Introduction
Multipotent mesenchymal stem cells (MSCs) have the self-renewal capacity and also differentiation potential into various cell types of mesodermal cell lineages [1]. Human MSCs can be derived from different sources such as adult bone marrow, adipose tissue, umbilical cord, cord blood, amniotic membrane, amniotic fluid, human embryonic stem cells (hESCs) and other sources [2-4]. Among the different MSCs sources, adipose-derived MSCs (AD-MSCs) has led to more attention due to its high abundance, an available and accessible application as well as sustainability [5].
On the other hand, several reports have indicated that reactive oxygen species (ROS) can cause the inhibition of the MSC proliferation and immunomodulation, senescence promotion. Moreover, ROS can increase adipogenic differentiation while reducing osteogenic. Therefore, the effect of the mitochondrial metabolism onto the differentiation capacity of the human MSCs was assessed [6].
Mitochondrial electron transport systems are the main source of ROS which produce by nicotinamide adenine dinucleotide phosphate oxidases, xanthine oxidase, cytochrome P450, nitric oxide synthases, lipoxygenases, heme oxygenase, cyclooxygenases, myeloperoxidase, and monoamine oxidases [7]. Thereby, antioxidants can be used to protect the body against free radicals. The antioxidant system includes natural enzymatic and non-enzymatic factors that neutralize the harmful effects of oxidant [8]. Based on the chemical structure, vitamin E can be used as an antioxidant in a group of potent, lipid-soluble, chain-breaking antioxidant, due to its lipid solubility, MSCs treatment with vitamin E protected against H2O2-induced apoptosis and promoted MSC survival via the AKT pathway and increase the proliferation and differentiation of MSCs [9-11]. In recent years, studies have shown the effect of vitamin E on the various stem cells such as embryonic stem cells, melanoma stem cells and mesenchymal stem cell have been investigated [12-15].
In addition, Selenium is known as an antioxidant and cofactor of many enzymes and the potential of Selenium to stimulated stem cell proliferation potency, For example, Selenium play a vital role in redox regulation in intracellular signaling via selenocysteine or Selenium-binding protein 1 (SELENBP1) is an indicator of cell differentiation/maturation adipocyte, and it can catalyze the oxidation of methanethiol [16-19]. Also, Selenium dioxide nanoparticles can improve the proliferation of ADSCs and bone marrow (BM-MSCs) [20]. Selenium can impact on normal myocardial differentiation and development by targeting miRNA [21].
Survival of MSCs, against oxidative stress is vital for stem cell therapy. Based on the AD-MSCs have shown potential for stem cell therapy owing to their antioxidant activity [13]. Due to the supportive effect of the antioxidants to induce proliferation and differentiation, this issue encouraged us to assess the best dosage of the vitamin E and Selenium for proliferation and differentiation of the AD-MSCs. In this study, the antioxidant influence of the Vitamin E and Selenium was investigated on the proliferation and viability of the AD-MSCs was evaluated using methyl thiazolyl diphenyl-tetrazolium bromide (MTT) technique and their effect on the osteogenic and adipogenic differentiation of the AD-MSCs.
Materials and Methods
AD-MSCs cell culture
AD-MSCs were purchased from the Yazd Cardiovascular Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran (Ebrahimi et al., unpublished data).
The AD-MSCs were passaged using Trypsin/EDTA (Shellmax, China) and seeded into the tissue culture flask containing Dulbecco's modified eagle medium+20% fetal bovine serum (DMEM+20% FBS) (Shellmax, China) and incubated in the humidified atmosphere at 37˚C and 5% CO2 (Heracell 150i, Thermo scientific, American). Following cell attachment, the culture medium was changed every other day.
MTT assay
Cell viability was assessed using MTT assay according to the company instruction (Biomatik, Germany) Initially, 5×103 cells/well were seeded into 96-well plates and incubated at 37°C, 5% CO2 humidified atmosphere for 24 hr. Then the medium was aspirated, and cells were treated whit 50, 100, 300, 500,700, and 750 µm of vitamin E and Selenium solution. 24 hr later, 0.5 mg/mL MTT was added for IC50 absorbance Measurement at 570 nm using the enzyme-linked immunosorbent assay reader.
Osteogenic and adipogenic analysis using alizarin red and oil red staining
To investigate the effects of vitamin E )Sigma, Germany (and Selenium )Sigma, Germany (on osteogenic differentiation, the 5 × 103 cells of AD-MSCs were cultured into each well of the 5-well plate by DMEM+20%FBS and incubated at 37°C, 5% CO2 humidified atmosphere. At 80% confluency, the medium was replaced with osteogenic medium (Bonyakhte, Iran) and adipogenic medium (Bonyakhte, Iran) while treated with optimal (IC50) dosages of vitamin E and Selenium compares whit control group (osteogenic or adipogenic medium without of Selenium or vitamin E). The differentiation medium was changed every three days for 17 days. Finally, matrix mineralization and lipid droplet accumulation of the differentiated cells analyzed using Alizarin red (Bonyakhte, Iran) and Oil-red (Bonyakhte, Iran) staining, respectively. Tests were repeated three times in each group.
Statistical analysis
Differences between groups were examined for statistical significance using a t-test with SPSS version 25. All data were representative of the mean±SD of the mean. The difference was considered to be significant for P<0.01.
Results
Effect of vitamin E and Selenium on AD-MSCs viability
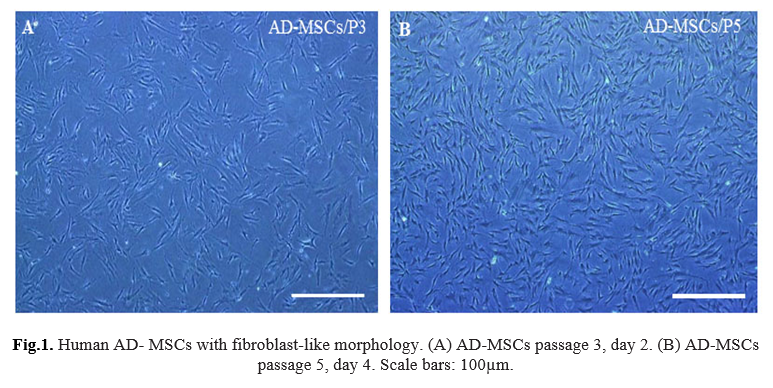
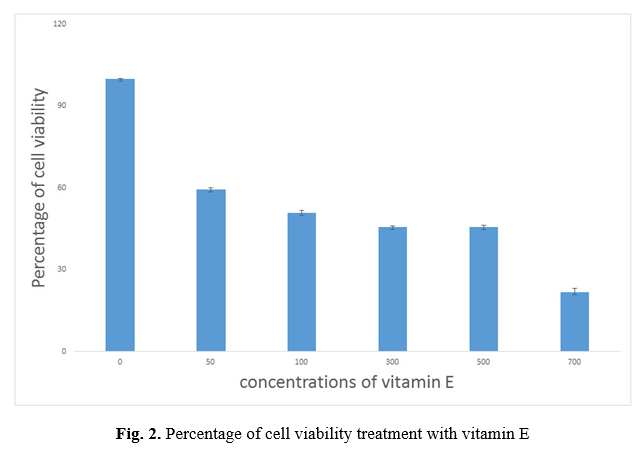
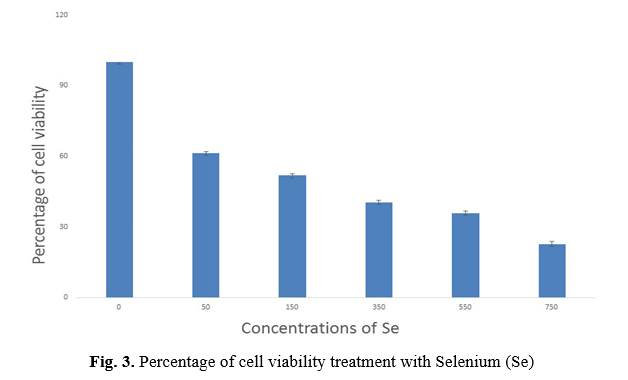
In this study, successful isolation and proliferation of human AD-MSCs with fibroblast-like morphology, was confirmed (Fig. 1 A, B). The antioxidant effect evaluation of vitamin E and Selenium on the viability of the AD-MSCs was assessed using MTT test. MTT data revealed a preferred concentration of vitamin E between 50 to 700 µM dosages, survival percentage of the cells reduced from 59.46% to 21.8%. IC50 was observed at 125 μM concentration of vitamin E (p <0.001) (Fig. 2) and Selenium between 50 to 750 µM dosages, the survival percentage of the cells reduced from 61.4% to 22.6%. IC50 was observed at 121 μM concentration of Selenium (p<0.001) (Fig. 3).
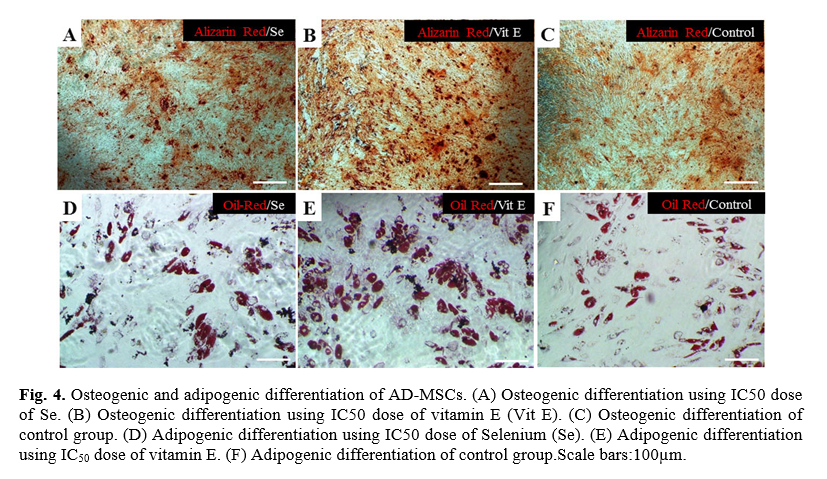
Osteogenic and adipogenic differentiation of MSCs whit vitamin E and Selenium treatment
Following the investigation, the effect of vitamin E and Selenium on cell proliferation, their impact on AD-MSCs differentiation potential was examined. Human AD-MSCs induced to differentiate in the presence and absence of the optimal dosages of vitamin E and Selenium. Following differentiation induction, Alizarin red staining showed mineralization of the matrix occurred in vitamin E, and Selenium treated cells more than the control group (Fig 4 A, B, C). Similarly, Oil-red staining confirmed better adipogenesis induction in the treated cells in comparison with the control group (Fig. 4 D, E, F).
Discussion
In this study, we have examined the antioxidant properties of the Selenium and vitamin E on the AD-MSCs stemness features. Our data indicate that both Selenium and vitamin E induce osteogenesis and adipogenesis in AD-MSCs in their IC50 dosages. Furthermore, it seems that Selenium and vitamin E can improve the proliferation condition of the AD-MSCs in vtiro.
Previous reports have shown that antioxidants such including vitamin E and Selenium have ROS scavenger potential, which can affect the proliferation and differentiation of MSCs. Moreover, the survival of MSCs against oxidative stress is vital for future cell therapy and tissue engineering [3]. Studies on other antioxidants such as melatonin, vitamin C, epigallocatechin-3-gallate have shown increase differentiation and proliferation of MSCs [22-25]. Here, we have investigated whether vitamin E and Selenium play a similar role in the proliferation and differentiation capacity of the AD-MSCs.
ROS is one of the essential biological elements with a critical role in the metabolic function of the cells. In this regard, interrupts in ROS production can cause cell damages including, apoptosis and release of inflammatory mediators and, subsequently, cell death [13, 26]. Besides, oxidative stress-induced the decline of cellular alkaline phosphatase an activity which caused decrease osteogenic differentiation potential of MSCs [23]. These ROS damaging effects mediated by antioxidant systems [26] so that treatment with antioxidants such as Vitamin E induced MSCs to deactivate the effects of H2O2-induced oxidative stress [27]. Several studies confirmed the effect of Vitamin C and D on the differentiation of MSCs into adipogenesis and osteogenesis [24, 28, 29]. Previous research showed supplementation with vitamin E can protect against free radical, also enhanced cell proliferation and gene expression such as alkaline phosphatase, transforming growth factor-beta 1, fibroblast growth factor receptor 1, when compared to MSCs cultured in media without vitamin E [30]. but some study indicated that vitamin E inhibits differentiation of osteoblasts isolated from rat [31].
Vitamin E also can enhance the efficiency of neural stem cell differentiation and promotes morphological maturation of the differentiated neurons [32]. Herein, it was investigated that vitamin E can improve the differentiation potential of the AD-MSCs to osteogenic and adipogenic cells. 125 μM concentration was the effective dosage for differentiation using vitamin E. Previous findings indicated the positive effect of the Selenium considering as another antioxidant for adipogenic differentiation of primary pig and rat preadipocytes [33-35].
Recently study specified that Selenium nanoparticles are used for stem cell research, shown that enhance the differentiation of MSCs toward osteogenic lineage over adipocytes by promoting osteogenic transcription [36]. Adipose tissue-derived stromal cells treated whit Selenium results in differentiation into mesodermal and neural lineage [37] . Moreover, Selenium containing agents which can be promoted regeneration and normalization of osteogenesis [38]. The present study has shown not only maximum lipid droplet accumulation at 121 μM concentration of Selenium but also higher matrix mineralization was detected with Selenium treatment in comparison with the control group.
Conclusion
Stem cells have generated a great deal in tissue engineering and regenerative medicine. In sum, the present study suggested vitamin E and Selenium due to antioxidant properties affect positively on the proliferation and differentiation induction of the AD-MSCs. According to our results, these antioxidants can be used to optimize the differentiation condition for osteogenesis and adipogenesis. AD-MSCs are considered as a suitable candidate for future cell therapy and subsequent regenerative medicine applications. We hope this study could be an approach to cell therapy.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
The current work was supported by grants from the department of clinical biochemistry, faculty of medicine, Pistachio Safety Research Center, Rafsanjan university of medical sciences, Rafsanjan, Iran, and Stem Cell Biology Research Center, Yazd Reproductive Sciences Institute, Shahid Sadoughi university of medical sciences, Yazd, Iran.
References
- Lin GL, Hankenson KD. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J Cell Biochem. 2011; 112(12): 3491-501.
- Ksiazek K. A comprehensive review on mesenchymal stem cell growth and senescence. Rejuvenation Res. 2009; 12(2): 105-16.
- Brandl A, Meyer M, Bechmann V, Nerlich M, Angele P. Oxidative stress induces senescence in human mesenchymal stem cells. Experl Cell Res. 2011; 317(11): 1541-547.
- Akyash F, Javidpou M, Nodoushan F, Aflatoonian B. Human embryonic stem cells derived mesenchymal stem/stromal cells and their use in regenerative medicine. J Stem Cell Res Ther. 2016; 1(7): 47.
- James AW. Review of signaling pathways governing MSC osteogenic and adipogenic differentiation. Scientifica 2013; 2013(1): 1-17.
- Denu RA, Hematti P. Effects of oxidative stress on mesenchymal stem cell biology. Oxidat Med Cel Longev. 2016; 2016(1): 1-9.
- Atashi F, Modarressi A, Pepper MS. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: a review. Stem Cell Dev. 2015; 24(10): 1150-163.
- Yehye WA, Rahman NA, Ariffin A, Hamid SBA, Alhadi AA, Kadir FA, et al. Understanding the chemistry behind the antioxidant activities of butylated hydroxytoluene (BHT): A review. Euro J Med Chem. 2015; 101: 295-312.
- Nazrun A, Norazlina M, Norliza M, Nirwana S. The anti-inflammatory role of vitamin E in prevention of osteoporosis. Adv Pharmacol Sci. 2012; 2012(1): 1-7.
- Godoy-Parejo C, Deng C, Zhang Y, Liu W, Chen G. Roles of vitamins in stem cells. Cell Mol Life Sci. 2020; 77(9): 1771-791.
- Brauer A, Pohlemann T, Metzger W. Osteogenic differentiation of immature osteo-blasts: Interplay of cell culture media and supplements. Biotech Histochem. 2016; 91(3): 161-69.
- Garg S, Sadhukhan R, Banerjee S, Savenka AV, Basnakian AG, McHargue V, et al. Gamma-tocotrienol protects the intestine from radiation potentially by accelerating mesen-chymal immune cell recovery. Antioxidants 2019; 8(3): 57-65.
- Bhatti FUR, Kim SJ, Yi AK, Hasty KA, Cho H. Cytoprotective role of vitamin E in porcine adipose-tissue-derived mesenchymal stem cells against hydrogen-peroxide-induced oxidative stress. Cell Tissue Res. 2018; 374(1): 111-20.
- Abd Jalil A, Khaza'ai H, Nordin N, Mansor N, Zaulkffali AS. Vitamin E-mediated modulation of glutamate receptor expression in an oxidative stress model of neural cells derived from embryonic stem cell cultures. Evid Based Complement Alternat Med. 2017; 2017: 6048936.
- Marzagalli M, Moretti RM, Messi E, Marelli MM, Fontana F, Anastasia A, et al. Targeting melanoma stem cells with the Vitamin E derivative δ-tocotrienol. Scientific Reports 2018; 8(1): 1-13.
- Prystupa A, Kiciński P, Luchowska-Kocot D, Błażewicz A, Niedziałek J, Mizerski G, et al. Association between serum Selenium concentrations and levels of proinflammatory and profibrotic cytokines-interleukin-6 and growth differentiation factor-15, in patients with alcoholic liver cirrhosis. Int J Environm Res Pub Hlth 2017; 14(437): 1-9.
- Lee MO, Cho YS. The role of selenium-mediated redox signaling by selenophosphate synthetase 1 (SEPHS1) in hESCs. Biochem Biophys Res Communic. 2019; 520(2): 406-12.
- Steinbrenner H, Micoogullari M, Hoang NA, Bergheim I, Klotz LO, Sies H. Selenium-binding protein 1 (SELENBP1) is a marker of mature adipocytes. Redox Biol. 2019; 20(1): 489-95.
- Park SH, Kim JH, Nam SW, Kim BW, Kim GY, Kim WJ, et al. Selenium improves stem cell potency by stimulating the proliferation and active migration of 3T3-L1 preadipocytes. Int J Oncol. 2014; 44(1): 336-42.
- Ahmed HH, Aglan HA, Mabrouk M, Abd-Rabou AA, Beherei HH. Enhanced mesenchymal stem cell proliferation through complexation of selenium/titanium nanocomposites. Journal of Materials Science: Materials Med. 2019; 30(2): 1-4.
- Cai J, Yang J, Liu Q, Gong Y, Zhang Y, Zhang Z. Selenium deficiency inhibits myocardial development and differentiation by targeting the mir-215-5p/CTCF axis in chicken. Metallomics 2019; 11(2): 415-28.
- Shin JW, Wu Y, Kang YG, Kim JK, Choi HJ, Shin JW. The effects of epigallocatechin-3-gallate and mechanical stimulation on osteogenic differentiation of human mesenchymal stem cells: individual or synergistic effects. Tissue Engineer Regener Med. 2017; 14(3): 307-15.
- Lee S, Le NH, Kang D. Melatonin alleviates oxidative stress-inhibited osteogenesis of human bone marrow-derived mesenchymal stem cells through AMPK activation. International journal of medical sciences. 2018; 15(10): 1083-1091.
- Wang Y, Singh A, Xu P, Pindrus MA, Blasioli DJ, Kaplan DL. Expansion and osteogenic differentiation of bone marrow-derived mesenchymal stem cells on a vitamin C functionalized polymer. Biomaterials 2006; 27(17): 3265-73.
- Rafat A, Roushandeh AM, Alizadeh A, Hashemi-Firouzi N, Golipoor Z. Comparison of the melatonin preconditioning efficacy between bone marrow and adipose-derived mesenchymal stem cells. Cell journal. 2019; 20(4): 450-58.
- Kim J. Effects of a potent antioxidant, platinum nanoparticle, on the lifespan of Caenorhabditis elegans. Mech Aging Dev. 2008; 129(6): 322-31.
- Bhatti F, Mehmood A, Latief N, Zahra S, Cho H, Khan S, et al. Vitamin E protects rat mesenchymal stem cells against hydrogen peroxide-induced oxidative stress in vitro and improves their therapeutic potential in surgically-induced rat model of osteoarthritis. Osteoarthritis Cartilage 2017; 25(2): 321-31.
- Basoli V, Santaniello S, Cruciani S, Ginesu G, Cossu M, Delitala A, et al. Melatonin and vitamin D interfere with the adipogenic fate of adipose-derived stem cells. Int J Mol Sci. 2017; 18(5): 981-89.
- Kolhe R, Mondal A, Pundkar C, Periyasamy-Thandavan S, Mendhe B, Hunter M, et al. Modulation of miRNAs by vitamin C in human bone marrow stromal cells. Nutrients 2018; 10(2): 186-99.
- Ahn KH, Jung HK, Jung SE, Yi KW, Park HT, Shin JH, et al. Microarray analysis of gene expression during differentiation of human mesenchymal stem cells treated with vitamin E in vitro into osteoblasts. Korean J Bone Metabolism 2011; 18(1): 23-32.
- Soeta S, Higuchi M, Yoshimura I, Itoh R, Kimura N, Amasaki H. Effects of vitamin E on the osteoblast differentiation. J Veterinary Med Sci. 2010: 174-80.
- Deng S, Hou G, Xue Z, Zhang L, Zhou Y, Liu C, et al. Vitamin E isomer δ-tocopherol enhances the efficiency of neural stem cell differentiation via L-type calcium channel. Neurosci lett. 2015; 585: 166-70.
- Hassan A, Ahn J, Suh Y, Choi YM, Chen P, Lee K. Selenium promotes adipogenic determination and differentiation of chicken embryonic fibroblasts with regulation of genes involved in fatty acid uptake, triacylglycerol synthesis and lipolysis. J Nutrit Biochem. 2014; 25(8): 858-67.
- Lee K, Hausman DB, Dean RG. Expression of CCAAT/enhancer binding protein C/EBPα, β and δ in rat adipose stromal-vascular cells in vitro. Biochimica et Biophysica Acta (BBA)-Mol Cell Res. 1999; 1450(3): 397-405.
- Chen X, Hausman D, Dean R, Hausman G. Hormonal regulation of leptin mrna expression and preadipocyte recruitment and differentiation in porcine primary cultures of S‐V cells. Obesity Res. 1998; 6(2): 164-72.
- Zheng C, Wang J, Liu Y, Yu Q, Liu Y, Deng N, et al. Functional selenium nanoparticles enhanced stem cell osteoblastic differentiation through BMP signaling pathways. Adv Func Mater. 2014; 24(43): 6872-883.
- Kim JH, Lee MR, Jee M, Kang S. IFATS collection: Selenium induces improvement of stem cell behaviors in human adipose-tissue stromal cells via SAPK/JNK and stemness acting signals. Stem Cells (Dayton, Ohio). 2008; 26(10): 2724-734.
- Kovalchuk PY, Gasko M, Tulyulyuk S. Morphological peculiarities of selenium influence on osteogenesis. Bukovynian Medical Bulletin 2015;19(3): 75-62.










































 , Alireza Khoshdel *
, Alireza Khoshdel * 

 , Mohammad Ali Fahmidehkar
, Mohammad Ali Fahmidehkar 

 , Mohammad Reza Hajizadeh
, Mohammad Reza Hajizadeh 

 , Mehdi Mahmoodi
, Mehdi Mahmoodi 

 , Mohammad Reza Mirzaei
, Mohammad Reza Mirzaei 

 , Fatemeh Akyash
, Fatemeh Akyash 

 , Behrouz Aflatoonian
, Behrouz Aflatoonian 






