignancy. Int J Nanomed. 2010; 5(1): 955.
- Farzan SF, Brickley EB, Li Z, Gilbert-Diamond D, Gossai A, Chen Y, et al. Maternal and infant inflammatory markers in relation to prenatal arsenic exposure in a U.S. pregnancy cohort. Environ Res. 2017; 156(1): 426-33.
- Asakawa S, Kishimoto Y, Takano T, Okita K, Takakuwa S, Sato T, et al. Nickel ions selectively inhibit lipopolysaccharide-induced interleukin-6 production by decreasing its mRNA stability. PloS One 2015; 10(3): 1-8.
- Turbica I, Gallais Y, Gueguen C, Tharinger H, Al Sabbagh C, Gorges R, et al. Ectosomes from neutrophil-like cells down-regulate nickel-induced dendritic cell maturation and promote Th2 polarization. J Leukocyte Biol. 2015; 97(4): 737-49.
- Luongo D, Russo R, Balestrieri A, Marzocco S, Bergamo P, Severino L. In vitro study of AFB1 and AFM1 effects on human lymphoblastoid Jurkat T-cell model. J Immunotoxicol. 2013; 11(4): 353-58.
- Kauf A, Rosenbusch R, Paape M, Bannerman DD. Innate immune response to intramammary Mycoplasma bovis infection. J Dairy Sci. 2007; 90(7): 3336-348.
Type of Study: Research |
Subject:
Immunology
Received: 2019/03/2 | Accepted: 2020/01/13 | Published: 2020/01/30
Received: 2019/03/2 | Accepted: 2020/01/13 | Published: 2020/01/30
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




