Wed, Feb 4, 2026
[Archive]
Volume 7, Issue 4 (November 2020)
IJML 2020, 7(4): 300-311 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Borooni S, Nourbakhsh F, Tajbakhsh E, Behshood P. The Effects of Aqueous Extract of Boswellia Serrata on Memory Impairment Induced by Lipopolysaccharide. IJML 2020; 7 (4) :300-311
URL: http://ijml.ssu.ac.ir/article-1-352-en.html
URL: http://ijml.ssu.ac.ir/article-1-352-en.html
Medical Toxicology Research Centre, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
Full-Text [PDF 580 kb]
(911 Downloads)
| Abstract (HTML) (1892 Views)
Boswellia serrata (B. serrata), commonly known as frankincense or olibanum-tree, is a tree in the Burseraceae family [1]. They are native to Arab countries and India. This plant has long been noticed as an herbal compound with a beneficial role for the treatment of inflammatory diseases such as arthritis, chronic colitis, as well as healing of wounds and improvement of the female endocrine system (the study of the co-administration of B. serrata and Dracocephalum on the elderly memory) [2, 3]. The anti-inflammatory effects of olibanum are attributed to terpenoid acids, particularly B. serrata, and other terrenes derivatives [4, 5]. The extensive spread experiments conducted to investigate B. serrata’s anti-inflammatory mechanism have demonstrated that they are selective inhibitors of 5-lipoxygenase, preventing leukotriene synthesis [6, 7]. Also, another inhibitory effect of B. serrata has been observed for the biosynthesis of glycosaminoglycan. Some evidence obtained from animal studies indicates the advantageous effects of B. serrata on memory function [8, 9]. According to findings, B. serrata can play a positive role in brain development, formation of axons and dendrites, and better neuronal communications. Lipopoly-saccharide (LPS) is a gram-negative bacteria-derived endotoxin, which induces the production of inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), Interleukin (IL)-1 beta, and IL-6 followed by impairment in synaptic plasticity, learning process, and memory [10,11]. Various researches indicated that B. serrata reduced anxiety symptoms. Besides, B. serrata can reduce the levels of inflammatory cytokines through the effect of the nuclear factor kappa enhancer binding protein (NF-κB) pathway that led to inhibition of hyperactivity and anxiety [12, 13]. Similarly, Sayed et al. indicated that frankincense has an anti-inflammatory effect. Using it triggered to diminish the level of IL-6. Also, in a behavioral test, an open arm’s presence in an elevated plus-maze increased [14]. The present study was aimed to investigate the effects of aqueous extract of B. serrata on LPS-induced memory impairment.
Materials and Methods
Animals and drugs
In this experiment, 60 male Wistar rats weighing between 200 and 250 g were prepared. Animals were kept under controlled situations, including temperature at 22±2˚C and lighting conditions with 12-h light: dark cycle. Additional food and water were available for each rat [15]. All experiments were admired by the Research Committee of Nourdanesh Institute of Higher Education, Meymeh, Iran. The oleo-gum resin of B. serrata was taken, and then 100 g of powder was added to 400 ml of ethyl acetate. Subsequently, it was shacked for 48 hours until the particles were completely dissolved. After filtration, we used the rotary equipment to remove the solvent. The residues were then maintained at 20 °C until use. The percent yield of the procedure was about 30% [15].
Groups and treatments
In this research, animal were divided into 6 groups (n=10). Group 1: control group saline – diluted Dimethyl sulfoxide (1mg/kg); group 2: LPS (1mg/kg) negative control group; group 3: LPS (1mg/kg)+aqueous extract (0.5 mg/kg); group 4: LPS (1mg/kg)+aqueous extract (1 mg/kg); group 5: aqueous extract (5 mg/kg) and group 6: Vitamin E 5 mg/kg+LPS (1mg/kg) were treated groups. In relation to conducting behavioral tests, the day after the injection the rats were subjected to behavioral tests such as Morris water maze (MWM) test, open-field and shuttle box one day after the injection.
Behavioral study
MWM apparatus and procedures
MWM test is suitable for the analysis of the spatial memory and learning of rats. A circular pot carried out the test with a diameter of 136 cm and a height of 30 cm, which is supposedly divided into four quadrants, north, south, right, and left [16]. At the center of the Northwest quadrant, a platform with a height of 28 cm and diameter of 10 cm is placed and the pot reaches a height of 1.5 cm above the surface of the platform with water with temperature of 23-25˚C. MWM testing took five days according to protocols [17].
Open-field test
This test is designed to test in vitro spatial memory in the rat. In this test, the animal is placed in a box environment without causing pleasant or unpleasant behavior. This box structurally consists of white wood, had a floor of 100×100 cm divided by red lines into 25 equal units of 20×20 cm and 50 cm high. A camera is mounted on top of the box to monitor the animal’s behavior closely. According to test protocols, the animal’s behavior is examined for its presence in different areas of the box (in the middle or around) according to test protocols [18,19].
Biochemical assessment
After learning and memory tests, animals were sacrificed, and the hippocampus tissues were dissected and kept at -80˚C for biochemical evaluations. The hippocampus samples were then homogenized in volumes of 9 g/L ice-cold normal saline (1:9 w/v). The supernatant was collected after centrifugation homogenates at 4000 rpm/min for 10 min at 40˚C. The supernatants were used for the evaluation of activities of malondialdehyde (MDA), glutathione (GSH), using a spectrophotometer (Jenway 6105 UV/Vis, UK); following the protocols provided with the assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, PR China). IL-6 levels were measured using a specific protocol for rats inside the kit (bioscience Co., San Diego, CA, USA) [20].
Histopathological study
At the end of the injection period and behavioral tests, the animals were killed, and the brains were maintained in 10% formalin. After five days of formalin storage, cannulated sections were examined for hemotoxin eosin and toluidine blue staining. The samples were fixed with ethanol and dried with xylene after leaving formalin. Finally, the samples were embedded in paraffin for tissue sections and staining. The paraffin blocks were cut from1–3 mm posterior from bregma by microtome (Leica Biosystems, Milan, Italy). Different sections of each brain sample were prepared at 2 μm intervals and prepared for staining with hemotoxin-eosin and toluidine blue. Optical microscopy (40 x) was used for microscopic examination (Olympus BX51, Japan). Images were captured digitally from different hippocampus subfields, including CA1 of both hemispheres [21].
Western blotting
For western blot analysis, the hippocampus was dissected from the brains of the rats. Tissues were homogenized at 4˚C in the lysis buffer. Then, lysates were sonicated on ice using a probe sonicator (UP100H, Germany). After centrifugation at 10000 g for 10 min at 4˚C, supernatants were collected and transferred to clean microtubes, and the protein concentrations were determined using a Bio-Rad protein assay kit.
All protocols were performed according to the reference and kit [22]. The primary antibodies were polyclonal BAX (Cell signaling, cat# 2772), monoclonal BCL2 (Cell signaling, cat# 2870), monoclonal Caspase 3-cleaved (Cell signaling, cat#9664), monoclonal Caspase 9-cleaved (Abcam, cat#7237) were considered to be involved in cell death and also apoptosis pathway [23, 24].
Statistical analysis
The time and distance data in MWM in five days were compared using repeated-measures analysis of variance (ANOVA) with Tukey’s post-hoc test. The biochemical analysis data, probe day data, and shuttle box were reported by one-way ANOVA followed by Tukey’s post-test. The differences level among groups were considered statistically significant when p<0.05. All data were presented as means±standard error of the mean (SEM).
Results
MWM results
LPS administration for five days increased elapsed time and traveled path to find the platform compared to the control group (p<0.05 to p<0.001). The time and distance mentioned were significantly reduced after the injection of aqueous extract of B. serrata 0.5 mg/kg and 1 mg/kg compared to the LPS group (p<0.01 to p<0.001) (Fig. 1 and 2). After the removal of the platform on probe day, the animals in the LPS-receiving group also spent less time and distance in the target quadrant (p<0.001), whereas the results in the B. serrata groups were opposite (P<0.001) (Fig. 3).
Open-field test results
On the first day, LPS alone or in combination with aqueous extract of B. serrata did not show a significant effect on total locomotion (Fig. 3A), and on the last day, LPS (1 mg/kg) reduced the peripheral, central and total locomotion’s compared to control group (p<0.001). Aqueous extract of B. serrata (0.5 mg/kg and 1 mg/kg) and Vitamin E plus LPS significantly increased the peripheral and total locomotion (p<0.001) (Fig. 3B). Also, treatment with B. serrata (1 mg/kg) significantly increased central locomotion compared to melatonin treated rats (p<0.001).
Effect of B. serrata on lipid peroxidation
As shown in Fig. 4A, exposure to LPS significantly increased the MDA level compared to control (p<0.001). Treatment with B. serrata (0.5 and 1 mg/kg) and vitamin E reduced MDA content (p<0.001). In the Och treated group, GSH content was decreased in the hippocampus (p<0.001) (Fig. 4B). Treatment with B. serrata (0.5 and 1 mg/kg) and vitamin E significantly increased GSH content compared to LPS treated rats (p<0.001).
Effect of B. serrata on inflammatory markers
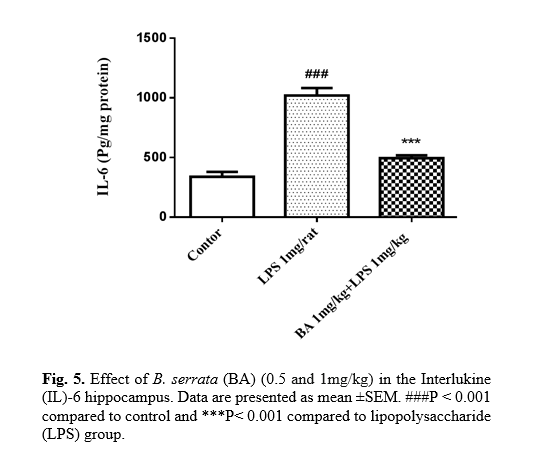
The role of inflammation in the pathogenesis of certain diseases, such as memory loss, has been documented. The results showed that the IL-6 level was increased in the hippocampus of LPS (1 mg/kg) treated rats compared to the control (P<0.001). As shown in Fig. 5, it was indicated that B. serrata (1 mg/kg) significantly decreased IL-6 level compared to the LPS group (p<0.001).
Effect of B. serrata on apoptotic factors (Bax/Bcl‑2, Caspase 3 and Caspase 9)
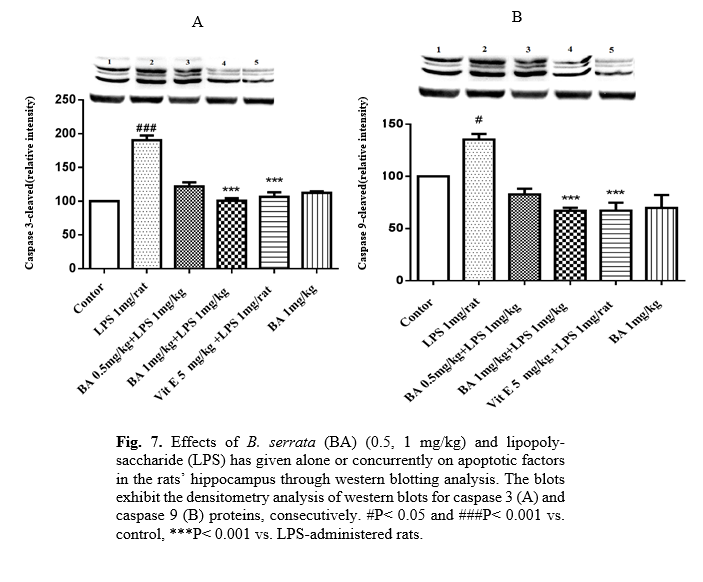
As indicated in Fig. 6, protein expression of Bax/Bcl2 was increased in the LPS group (p<0.001). Besides, the protein levels of cleaved caspases 3 and 9 were up-regulated by LPS. Co-treatment of LPS with B. serrata (1 mg/kg) or vitamin E significantly decreased the ratio of Bax/Bcl2 (p<0.001 and p<0.001, respectively). Furthermore, B. serrata (0.5 and 1 mg/kg) or vitamin E plus LPS inhibited the activation of caspases 3 and 9 (Fig. 7A, 7B).
Histology
LPS (1 mg/kg) reduced the number of degenerating neurons in the CA1 subfields (Fig. 8), compared to the control group. Also, B. serrata (0.5 and 1 mg/kg) increased the number of positive neurons in the CA1 subfields, p<0.01, and p<0.001) in comparison with the control group.
.png)
.png)
.png)
.png)
.png)

Full-Text: (2346 Views)
Introduction
Boswellia serrata (B. serrata), commonly known as frankincense or olibanum-tree, is a tree in the Burseraceae family [1]. They are native to Arab countries and India. This plant has long been noticed as an herbal compound with a beneficial role for the treatment of inflammatory diseases such as arthritis, chronic colitis, as well as healing of wounds and improvement of the female endocrine system (the study of the co-administration of B. serrata and Dracocephalum on the elderly memory) [2, 3]. The anti-inflammatory effects of olibanum are attributed to terpenoid acids, particularly B. serrata, and other terrenes derivatives [4, 5]. The extensive spread experiments conducted to investigate B. serrata’s anti-inflammatory mechanism have demonstrated that they are selective inhibitors of 5-lipoxygenase, preventing leukotriene synthesis [6, 7]. Also, another inhibitory effect of B. serrata has been observed for the biosynthesis of glycosaminoglycan. Some evidence obtained from animal studies indicates the advantageous effects of B. serrata on memory function [8, 9]. According to findings, B. serrata can play a positive role in brain development, formation of axons and dendrites, and better neuronal communications. Lipopoly-saccharide (LPS) is a gram-negative bacteria-derived endotoxin, which induces the production of inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), Interleukin (IL)-1 beta, and IL-6 followed by impairment in synaptic plasticity, learning process, and memory [10,11]. Various researches indicated that B. serrata reduced anxiety symptoms. Besides, B. serrata can reduce the levels of inflammatory cytokines through the effect of the nuclear factor kappa enhancer binding protein (NF-κB) pathway that led to inhibition of hyperactivity and anxiety [12, 13]. Similarly, Sayed et al. indicated that frankincense has an anti-inflammatory effect. Using it triggered to diminish the level of IL-6. Also, in a behavioral test, an open arm’s presence in an elevated plus-maze increased [14]. The present study was aimed to investigate the effects of aqueous extract of B. serrata on LPS-induced memory impairment.
Materials and Methods
Animals and drugs
In this experiment, 60 male Wistar rats weighing between 200 and 250 g were prepared. Animals were kept under controlled situations, including temperature at 22±2˚C and lighting conditions with 12-h light: dark cycle. Additional food and water were available for each rat [15]. All experiments were admired by the Research Committee of Nourdanesh Institute of Higher Education, Meymeh, Iran. The oleo-gum resin of B. serrata was taken, and then 100 g of powder was added to 400 ml of ethyl acetate. Subsequently, it was shacked for 48 hours until the particles were completely dissolved. After filtration, we used the rotary equipment to remove the solvent. The residues were then maintained at 20 °C until use. The percent yield of the procedure was about 30% [15].
Groups and treatments
In this research, animal were divided into 6 groups (n=10). Group 1: control group saline – diluted Dimethyl sulfoxide (1mg/kg); group 2: LPS (1mg/kg) negative control group; group 3: LPS (1mg/kg)+aqueous extract (0.5 mg/kg); group 4: LPS (1mg/kg)+aqueous extract (1 mg/kg); group 5: aqueous extract (5 mg/kg) and group 6: Vitamin E 5 mg/kg+LPS (1mg/kg) were treated groups. In relation to conducting behavioral tests, the day after the injection the rats were subjected to behavioral tests such as Morris water maze (MWM) test, open-field and shuttle box one day after the injection.
Behavioral study
MWM apparatus and procedures
MWM test is suitable for the analysis of the spatial memory and learning of rats. A circular pot carried out the test with a diameter of 136 cm and a height of 30 cm, which is supposedly divided into four quadrants, north, south, right, and left [16]. At the center of the Northwest quadrant, a platform with a height of 28 cm and diameter of 10 cm is placed and the pot reaches a height of 1.5 cm above the surface of the platform with water with temperature of 23-25˚C. MWM testing took five days according to protocols [17].
Open-field test
This test is designed to test in vitro spatial memory in the rat. In this test, the animal is placed in a box environment without causing pleasant or unpleasant behavior. This box structurally consists of white wood, had a floor of 100×100 cm divided by red lines into 25 equal units of 20×20 cm and 50 cm high. A camera is mounted on top of the box to monitor the animal’s behavior closely. According to test protocols, the animal’s behavior is examined for its presence in different areas of the box (in the middle or around) according to test protocols [18,19].
Biochemical assessment
After learning and memory tests, animals were sacrificed, and the hippocampus tissues were dissected and kept at -80˚C for biochemical evaluations. The hippocampus samples were then homogenized in volumes of 9 g/L ice-cold normal saline (1:9 w/v). The supernatant was collected after centrifugation homogenates at 4000 rpm/min for 10 min at 40˚C. The supernatants were used for the evaluation of activities of malondialdehyde (MDA), glutathione (GSH), using a spectrophotometer (Jenway 6105 UV/Vis, UK); following the protocols provided with the assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, PR China). IL-6 levels were measured using a specific protocol for rats inside the kit (bioscience Co., San Diego, CA, USA) [20].
Histopathological study
At the end of the injection period and behavioral tests, the animals were killed, and the brains were maintained in 10% formalin. After five days of formalin storage, cannulated sections were examined for hemotoxin eosin and toluidine blue staining. The samples were fixed with ethanol and dried with xylene after leaving formalin. Finally, the samples were embedded in paraffin for tissue sections and staining. The paraffin blocks were cut from1–3 mm posterior from bregma by microtome (Leica Biosystems, Milan, Italy). Different sections of each brain sample were prepared at 2 μm intervals and prepared for staining with hemotoxin-eosin and toluidine blue. Optical microscopy (40 x) was used for microscopic examination (Olympus BX51, Japan). Images were captured digitally from different hippocampus subfields, including CA1 of both hemispheres [21].
Western blotting
For western blot analysis, the hippocampus was dissected from the brains of the rats. Tissues were homogenized at 4˚C in the lysis buffer. Then, lysates were sonicated on ice using a probe sonicator (UP100H, Germany). After centrifugation at 10000 g for 10 min at 4˚C, supernatants were collected and transferred to clean microtubes, and the protein concentrations were determined using a Bio-Rad protein assay kit.
All protocols were performed according to the reference and kit [22]. The primary antibodies were polyclonal BAX (Cell signaling, cat# 2772), monoclonal BCL2 (Cell signaling, cat# 2870), monoclonal Caspase 3-cleaved (Cell signaling, cat#9664), monoclonal Caspase 9-cleaved (Abcam, cat#7237) were considered to be involved in cell death and also apoptosis pathway [23, 24].
Statistical analysis
The time and distance data in MWM in five days were compared using repeated-measures analysis of variance (ANOVA) with Tukey’s post-hoc test. The biochemical analysis data, probe day data, and shuttle box were reported by one-way ANOVA followed by Tukey’s post-test. The differences level among groups were considered statistically significant when p<0.05. All data were presented as means±standard error of the mean (SEM).
Results
MWM results
LPS administration for five days increased elapsed time and traveled path to find the platform compared to the control group (p<0.05 to p<0.001). The time and distance mentioned were significantly reduced after the injection of aqueous extract of B. serrata 0.5 mg/kg and 1 mg/kg compared to the LPS group (p<0.01 to p<0.001) (Fig. 1 and 2). After the removal of the platform on probe day, the animals in the LPS-receiving group also spent less time and distance in the target quadrant (p<0.001), whereas the results in the B. serrata groups were opposite (P<0.001) (Fig. 3).
Open-field test results
On the first day, LPS alone or in combination with aqueous extract of B. serrata did not show a significant effect on total locomotion (Fig. 3A), and on the last day, LPS (1 mg/kg) reduced the peripheral, central and total locomotion’s compared to control group (p<0.001). Aqueous extract of B. serrata (0.5 mg/kg and 1 mg/kg) and Vitamin E plus LPS significantly increased the peripheral and total locomotion (p<0.001) (Fig. 3B). Also, treatment with B. serrata (1 mg/kg) significantly increased central locomotion compared to melatonin treated rats (p<0.001).
Effect of B. serrata on lipid peroxidation
As shown in Fig. 4A, exposure to LPS significantly increased the MDA level compared to control (p<0.001). Treatment with B. serrata (0.5 and 1 mg/kg) and vitamin E reduced MDA content (p<0.001). In the Och treated group, GSH content was decreased in the hippocampus (p<0.001) (Fig. 4B). Treatment with B. serrata (0.5 and 1 mg/kg) and vitamin E significantly increased GSH content compared to LPS treated rats (p<0.001).
Effect of B. serrata on inflammatory markers
The role of inflammation in the pathogenesis of certain diseases, such as memory loss, has been documented. The results showed that the IL-6 level was increased in the hippocampus of LPS (1 mg/kg) treated rats compared to the control (P<0.001). As shown in Fig. 5, it was indicated that B. serrata (1 mg/kg) significantly decreased IL-6 level compared to the LPS group (p<0.001).
Effect of B. serrata on apoptotic factors (Bax/Bcl‑2, Caspase 3 and Caspase 9)
As indicated in Fig. 6, protein expression of Bax/Bcl2 was increased in the LPS group (p<0.001). Besides, the protein levels of cleaved caspases 3 and 9 were up-regulated by LPS. Co-treatment of LPS with B. serrata (1 mg/kg) or vitamin E significantly decreased the ratio of Bax/Bcl2 (p<0.001 and p<0.001, respectively). Furthermore, B. serrata (0.5 and 1 mg/kg) or vitamin E plus LPS inhibited the activation of caspases 3 and 9 (Fig. 7A, 7B).
Histology
LPS (1 mg/kg) reduced the number of degenerating neurons in the CA1 subfields (Fig. 8), compared to the control group. Also, B. serrata (0.5 and 1 mg/kg) increased the number of positive neurons in the CA1 subfields, p<0.01, and p<0.001) in comparison with the control group.
.png)
.png)
.png)
.png)

.png)


Discussion
The results of current research showed that, B. serrata (0.5 and 1 mg/kg) improve learning and memory impairment induced by LPS. According to previous studies, LPS as a cell wall of gram negative bacteria lead to learning and memory impairment through induction of neuroinflammation. A possible mechanism of LPS that suggested by our data that are coordinate with previous studies were an increase in inflammatory (IL-6) and oxidative stress (MDA) factors. As a result of such changes, LPS lead to learning and memory loss in behavioral tests.
B. serrata is a major component of olibanum which is a resin of B. serrata which is an indigenous Arab plant, East African and Indian, used for therapeutic purposes. Long-standing studies have suggested that olibanum has been used as a useful compound in treating neuroinflammation disease. These anti-inflammatory properties are linked to inhibition of the lipoxygenase, a key enzyme in leukotriene synthesis. Clinical therapeutic effects of frankincense on the treatment of stroke and decreasing edema have also been mentioned in previous studies. Administration of 500 mg/kg of frankincense in capsule form to patients with stroke every 6 hours for one-month results in restoring muscle strength in the left limbs [25]. Daily administration of 100 mg frankincense extract in lactating rats improved hippocampal function through increased dendritic fractions and increased hippocampal neuronal cell volume in neonates [26]. Dosage of 50 mg for 21 to 42 days consistently improved memory impairment due to streptozotocin injection. As delay time to enter to dark compartment increased and frequency of entry into the dark chamber decreased [27].
Based on Hosseini-Sharifabad et al., oral administration of olibanum due to the presence of boswellic acid at a daily dose of 100 mg/kg for four weeks to 24-month-old rats resulted in augmented growth of dendritic branches, increased volume of the hippocampal pyramidal layer, and reduce the speed of changes to dendrite win analysis. In their study, it was found that the resin of B.serrata improved spatial memory performance in the water maze test, which was attributed to the protective effect of boswellic acid against oxidative stress in the brain [28]. As a result of our finding, boswellic acid has beneficial effects on learning and memory loss induced by LPS in rats because this useful material of olibanum decrease delay time and distance to reach the escape platform in MWM. As well as in probe day, the rats spent more time and distance in the goal quarter. According to the data obtained from the plan, we propose that boswellic acid by blocking the production of inflammatory cytokines such as IL-6, as well as by strengthening the brain’s antioxidant system with increase the levels of superoxide dismutase, catalase, and total thiol groups. Also, boswellic acid weakened the oxidative system and prevented toxic metabolites like nitric oxide metabolite. This effective constituent of olibanum leads to increasing the level of factors affecting neurogenesis leading to improved harmful effects of LPS administration.
The results of current research showed that, B. serrata (0.5 and 1 mg/kg) improve learning and memory impairment induced by LPS. According to previous studies, LPS as a cell wall of gram negative bacteria lead to learning and memory impairment through induction of neuroinflammation. A possible mechanism of LPS that suggested by our data that are coordinate with previous studies were an increase in inflammatory (IL-6) and oxidative stress (MDA) factors. As a result of such changes, LPS lead to learning and memory loss in behavioral tests.
B. serrata is a major component of olibanum which is a resin of B. serrata which is an indigenous Arab plant, East African and Indian, used for therapeutic purposes. Long-standing studies have suggested that olibanum has been used as a useful compound in treating neuroinflammation disease. These anti-inflammatory properties are linked to inhibition of the lipoxygenase, a key enzyme in leukotriene synthesis. Clinical therapeutic effects of frankincense on the treatment of stroke and decreasing edema have also been mentioned in previous studies. Administration of 500 mg/kg of frankincense in capsule form to patients with stroke every 6 hours for one-month results in restoring muscle strength in the left limbs [25]. Daily administration of 100 mg frankincense extract in lactating rats improved hippocampal function through increased dendritic fractions and increased hippocampal neuronal cell volume in neonates [26]. Dosage of 50 mg for 21 to 42 days consistently improved memory impairment due to streptozotocin injection. As delay time to enter to dark compartment increased and frequency of entry into the dark chamber decreased [27].
Based on Hosseini-Sharifabad et al., oral administration of olibanum due to the presence of boswellic acid at a daily dose of 100 mg/kg for four weeks to 24-month-old rats resulted in augmented growth of dendritic branches, increased volume of the hippocampal pyramidal layer, and reduce the speed of changes to dendrite win analysis. In their study, it was found that the resin of B.serrata improved spatial memory performance in the water maze test, which was attributed to the protective effect of boswellic acid against oxidative stress in the brain [28]. As a result of our finding, boswellic acid has beneficial effects on learning and memory loss induced by LPS in rats because this useful material of olibanum decrease delay time and distance to reach the escape platform in MWM. As well as in probe day, the rats spent more time and distance in the goal quarter. According to the data obtained from the plan, we propose that boswellic acid by blocking the production of inflammatory cytokines such as IL-6, as well as by strengthening the brain’s antioxidant system with increase the levels of superoxide dismutase, catalase, and total thiol groups. Also, boswellic acid weakened the oxidative system and prevented toxic metabolites like nitric oxide metabolite. This effective constituent of olibanum leads to increasing the level of factors affecting neurogenesis leading to improved harmful effects of LPS administration.
In the study of Ebrahimpour et al., B. serrata [40, 80 and 160 mg/kg, i.p.) improve learning and memory in water maze task in trimethyltin-induced neuronal toxicity model through inhibition of acetylcholinesterase, increased glutathione levels, reduced malondialdehyde levels in the cerebral cortex [29]. Besides, boswellic acid can reduce apoptosis of hippocampus memory-related cells by blocking the production of free radicals [28]. After memory impairment induced by scopolamine, B.serrata extract due to its resin’s main composition, boswellic acid leads to suppressing the generation of leukotrienes and inflammatory cytokines, inhibition of 5-lipooxygenase activity and reduction of prostaglandin E2 formation and cerebral edema discount [9].
In parkinsonian rats, boswellic acid delays the processes associated with aging of the
brain with inhibition of expression of inflammatory cytokines like IL-6, IL-1, and TNF-α. Also, boswellic acid suppresses inducible nitric oxide synthesizes, and COX2 acts as a neuroprotection factor for improving motor dysfunction in Parkinson’s disease [30].
In parkinsonian rats, boswellic acid delays the processes associated with aging of the
brain with inhibition of expression of inflammatory cytokines like IL-6, IL-1, and TNF-α. Also, boswellic acid suppresses inducible nitric oxide synthesizes, and COX2 acts as a neuroprotection factor for improving motor dysfunction in Parkinson’s disease [30].
Sayed and collogues showed that administration of 3-acetyl-11-keto-β-boswellic acid (AKBA) 5 mg/kg for seven days showed anti-apoptotic, and anti-amyloidogenic effects in LPS-injected mice. Evidence suggests that the mechanism of the effect of AKBA is through decreased expression of brain-related genes like phosphorylated inhibitory protein for NF-κB, IκB-α (P-IκB-α), inflammatory microRNA-155, and reduced oxidative stress content as carbonyl protein content. In addition, AKBA caused an increase in the SOCS-1 expression level. This experiment also showed that AKBA could decrease apoptosis and amyloidogenesis and act as a therapeutic drug for relieving the symptoms of the neuroinflammatory disorder like Alzheimer [14].
Conclusion
The current study proposed that spatial learning and memory enhanced during the administration of two doses of B. serrata (5 and 10 mg/kg) in the LPS-treatment groups. This finding is in agreement with the results reported by the researcher. Results were reported by the researcher that high antioxidant agents.
Conflict of Interest
The authors declare that there is no conflict of interest.
Acknowledgments
The authors didn't declare any acknowledgments.
References
The current study proposed that spatial learning and memory enhanced during the administration of two doses of B. serrata (5 and 10 mg/kg) in the LPS-treatment groups. This finding is in agreement with the results reported by the researcher. Results were reported by the researcher that high antioxidant agents.
Conflict of Interest
The authors declare that there is no conflict of interest.
Acknowledgments
The authors didn't declare any acknowledgments.
References
- Safayhi H, Mack T, Sabieraj J, Anazodo MI, Subramanian LR, Ammon H. Boswellic acids: novel, specific, nonredox inhibitors of 5-lipoxygenase. J Pharmacol Exper Therapeut. 1992; 261(3): 1143-146.
- Safayhi H, Rall B, Sailer ER, Ammon HPT. Inhibition by boswellic acids of human leukocyte elastase. J Pharmacol Exper Therapeut. 1997; 281(1): 460-63.
- Poeckel D, Werz O. Boswellic acids: biological actions and molecular targets. Curr Med Chem. 2006; 13(28): 3359-369.
- Banno N, Akihisa T, Yasukawa K, Tokuda H, Tabata K, Nakamura Y, et al. Anti-inflammatory activities of the triterpene acids from the resin of Boswellia carteri. J Ethnopharmacol. 2006; 107(2): 249-53.
- Anthoni C, Laukoetter MG, Rijcken E, Vowinkel T, Mennigen R, Muller S, et al. Mechanisms underlying the anti-inflammatory actions of boswellic acid derivatives in experimental colitis. Am J Physiol-Gastrointest Liver Physiol. 2006; 290(6): 1131-137.
- Singh G, Atal C. Pharmacology of an extract of salai guggal ex-Boswellia serrata, a new non-steroidal anti-inflammatory agent. Agents Act. 1986; 18(3-4): 407-12.
- Singh S, Khajuria A, Taneja S, Johri R, Singh J, Qazi G. Boswellic acids: A leukotriene inhibitor also effective through topical application in inflammatory disorders. Phytomed. 2008; 15(6-7): 400-407.
- Mahmoudi A, Hosseini-Sharifabad A, Monsef-Esfahani HR, Yazdinejad AR, Khanavi M, Roghani A, et al. Evaluation of systemic administration of Boswellia papyrifera extracts on spatial memory retention in male rats. J Nat Med. 2011; 65(3-4): 519.
- Mahboubi M, Taghizadeh M, Talaei SA, Firozeh SMT, Rashidi AA, Tamtaji OR. Combined administration of Melissa officinalis and Boswellia serrata extracts in an animal model of memory. Iran J Psychiat Behavior Sci. 2016;10(3): 681-90.
- Tanaka S, Ide M, Shibutani T, Ohtaki H, Numazawa S, Shioda S, et al. Lipopolysaccharide‐induced microglial activation induces learning and memory deficits without neuronal cell deathin rats. J Neurosci Res. 2006; 83(4): 557-66.
- Bilbo SD, Biedenkapp JC, Der-Avakian A, Watkins LR, Rudy JW, Maier SF. Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. J Neurosci. 2005; 25(35): 8000-8009.
- Gupta I, Parihar A, Malhotra P, Singh G, Lüdtke R, Safayhi H, et al. Effects of Boswellia serrata gum resin in patients with ulcerative colitis. Euro J Med Res. 1997; 2(1): 37-43.
- Nourbakhsh F, Borooni S, Tajbakhsh E. The protective effect of silymarin on lipopolysac-charideinduced liver toxicity in male wistar rat. International Journal of Medical Laboratory 2019 ;6(4): 275-87.
- Sayed AS, Gomaa IEO, Bader M, Sayed ND. Role of 3-Acetyl-11-Keto-Beta-Boswellic acid in counteracting LPS-induced neuro inflammation via modulation of miRNA-155. Mol Neurobiol. 2018; 55(7): 5798-808.
- Tahamtan M, Allahtavakoli M, Abbasnejad M, Roohbakhsh A, Taghipour Z, Taghavi M, et al. Exercise preconditioning improves behavioral functions following transient cerebral ischemia induced by 4-vessel occlusion (4-VO) in rats. Archiv Iran Med. 2013; 16(12): 696-704.
- Hosseini M, Feizpour A, Rezaeipour M, Amani A, Saffarzadeh F, Farrokhi E. Chronic treatment by L-NAME differently affects morris water maze tasks in ovariectomized and naïve female rats. Basic Clin Neurosci. 2011; 2(4): 47-52.
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nature protocols 2006; 1(2): 848-52.
- Abbasi Y, Mousavizadeh K, Shabani R, Katebi M, Mehdizadeh M. Behavioral changes in combination therapy of ethanol and modafinil on rats focal cerebral ischemia. Basic Clin Neurosci. 2020; 11(3): 269-78.
- Rezvani ME, Roohbakhsh A, Mosaddegh MH, Esmailidehaj M, Khaloobagheri F, Esmaeili H. Anticonvulsant and depressant effects of aqueous extracts of Carum copticum seeds in male rats. Epilepsy Behavior. 2011; 22(2): 220-25.
- Piri H, Haghdoost-Yazdi H, Fraidouni N, Dargahi T, Yaghoubidoust M, Azadmehr A. The Anti-Parkinsonism effects of katp channel blockade in the 6-hydroxydopamine-induced animal model: the role of oxidative stress. Basic Clin Neurosci. 2017; 8(3): 183-90.
- Hussein HA, Moghimi A, Roohbakhsh A. Anticonvulsant and ameliorative effects of pioglitazone on cognitive deficits, inflammation and apoptosis in the hippocampus of rat pups exposed to febrile seizure. Iran J Basic Med Sci. 2019; 22(3): 267.
- Li W, Murai Y, Okada E, Matsui K, Hayashi S, Horie M, et al. Modified and simplified western blotting protocol: use of intermittent microwave irradiation (IMWI) and 5% skim milk to improve binding specificity. Pathol Int. 2002; 52(3): 234-38.
- Aboutaleb N, Shamsaei N, Rajabi H, Khaksari M, Erfani S, Nikbakht F, et al. Protection of hippocampal CA1 neurons against ischemia/ reperfusion injury by exercise preconditioning via modulation of Bax/Bcl-2 ratio and prevention of caspase-3 activation. Basic Clin Neurosci. 2016; 7(1): 21-9.
- Moghimi A. Protective effects of NUCB2 after cerebral ischemia via modulation of Bcl-2/Bax ratio and reducing GFAP expression. Basic Clin Neurosci. 2019; 10(5): 451-60.
- Jivad N, Rafieian-Kopaei M, Rezaei-Kheirabadi F, Khosravi S, Azizi M. A study of the clinical efficacy of frankincense in the acute phase of ischemic stroke. Adv Herbal Med. 2015; 1(2): 4-10.
- Hosseini-Sharifabad M, Esfandiari E. Effect of Boswellia serrata triana and planch. gum resin administration during lactation on morphology of pyramidal neurons in hippocampus of rat. Journal of Herbal Drugs 2011; 2(1): 45-52.
- Beheshti S, Aghaie R. Therapeutic effect of frankincense in a rat model of Alzheimer’s disease. Avicenna journal of phytomedicine 2016; 6(4): 468-74.
- Hosseini-sharifabad M, Esfandiari E. Effect of Boswellia serrata gum resin on the morphology of hippocampal CA1 pyramidal cells in aged rat. Anatom Sci Int. 2015; 90(1): 47-53.
- Ebrahimpour S, Fazeli M, Mehri S, Taherianfard M, Hosseinzadeh H. Boswellic acid improves cognitive function in a rat model through its antioxidant activity:-neuroprotective effect of boswellic acid. J Pharmacopunc. 2017; 20(1): 10-18.
- Ameen AM, Elkazaz AY, Mohammad HM, Barakat BM. Anti-inflammatory and neuro-protective activity of boswellic acids in rotenone parkinsonian rats. Canad J Physiol Pharmacol. 2017; 95(7): 819-29.
Type of Study: Research |
Subject:
Biochemistry
Received: 2020/03/14 | Accepted: 2020/11/18 | Published: 2020/11/30
Received: 2020/03/14 | Accepted: 2020/11/18 | Published: 2020/11/30
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







