Introduction
In cartilage tissue engineering, it is important to supply a template for three-dimensional cultures of chondrogenic cells to construct biocompatible and biodegradable scaffolding. If conceivable, scaffolding should direct and, differentiation, cell survival, and generation of extracellular matrix, should modify controlled degradation, and provide mechanical integrity depending on the extent and site of the deficiency, and permit the release of nutrients and materials and waste products. [
1]. In Poly D, L-Lactic-Colic Acid (PLGA), the acid-and-lactic acid forms are in equal proportions. PLGA can be processed in about any configuration and extent. It is soluble in a wide range of common solvents including, tetrahydrofuran, chlorinated solvents, acetone or ethyl acetate. Mechanical strength, capacity of the hydrolysis, rapidity of degradation, and conduct of the polymer swelling are instantly affected by the degree of crystallization of the PLGA, determined by more on the molar ratio and the type of single monomer ingredients in the copolymer chain. PGA crystals, when polymerized with PLA, decrease the crystallinity of PLGA crystallization and, consequently, enhance hydrolysis and hydration [
2].
Fibrin scaffold is a network of proteins that holds a variety of living tissues together. Fibrin has unique viscoelasticity and biocompatibility properties but its sustainability is weak and is destroyed quickly. Fibrin scaffold facilitates transfer of molecules and food as well as disposal of metabolites, the cell proliferation, and migration. Numerous studies suggest that fibrin scaffolds support survival and proliferation of mesenchymal cells (MSCs) [
3].
Hyaluronic acid (HA) scaffolds have been well established as a biomaterial for MSC delivery. HA hydrogels have been used to induce MSC adipogenesis, osteogenesis and keratinogenesis
in vitro [
4]. Researchers report that delivery of MSCs in HA hydrogels promotes chondrogenic gene expression [
5]. HA is recognized to interact instantly with fibrinogen precursor fibrin by complex reversible ion interactions; however, it constructs an adequate adhesive surface that permits cells to migrate more freely [
6].
In terms of accessibility, adipose tissue is at the forefront of stem cell resources due to its less painful and abundance and ease of collection from sources. Its adipose-derived stem cells (ADSCs) can be stored and propagated for a long time without losing their differentiating capacity, leading to the fact that large amounts of cells are increasingly being administered for cell therapy objectives. Several studies demonstrate that ADSCs-based cell therapy products show optimum efficiency and efficacy in some medical indications for even allogeneic and autologous objectives hence becoming as probable tools for reconstructing, replacing and regenerating damaged or dead cells [
7].
Materials and Methods
Chemicals
All chemicals were provided by Sigma-Aldrich (St. Louis, MO, USA).
Isolation and culture of human ADSCs
The samples of subcutaneous adipose tissue were harvested from three people aged 30–35 years who underwent scheduled liposuction. The hADSC was obtained from human adipose tissue with ethical approval from the health research Ethics Committee of Isfahan university of medical sciences. The adipose tissue was isolated from human subcutaneous adipose tissue by enzymatic digestion of 1 mg collagenase IA per 1 g adipose tissue at 37°C for 30 min. Later, a complete cell culture medium [DMEM medium supplemented with 1% penicillin/streptomycin (Gibco) and 10% fetal bovine serum] was added to the cell suspension to neutralize the activity of the enzyme. Then, cell suspension was centrifuged for 7 min at 177 g and the supernatant was removed along with adipocytes. Finally, the resulting cellular pellet was cultured in a culture medium at 5% CO2, 37˚C conditions [
3]. Additional cells were removed by changing the medium after 24 hr.
Experimental design and in vitro chondrogenic differentiation
After isolating hADSCs and three passages, stem cells were subdivided into four subgroups: 1- PLGA+hADSCs+chondrogenic medium, 2- PLGA/Fibrin+hADSCs+chondrogenic medium, and 3- PLGA/Fibrin/HA+hADSCs+chondrogenic medium. Triplicate of each subgroup was prepared and cultured for 14 days [
3]. At the end of the treatment period, cells were isolated from all scaffolds. Cell viability was assessed by MTT assay and gene expression [(
SOX-9,
Aggrecan (
AGG),
type II collagen (
Col II),
type X collagen (
Col X)] was evaluated by real-time polymerase chain reaction (PCR).
Harvested hADSCs from the 3
rd passage, were resuspended in chondrogenic medium (high glucose Dulbecco's modified Eagle medium, supplemented with 1% ITS +Premix, 40 μg/ml proline, 100 nM dexamethasone, 50 μg/ml ascorbate -2-phosphate, 100 μg sodium pyruvate, 1% penicillin-streptomycin, linoleic acid 5 μg/ml and bovine serum albumin 0.5 mg/ml) [
8]. For loading the cells on scaffolds (3 mm in height and 7 mm in diameter), 2×10
6 cells in 200 ml of medium were loaded on each scaffold and then plate incubated in %5 CO2 and 37ºC for 2 hrs. Finally, 500 μml of the chondrogenic medium was added to each well. The half amount of medium was changed twice a week.
Fabrication of the PLGA scaffold
3-D PLGA (48/52 wt % poly (lactide)/poly (glycolide) scaffold was prepared via solvent casting and particulate leaching methods. Briefly, polymer/solvent solution (8% w/v concentration of PLGA in methylene chloride) was cast in cylindrical silicon molds (7×3 mm) in which sodium chloride (approximately 180 micrometers in particle size) was filled with salt particles. Then the scaffolds were placed at room temperature for 12 hours to dry. To leach out NaCl particles, the samples were immersed in deionized water for 3rd in 2 days to produce highly porous structure [
9].
Fibrinogen and thrombin preparation
Fibrinogen and Fresh frozen plasma (FFP) were achieved from the Isfahan Blood Transfusion Organization. The FFP (15ml) was mixed with gluconate calcium (10 ml) for 3 hr at 37°. It was incubated and then centrifuged for 10 min at 565 g. The supernatant was collected as a thrombin.
Cell viability assay by MTT (3-(4,5-dimethyl) thiazol-2-yl-2,5-dimethyltetrazolium bromide)
The viability and proliferation of differentiated cells from PLGA/Fibrin, PLGA/HA and PLGA/Fibrin/HA scaffolds were estimated with MTT assay. The chondrogenic medium was removed and the MTT solution (4mg/ml) was added to each well and incubated at 37˚C for 4hr. Then the medium was omitted, and intracellular formazan was solubilized by adding 400 μl of dimethyl sulfoxide. The absorbance of each well was read at 570 nm by enzyme-linked immunosorbent assay plate reader (Hiperion MPR4, Germany) [
10]. This analysis was repeated in triplicate.
Real-time polymerase chain reaction assay
At first, PLGA/Fibrin, PLGA/HA and PLGA/Fibrin/HA scaffolds, on day 14, were washed with PBS and for digestion, they were placed in 0.15 mM NaCl (Merck) and 1.5% 55 mM citrate sodium (Sharlau). The resultant solution was centrifuged at 1200 rpm for 10 min and the derived cell pellet was used for the extraction of RNA with RNeasy mini kit (Qiagen). For leasing cells, a mixture of 10 microliters of 2-mercaptoethanol (Sigma) and 990 microliters of Invitrogen was first kept at room temperature for 5 minutes. In the subsequent phase, 200 μl of chloroform was added for 2–3 min at room temperature, shaken extremely, and centrifuged at C.4° at 12000 g for 15 min. The supernatant aqueous phase was transported into a 1.5 ml microtube and the same volume of ethanol 70% was added and then mixed. The resultant solution was transferred to columns in the kit and the rest of the instruction was carried out according to the kit protocol in which RNase free DNase set (Qiagen) was applied for elimination of possible DNA contamination. The extent of derived RNA was measured by spectrophotometer (Biophotometer, Eppendorf) at 260/280 nm wavelength. Reverse transcription for cDNA, 100 ng RNA was used by recruitment of RevertAid
TM First Strand cDNA Synthesis Kit (Fermentas) according to manufacturer’s protocol. Relative quantification of the expression
AGG,
Col II,
SOX9, and
Col X was measured, using Maxima SYBR® Green/Roxq PCR Master Mix 2X (Fermentas), with GAPDH primer as an internal control. Real-time PCR reactions were performed using the Comparative Ct (∆∆Ct) method. The reactions were conducted in 20 µl with, 10 µl SYBR® Green, 7.5 µl H
2o, 0.25 µM forward and reverse primers and 1.5 µl cDNA as planned by Step One Plus Real-Time PCR system (Applied Biosystem): primary denaturation in 95° for 10 min, denaturation in 95° for 15 secs, Annealing and Extension in 60° for 1 min –the whole process was done 40 cycles- and finally melt curve (increment 0.3°C, 60°C→95°C) was depicted. All experiments were performed in triplicates for each specimen [
11]. The applied primers for Real-Time PCR are indicated in table 1.
Statistical analysis
All statistical analyses were performed using SPSS software for windows (Statistical Package for the Social Sciences, version 19, SPSS Inc., Chicago, Illinois, USA). Data were expressed as mean±SEM. To verify the normal distribution of the data, the Kolmogorov-Smirnov test was used. Data were then analyzed using one-way ANOVA and LSD post hoc tests. Values with P<0.05 were considered statistically significant.
Results
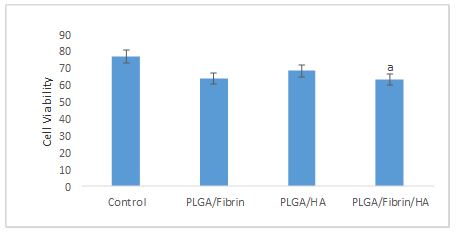
MTT assay
The MTT results indicated the viability being significantly higher in the control group (77±4.61), than the PLGA/Fibrin/HA group (63.34±2.96) (Fig. 1).
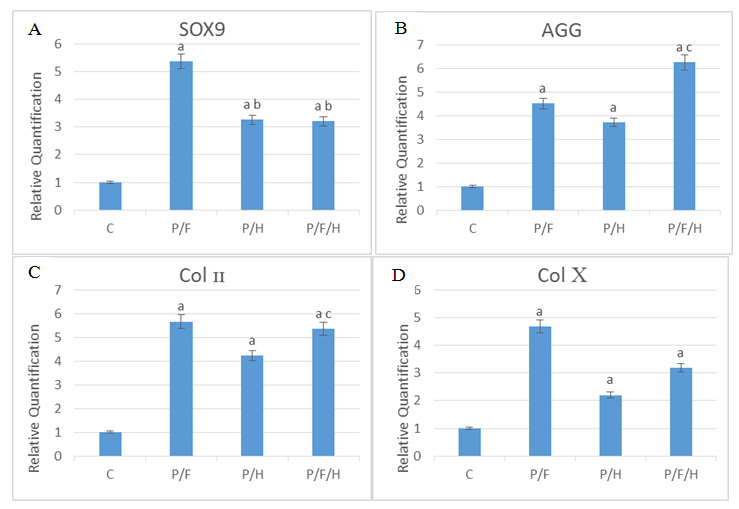
Real-time PCR analysis
The results of the real-time PCR indicated
SOX9,
AGG and
Col X gene expression in the control groups being significantly lower than the other groups (P≤0.05). It also revealed
Col II gene expression in the control group being significantly lower than the PLGA/Fibrin and PLGA/Fibrin/HA groups (P≤0.05). The results demonstrated
SOX9 gene expression in the PLGA/Fibrin (5.38±0.4) group being significantly higher than the PLGA/HA (3.26±0.47) and PLGA/Fibrin/HA (3.21±63) groups. However, no significant differences were found between PLGA/HA and PLGA/Fibrin/HA groups (P≤0.05). The results of the real-time PCR showed
AGG gene expression in the PLGA/HA (3.72±0.32) group being significantly lower than the PLGA/Fibrin/HA (6.26±0.98) group (P≤0.05). The
Col X gene expression of cells in PLGA/HA (2.2±0.17) and PLGA/Fibrin/HA (3.18±.65) groups significantly decreased in comparison with the PLGA/Fibrin (4.68±0.32) group (P≤0.05) (Fig. 2).
Table 1. Gene sequence of primers
| Primer sequences (forward and reverse) |
Gene |
| CTGGTGATGATGGTGAAG |
collagen II-F |
| CCTGGATAACCTCTGTGA |
collagen II –R |
| TTCAGCAGCCAATAAGTG |
sox-9 –F |
| TTCAGCAGCCAATAAGTG |
sox-9 –R |
| AGAATCCATCTGAGAATATGC |
collagen x –F |
| CCTCTTACTGCTATACCTTTAC |
collagen x – R |
| GTGGGACTGAAGTTCTTG |
Aggrecan-F |
| GTTGTCATGGTCTGAAGTT |
Aggrecan-R |
| AAGCTCATTTCCTGGTATG |
GAPDH-F |
| CTTCCTCTTGTGCTCTTG |
GAPDH-R |
Fig. 1. Comparison of MTT assay results between control, PLGA, PLGA/Fibrin, PLGA/HA and PLGA/Fibrin/HA groups.
a: Significant compared to the Control group (p≤0.05)
Fig. 2. The results of SOX9 (A), AGG (B), Col II (C) and Col X (D) gene expression in the control, PLGA, PLGA/Fibrin, PLGA/HA and PLGA/Fibrin/HA groups. C=Control; P/F=PLGA/Fibrin; P/H=PLGA/ Hyaluronic Acid; P/F/H=PLGA/Fibrin/ Hyaluronic Acid
a: Significant compared to the Control group
b: Significant compared to PLGA/Fibrin group
c: Significant compared to PLGA/HA group (P≤0.05)
Discussion
Although bone marrow-derived mesenchymal stem cells (BM-MSCs) have been the focus of attention for cartilage repair, human adipose-derived stem cells might be a more suitable alternative for cartilage cell therapy. Compared to BM-MSC, they are easier to harvest at elevated frequencies and more permanent in long-term cultivation [
12]. Tissue engineering is specified as an interdisciplinary field that has been applied ranging from life sciences and engineering to the progression of biological possible availability both functionally and physically reconstructing damaged tissue. [
13]. Porous scaffolds made of biodegradable and biocompatible polymers play an important role in tissue engineering and regenerative medicine. Among diverse types of scaffolding matrices, PLGA is significant and very favorite because of its modifiable demolition rate, processing, approvable mechanical properties, etc. [
14]. One study indicated that MSCs seeded on PLGA can differentiate into chondrocytes without cytokine stimulation [
15]. We used hybrid scaffolds in this study, because PLGA mesh acts as the skeleton of the scaffold and Fibrin, and HA provides accommodation for the cells.
HA [
16] and Fibrin [
3] available ECM for cross-cell-to-cell cross-linking is very significant for the accumulation of cells before precartilagous compaction. SOX9, but then, is one of the main markers to be expressed in precartilage-dense cells. in addition, SOX9 is a transcription factor needed for the expression of Col II and AGG, which is expressed throughout cell density to differentiation into condrocytes. [
17]. In this study, we demonstrated that the PLGA with Fibrin and HA induces cell promotes and aggregation the expressions of the cartilage-specific gene including
SOX9,
Col II, and
AGG in hADSCs.
Recently, several natural and synthetic formatives have been extensively used to construct scaffolding for tissue engineering objectives. Multiple studies have effectively cultured cartilage cells, reconstituted tissue-engineered cartilage, and transplanted engineered cartilage. Accordingly, compatible scaffolds that bear potential for cell proliferation and matrix aggregation have been studied. The advantages of artificial scaffolding include high repeatability, controllable destruction rate, and easy construction in certain shapes while natural scaffolding usually mimics the main elements of natural texture [
18].
Concerning the cell viability, our results are contrary to those of Hashemibeni's et al study; they concluded that fibrin promotes the viability and proliferation of the hADSCs in fibrin scaffold compared to other scaffolds [
3]. In our study, declining proliferation in the PLGA/Fibrin/HA group may be due to a decrease in proliferation and increased cell differentiation in the PLGA/Fibrin/HA group compared to the control group.
In the last years, HA has been shown to protect both MSC and cartilage cells as well as encourage their proliferation in laboratory conditions
(in vitro) [
19]. HA can reinforce the preservation of the cellulose phenotype of chondrocyte osteoarthritis and at the same time enhance the generation of glucosamine molecules in each cell [
20]. Additionally, study by zhu et al. The success of HA-based scaffolds in combination with calcium I or other glycosaminoglycans is permitted. These changes increase the differentiation of mesenchymal stem cells into a chronic phenotype and reduce the expression of genes associated with hypertrophy with the results confirmed in vitro and in vivo conditions using naked mouse models [
19]. Gene expression of
Col X has been described to be the marker of hypertrophic chondrocytes [
21]. In our study, HA also reduced
Col X, which is one of the markers of hypertrophy. HA is a composition of the extracellular matrix (ECM) that can provide cells with a 3-D microenvironment the same as natural conditions. Liu et al. made an HA to encapsulate the MSCs administrating hydrosulfonic acid, HA and bisacrylamide PEG to repair the full-thickness cartilage defect of the femoral trochlea of rabbit [
22]. The sulfated HA exhibits significantly enhanced chondrogenesis, gradual degradation and suppresses the hypertrophy of encapsulated MSCs both
in vitro and
in vivo. also, It can inhibit cartilage hypertrophy and abrasion in the osteoarthritic joints of the animal [
23]. in the present study, HA also increased the expression of specific cartilage genes (
Sox9,
Col II,
AGG). Moreover, the conjugation of N-cadherin peptides onto HA stimulates both cartilage-specific matrix production and early chondrogenesis of MSCs, Similarly, it was shown in this study that HA in PLGA/HA and PLGA/Fibrin/HA upregulates the expression levels of these genes significantly [
24]. Lysine methyl ester modified HA scaffolding cross-linking exhibits morphologically, mechanically, water absorption and adequate stability to preserve the comparative identity of the chondrocytes [
25]. Dragoo et al. showed that fibrin is the most suitable scaffold for the retrieval of rabbit articular cartilage [
26]. Moreover, Girandon et al. showed that ADSCs can reproduce and survive in a fibrin scaffold [
27]. Human source fibrin was manually made and used in this study. Although fibrin has unique viscoelastic properties, it is weak in terms of sustainability and also degrades rapidly. Cakmak et al. transplanted chondrocytes with injectable fibrin and established that this method can achieve cartilage tissue renewal. They injected chondrocyte-fibrin into the forehead and interocular regions of rabbits and after 8 weeks they showed neocartilage reconstruction [
28]. Lee et al. stated that injecting synovium-derived MSCs with injectable collagen/HA/fibrinogen composite gel into the rabbit model regenerates and repaires osteochondral defect in knee. Through histological analysis, they showed that GAG and Col II are accumulated in the ECM. Further, a hyaline-like cartilage was generated. The defects had been repaired with hyaline-like cartilage tissue after 24 weeks [
29]. Regarding
AGG and
Col II, evidence showed that in the PLGA/Fibrin/HA group compared to control and PLGA/HA is recognized significantly higher in expression. Although there were remarkable
SOX9 differences in PLGA/Fibrin hybrid scaffold with the PLGA/Fibrin and PLGA/Fibrin/HA groups, there was no significant variation in the semi-quantitative gene expression assessment for
Col II,
AGG, and
Col X.
Conclusion
In this study, compariotion of synthetic and natural matrices were applied as scaffolds. PLGA/fibrin and PLGA/HA scaffolds, especially PLGA/Fibrin/HA hybrid scaffold promote early
in vitro chondrogenesis of hADSCs proven using chondrogenic differentiation markers, including
Col II,
AGG, and
SOX9. This study suggests that PLGA/Fibrin/HA hybrid scaffold may serve as a potential structural basis for the
in vitro tissue-engineered articular cartilage construct.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
The authors thank all their colleagues at Isfahan University of Medical Sciences, Isfahan, Iran for their cooperation.
References
- Paduszyński P, Aleksander-Konert E, Zajdel A, Wilczok A, Jelonek K, Witek A, et al. Changes in expression of cartilaginous genes during chondrogenesis of Wharton’s jelly mesenchymal stem cells on three-dimensional biodegradable poly (L-lactide-co-glycolide) scaffolds. Cell mol biol lett. 2016; 21(1): 14.
- Makadia HK, Siegel SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers. 2011; 3(3): 1377-397.
- Hashemibeni B, Valiani A, Esmaeli M, Kazemi M, Aliakbari M, Iranpour FG. Comparison of the efficacy of piascledine and transforming growth factor β1 on chondrogenic differentiation of human adipose-derived stem cells in fibrin and fibrin-alginate scaffolds. Iran J Basic Med Sci. 2018; 21(2): 212.
- Snyder TN, Madhavan K, Intrator M, Dregalla RC, Park D. A fibrin/hyaluronic acid hydrogel for the delivery of mesenchymal stem cells and potential for articular cartilage repair. J Biologic Eng. 2014; 8(1): 10.
- Chung C, Burdick JA. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng Part A. 2008; 15(2): 243-54.
- LeBoeuf RD, Raja R, Fuller G, Weigel P. Human fibrinogen specifically binds hyaluronic acid. J Biologic Chem. 1986; 261(27): 12586-2592.
- Mazini L, Rochette L, Amine M, Malka G. Regenerative capacity of adipose derived stem cells (ADSCs), comparison with mesenchymal stem cells (MSCs). Int J Mol Sci. 2019; 20(10): 2523.
- Hashemibeni B, Razavi S, Esfandiary E, Karbasi S, Mardani M, Nasresfahani M. Induction of chondrogenic differentiation of human adipose-derived stem cells with TGF-β3 in pellet culture system. Iran J Basic Med Sci. 2008; 11(1): 10-7.
- Tavakoli E, Mehdikhani-Nahrkhalaji M, Hashemi-Beni B, Zargar-Kharazi A, Kharaziha M. Preparation, characterization and mechanical assessment of poly (lactide-co-glycolide)/ hyaluronic acid/fibrin/bioactive glass nano-composite scaffolds for cartilage tissue engineering applications. Procedia Mater Sci. 2015; 11: 124-30.
- Hashemibeni B, Pourentezari M, Valiani A, Zamani M, Mardani M. Effect of icariin on the chondrogenesis of human adipose derived stem cells on poly (lactic-co-glycolic) acid/fibrin composite scaffold. Int J Adv Biotech Res. 2017; 8(2): 595-605.
- Mardani M, Hashemibeni B, Ansar MM, Esfahani SHZ, Kazemi M, Goharian V, et al. Comparison between chondrogenic markers of differentiated chondrocytes from adipose derived stem cells and articular chondrocytes in vitro. Iran J Basic Med Sci. 2013; 16(6): 763.
- D'andrea F, De Francesco F, Ferraro GA, Desiderio V, Tirino V, De Rosa A, et al. Large-scale production of human adipose tissue from stem cells: a new tool for regenerative medicine and tissue banking. Tissue Eng Part C: Methods. 2008; 14(3): 233-42.
- Dai W, Kawazoe N, Lin X, Dong J, Chen G. The influence of structural design of PLGA/collagen hybrid scaffolds in cartilage tissue engineering. Biomaterials. 2010; 31(8): 2141-152.
- Pan Z, Ding J. Poly (lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine. Interface Focus 2012; 2(3): 366-77.
- Sonomoto K, Yamaoka K, Kaneko H, Yamagata K, Sakata K, Zhang X, et al. Spontaneous differentiation of human mesenchymal stem cells on poly-lactic-co-glycolic acid nano-fiber scaffold. PLoS One. 2016; 11(4): e0153231.
- Knudson CB. Hyaluronan and CD44: strategic players for cell-matrix interactions during chondrogenesis and matrix assembly. Birth Defect Res Part C. 2003; 69(2): 174-96.
- Kawakami Y, Rodriguez-León J, Belmonte JCI. The role of TGFβs and Sox9 during limb chondrogenesis. Curr Opinion Cell Biol. 2006; 18(6): 723-9.
- Davies RL, Kuiper NJ. Regenerative medicine: a review of the evolution of autologous chondrocyte implantation (ACI) therapy. Bioengineering. 2019; 6(1): 22.
- Zhu M, Feng Q, Sun Y, Li G, Bian L.
Effect of cartilaginous matrix components
on the chondrogenesis and hypertrophy of mesenchymal stem cells in hyaluronic acid hydrogels. Journal of Biomedical Materials Research Part B: App Biomater. 2017; 105(8): 2292-300.
- Bauer C, Berger M, Baumgartner RR, Höller S, Zwickl H, Niculescu-Morzsa E, et al. A novel cross-linked hyaluronic acid porous scaffold
for cartilage repair: an in vitro study with osteoarthritic chondrocytes. Cartilage. 2016; 7(3): 265-73.
- Wu SC, Chang JK, Wang CK, Wang GJ, Ho ML. Enhancement of chondrogenesis of human adipose derived stem cells in a hyaluronan-enriched microenvironment. Biomaterials. 2010; 31(4): 631-40.
- Liu Y, Shu XZ, Prestwich GD. Osteochondral defect repair with autologous bone marrow-derived mesenchymal stem cells in an injectable, in situ, cross-linked synthetic extracellular matrix. Tissue Eng. 2006; 12(12): 3405-416.
- Feng Q, Lin S, Zhang K, Dong C, Wu T, Huang H, et al. Sulfated hyaluronic acid hydrogels with retarded degradation and enhanced growth factor retention promote hMSC chondrogenesis and articular cartilage integrity with reduced hypertrophy. Acta Biomaterialia. 2017; 53: 329-42.
- Bian L, Guvendiren M, Mauck RL, Burdick JA. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proceed Nation Acad Sci. 2013; 110(25): 10117-10122.
- La Gatta A, Ricci G, Stellavato A, Cammarota M, Filosa R, Papa A, et al. Hyaluronan hydrogels with a low degree of modification as scaffolds for cartilage engineering. Int J Biologic Macromol. 2017; 103: 978-89.
- Dragoo JL, Carlson G, McCormick F, Khan-Farooqi H, Zhu M, Zuk PA, et al. Healing full-thickness cartilage defects using adipose-derived stem cells. Tissue Eng. 2007; 13(7): 1615-621.
- Girandon L, Kregar-Velikonja N, Bozikov K, Barlic A. In vitro models for adipose tissue engineering with adipose-derived stem cells using different scaffolds of natural origin. Folia Biol (Praha). 2011; 57(2): 47-56.
- Cakmak O, Babakurban ST, Akkuzu HG, Bilgi S, Ovalı E, Kongur M, et al. Injectable tissue‐engineered cartilage using commercially available fibrin glue. Laryngoscope 2013; 123(12): 2986-92.
- Lee JC, Lee SY, Min HJ, Han SA, Jang J, Lee S, et al. Synovium-derived mesenchymal stem cells encapsulated in a novel injectable gel can repair osteochondral defects in a rabbit model. Tissue Eng Part A. 2012; 18(19-20): 2173-86.









































 , Batool Hashemibeni
, Batool Hashemibeni 

 , Majid Pourentezari *
, Majid Pourentezari * 

 , Ali Valiani
, Ali Valiani 

 , Mohammad Mardani
, Mohammad Mardani 

 , Mohammad Zamani Rarani
, Mohammad Zamani Rarani 

 , Hamidreza Pourentezari
, Hamidreza Pourentezari