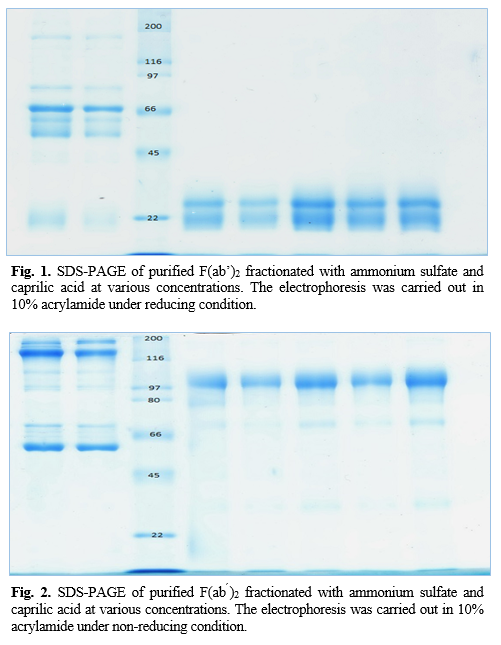
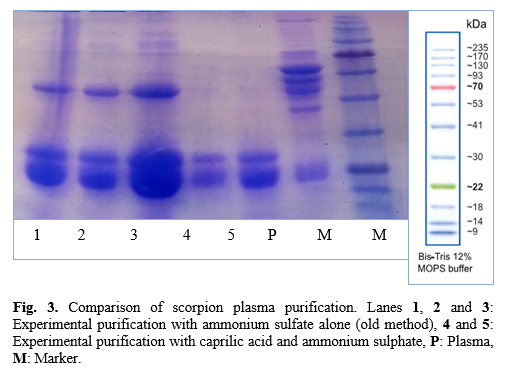
Values are expressed as mean±SD (n=5), and values in the same line with different superscripts (a-e) are differences significant at p<0.05 by Duncan test using SPSS. (All of reaction voloum:1000 mL).
AS=Ammonium sulfate; CA= Caprilic acid
Discussion
Large scale production of therapeutic antivenom against scorpion sting generally includes pepsin digestion of equine hyperimmune plasma and then fractionation of the F(ab ́)
2 fragment with ammonium sulfate. [22]. Intravenous administration of antivenoms is connected with a high (10-76%) prevalence of negative effects [3]. Additional contaminants considered to result in negative effects consisting of serum proteins and other fragments or excessive mulecular weight aggregates. Consequently, fractionation of antivenom antibody has aimed at clearing the Fc portion by pepsin digestion and also eliminating additional plasma protein impurities or their digestion products by numerous procedures [18,19]. Pepsin digestion has been utilized since 1939 to eliminate the greatly immunogenic Fc of heterologous antibody. Nevertheless, it is essential for the pepsin to be entirely inactivated or eliminated in subsequent fractionation procedures because the enzyme can influence the stability of the antivenom [14]. Ammonium sulfate is commonly used in large-scale fractional precipitation of IgG or F(ab ́)
2. The negative aspect of ammonium sulfate precipitation is usually that the F(ab ́)
2 product needs to be recovered and resolubilized. This method is hard to perform on a large scale under aseptic situations, and endproduct pollution is usually discovered. The procedure can also involve an important decrease of antibody activity [1]. Incorporation of caprilic acid with ammonium sulfate fractionation has the benefit of precipitating numerous serum proteins or their fragments. Considerably, the high mulecular weight aggregates were not seen in the F(ab ́)
2 end product purified by caprylic acid precipitation. The risk of viral contamination of biological products is a subject of great interest [7]. In this regard, the fractionation studied regarding pepsin digestion and also treatment with caprylic acid might inactivate several viruses because of the acidic situation and the detergent action of the organic acid [22]. Since the purification of antibody is generally performed by dialysis in cellulose bags, this procedure is time-consuming and can take several days. With regards to the above notes, the aim of the present study was to prepare equine antivenom using combination of caprilic acid and ammonium sulfate methods as a novel and effective combinative method in improving of F(ab ́)
2 antivenom.
Conclusion
The present study made an attempt to purify equine antivenom using combination of caprilic acid and ammonium sulfate fractionation method in improving pepsin digested horse F(ab ́)
2 antivenom. The results of this study showed that using caprilic acid and then ammonium sulfate can be effective in removal of impurities and extraction of F(ab')
2 at high volumes in shorter period of time, which is an important factor in production of biological products. we used this method for purification of 50 liters of hyperimmune plasma. The most important benefits of this method are 1) cheaper production cost which is an important factor in poor developing countries, 2) The shorter purification time which is the most important factor in biological product purification, and 3) removal or inactivation of progeny and protein aggregation and viral. Our results was consistent with those of other researchers who asserted this method as effective in antivenom large scale purification.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgements
We gratefully acknowledge financial support from the Research Council of the Islamic Azad University of Yazd and
Razi Vaccine and Serum Research Institute in Ahvaz.
References
[1]. Gutiérrez JM, León G, Burnouf T. Antivenoms for the treatment of snakebite envenomings: the road ahead. Biologicals 2011; 39(3): 129-42.
[2]. Redwan ER. Comparison between therapeutic antitoxin F(ab)
2 fractionated with ammonium sulfate and caprylic acid. J Immunoassay Immunochem. 2006; 27(4): 319-29.
[3]. Otero R, Gutiérrez JM, Rojas G, Núñez V, Dıaz A, Miranda E, et al. A randomized blinded clinical trial of two antivenoms, prepared by caprylic acid or ammonium sulphate fractionation of IgG, in Bothrops and Porthidium snake bites in Colombia: correlation between safety and biochemical characteristics of antivenoms. Toxicon.1999; 37(6): 895-908.
[4]. Rojas G, Jiménez J, Gutiérrez J. Caprylic acid fractionation of hyperimmune horse plasma: description of a simple procedure for antivenom production. Toxicon. 1994; 32(3): 351-63.
[5]. Krifi M, Ayeb MEl, Dellagi K, Venom J. The improvement and standardization of antivenom production in developing countries: comparing antivenom quality, therapeutical efficiency, and cost. J Venom Anim Toxins. 1999; 5(1-2): 128-41.
[6]. Dos Santos M, Lima MDI, Furtado G, Colletto G, Kipnis T, Da Silva WD. Purification of F(ab)
2 anti-snake venom by caprylic acid: a fast method for obtaining IgG fragments with high neutralization activity, purity and yield. Toxicon. 1989; 27(3): 297-303.
[7]. Burnouf T, Griffiths E, Padilla A, Seddik S, Stephano MA, Gutiérrez JM. Assessment of the viral safety of antivenoms fractionated from equine plasma. Biologicals 2004; 32(3): 115-28.
[8]. Fernande AS, Kaundinya JO, Daftary G, Saxena L, Banerjee S, Pattnaik P. Chromatographic purification of equine immunoglobulin GF(ab)
2 from plasma. J Chromatogr B. 2008; 876(1): 109-115.
[9]. Perosa F, Carbone R, Ferrone S, Dammacco F. Purification of human immunoglobulins by sequential precipitation with caprylic acid and ammonium sulphate. J Immunol Methods 1990; 128(1): 9-16.
[10]. Reik LM, Maines SL, Ryan DE, Levin W, Bandiera S, Thomas PE. A simple, non-chromatographic purification procedure for monoclonal antibodies Isolation of monoclonal antibodies against cytochrome P450 isozymes. J Immunol Methods 1987; 100(1-2): 123-30.
[11]. Temponi M, Kageshita T, Perosa F, Ono R, Okada H, Ferrone S. Purification of murine IgG monoclonal antibodies by precipitation with caprylic acid: comparison with other methods of purification. Hybridoma 1989; 8(1): 85-95.
[12]. McKinney MM, Parkinson A. A simple, non-chromatographic procedure to purify immunoglobulins from serum and ascites fluid. J Immunol Methods 1987; 96(2): 271-80.
[13]. Saetang T, Suttijitpaisal P, Ratanabanangkoon K, Nat J. Preparations of toxic components from Naja kaouthia venom by selective heat denaturation. Toxins 1998; 7(1): 37-44.
[14]. Laemmli UK. leavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227(5259): 680-85.
[15]. Slovis N, Murray G. How to approach whole blood transfusions in horses. AAEP Proceedings 2001; 47(8): 266-69.
[16]. Morais V, Massaldi H. Effect of pepsin digestion on the antivenom activity of equine immunoglobulins. Toxicon. 2005; 46(8): 876-82.
[17]. Rial A, Morais V, Rossi S, Massaldi H. A new ELISA for determination of potency in snake antivenoms. Toxicon. 2006; 48(4): 462-66.
[18]. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193(1): 265-75.
[19]. Baldwin RL. How Hofmeister ion interactions affect protein stability. Biophys J. 1996; 71(4): 2056-63.
[20]. Bernard N, Jolivalt C, Schwartzentruber J. Protein precipitation by caprylic acid: equilibrium composition data. Biotechnol Bioeng. 1996; 49(4): 405-11.
[21]. Kukongviriyapan V, Poopyruchpong N, Ratanabanangkoon K. Some parameters of affinity chromatography in the purification of antibody against Naja naja siamensis toxin 3. J immunol methods. 1982; 49(1): 97-104.
[22]. Raweerith R, Ratanabanangkoon K. Frac-tionation of equine antivenom using caprylic acid precipitation in combination with cationic ion-exchange chromatography. J Immunol Methods 2003; 282(1-2): 63-72.
[23]. World Health Organization. WHO guidelines for the production, control and regulation of snake antivenom immunoglobulins. Geneva: WHO. 2010; p.134.