Introduction
Skin is a one of the body’s extensive organ and important physical barrier amongst a creature and its surrounding [1]. This organ protects the human body against various harmful agents. The wound healing phases includes inflammation, hemostasis, differentiation, proliferation, and reformation. Hemostasis initiated before wound inflammation. Macrophages role in proliferation, matrix formation, and angiogenesis through secreting mediators comprises transforming growth factor beta, epithelial growth factor, insulin-like growth factor, and vascular endothelial growth factor has been demonstrated. Proliferation is another phase of wound healing [1-3]. Endothelial cells and fibroblasts are the preceding cell population that infiltrated into the healing wound site. The platelet-derived growth factor as a major chemotactic growth factor imbibes fibroblasts into the wound site. In this phase, proteoglycans and collagens are generating. Indeed, collagen type I and III are the important collagens engaged in the regeneration process [2, 3]. The ultimate stage of wound healing is scar formation that taken place in fibroblastic phase of wound healing [1, 2, 4].
Thermal wounds are continuously considers as one of the hazards that threats human health [5-7]. In burn, the skin integrity is losing. Indeed, in the second-and third-degree burns, the wound healing procedure is regularly a long-term. Consequently, evaluating new treatment options might be capable to accelerate the wound healing progression of burn wounds and have less side effects that is excessive prominence. Perhaps, it is the purpose why despite the presence of various therapeutic agents and approaches for the burn wounds treatment, there are numerous novel investigations for finding further effective therapeutic agents for the wound management [8, 9].
Royal jelly (RJ) is a bee product. There are many reports on pharmacological activity of RJ on experimented animals. The previous study indicated that daily application of RJ possesses betters wound healing effects than nitrofurazone [10].
To our knowledge, in spite of many investigations that have assessed the effects of administration of RJ on skin problems, there are insufficient experimentations about the topical use of RJ for treatment of burn wounds. The aim of this study was to evaluate the repairing effects of RJ on tissue damage caused by burns and effect of RJ on the induction of wound healing response in Wistar rats. Considering the pathogenesis of second-degree burn wound and healing mechanisms based on that, the existing management has been designed with usage of RJ on these wounds that may be effective and be an appropriate dressing with tissue-healing characteristics.
Materials and Methods
In this study, 40 healthy, intact male weighing 200-250 gr Wistar rats were enrolled. The experimental animals were purchased from Animal Breeding Center, Bushehr University of Medical Sciences, Iran. The animals placed on each group, kept in a standard animal room (Temperature 23˚C, 12-12 dark-light cycle). All the animals had free access to food and water pellets earlier and throughout the experimental tests. The number of each group was obtained as follows:
α = 0.05
β = 0.2
N = (Z α+ Z β)2 × β2S2/d2
N = (1.96+0.8)2 × 2 × 0.932/6.72
RJ was prepared from Tajdini Natural Honey Company, Kangavar, Kermanshah Province. Topical doses of RJ were selected as the effective dose based on the pilot results. For this purpose, RJ administered as an ointment.
Second-degree burn wounds induction
All the studied protocols were carried out according to the Guide for the Care and Use of Laboratory Animals [11]. For induction of second-degree burn injuries in first, the animals were anesthetized with the intraperitoneal injection of a mixture of xylazine (10 mg/ kg) and ketamine (90 mg/ kg) and the backside hair of the rats, removed. In following the area were antisepticised with the 70% ethanol. Then, a solid aluminum bar with the temperature of 150˚C were employed on the area. Two second-degree burn wounds of 1 cm2 were made at full thickness of the skin at intervals of 1 cm. The burn plaque plate was placed on rat skin for 15 seconds.
The project consisted of five groups of eight male Wistar rats each weighing 200-250 g, previously weighed in the scales and placed in groups, with the mean weight close to each other. After 1 week of habituation, using a randomized complete block design with weight, they were divided into 5 groups including 8 subjects.
Experimental design
After induction of the second-degree wounds (day 0), the animals randomly were divided to five group. In the experimental groups, rats received an ointment that was a uniform mixture of RJ (1% and 3%) after the wound induction. Rats in negative control group received daily Eucerin ointment for the similar condition as the experimental groups and the rats in positive control group, received silver sulfadiazine treatments. In addition, eight control healthy rats were studied to compare factors in healthy rats with treatment groups. During the treatment, the changes of the wounds appearance were evaluated with imaging by physical examination. To evaluate wound healing process, various parameters such as the dynamic measure of wound healing, the wound healing percent or contraction, were assessed in all groups, in postburn. The percent of contraction in wound surface area was computed.
Microscopic examination of wound area
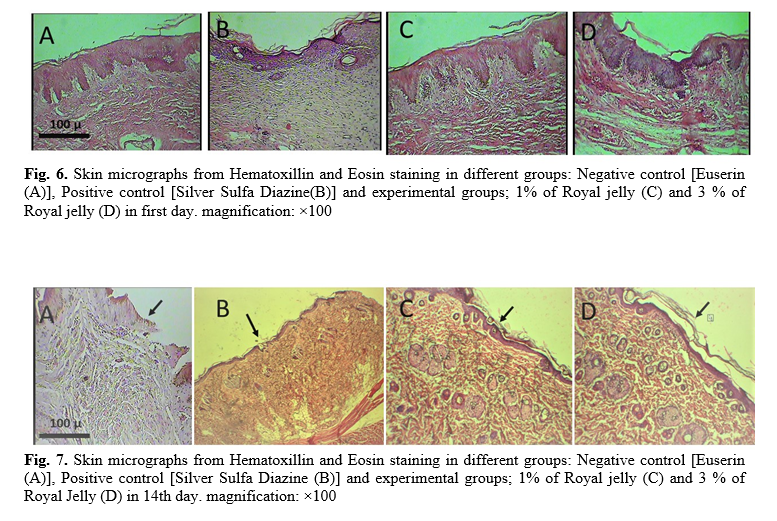
In the last day of the tests (day 14 for all groups), the wound surface areas, wound healing rate, were assessed. Wound length changes were measured with image G software. The skin samples were applied to measure the amount of inflammatory factors. In addition, the morphological and morphometric examination were studied. In following, the area of wounds fixed in the 10% formalin solution. After skin tissues, processing, the samples embedded in paraffin and the blocks were cut. The sections were stained with Hematoxylin & Eosin and Mason Trichrome methods. The specimens were investigated for the fibroblasts migration, granulation tissue formation and inflammatory response. The study protocols approved by ethical committee of Bushehr University of medical sciences, Iran by ethic code; Ir.bpums.rec.1396. 193 and Approval code:520.
Results
Macroscopic examination of wound area
After induction of second-degree burn wound in the studied groups, wound surface area pictures were recorded by digital camera in 1, 7, 14 and 21 days after wound induction. As presented in figure 1 and table 1, the wound surface area examined carefully to determine the general appearance of the wound comprising color, consistency, wound discharge and area of wound closure. Figure 2 showed the wound surface after 7 day of treatment. The skin tissue of negative control group has necrotic pattern and had secretion and much larger wound area in compared to RJ treated groups. In other groups, partial healing was presented and the wound showed clear red appearance that indicate the partial healing progression.
Results showed that the wound surface in 14 days after treatment, in the negative control group had a red inflamed tissue and greater wound area versus RJ treatment groups. In other groups, partial healing and the closed wound pattern was observed (Fig. 3).
.png)

.png)
.png)
As presented in Figure 4, wound surface in 21 days after treatment in all experimental groups relatively improved, but the color, extent and appearance of the wounds in treatment groups, particularly those treated with RJ, were more improved and show partially closed pattern. As shown in Figure 5, wound surface in 28 days after treatment in all experimental groups were improved, but the color, extent and appearance of the wounds in treatment groups, particularly those treated with RJ, were more recovered and show complete closed pattern.
Macroscopic examination of wound area
Microscopic examination in 1, 14 and 28 days after wound induction were performed. Correspondingly, wound structure examined by two conventional staining methods to evaluate the wound healing structure, including the re-epithelialization. Indeed, specific staining for evaluate the regeneration and maturation of the collagen fibers are considered to study the microscopic wound structures at three consecutive time-intervals; beginning, middle and end of treatment period. Skin tissue micrographs in different groups presented in figure 6. The skin tissue in all groups have uniformly normal microscopic patterns and healthy epithelium with the normal tissue structure. Skin tissue micrographs in different groups, after 14 day of treatment were studied. Our data showed that the skin tissue in treatment groups including Silver Sulfa Diazine ointment and also particularly in RJ treated cases had relatively normal uniform microscopic structure and epithelium have relatively good thickness and growth without any histopathologic changes. In negative control group, collagen fibers repaired but epithelium is not formed (Fig. 7). As presented in figure 8, at the end of treatment (28th day), skin tissue in all groups has relatively normal microscopic structure, good thickness of epitheliums, growth and repair pattern with any specific histopathological changes. Nevertheless, in the positive control groups (Silver Sulfa Diazine ointment) and RJ treatment, there are thicker epithelium structure versus other groups.
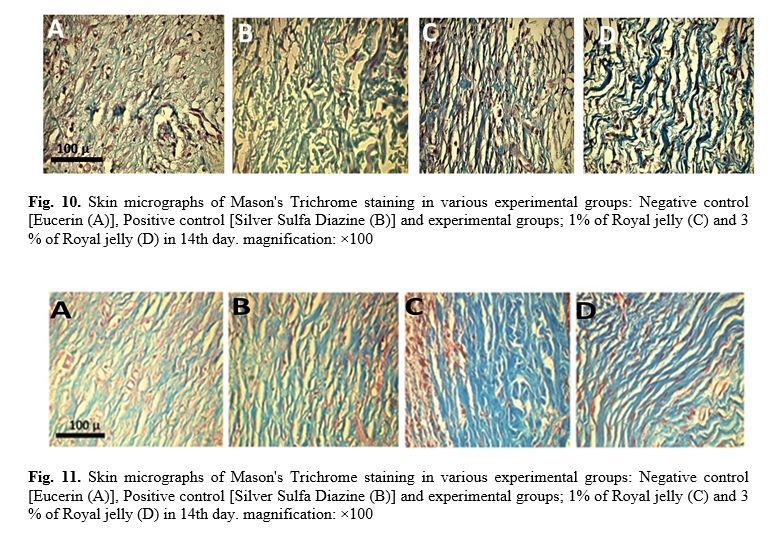
According to our results from Mason's Trichrome staining (figure 9), in all experimental groups the skin tissue with strands of graft collagen have uniformly normal microscopic structures. Figure 10 showed the micrograph of skin tissue (dermis area) in different groups on the 14th day. As shown in figure 10, skin tissue with strands of graft collagen have not regular normal microscopic structure in the negative control group but in the other positive control groups (Silver Sulfa Diazine ointment) and the RJ treated groups (1% and 3% of RJ), skin structure is completely normal with mature collagen fibers.
Table 1. Qualitative changes in the wound’s appearance in experimental groups
| Parameters |
Date(day) |
I
(Negative control) |
II
(Positive control) |
III
(Test) |
| Epithelium thickness (Micron)* |
1 |
149.11±43 |
151.11±23 |
152.45±13 |
| 14 |
No epithelium |
34.11±3 * |
31.21±5* |
| 28 |
160.53±22 |
158.36±21 |
159.46±22* |
| The number of blood vessels** |
1 |
10.21± 2 |
11.17±(2) |
9.31±(0.9) |
| 14 |
7.15±(0.8) |
12.22±(1)* |
15.10±(1)** |
| 28 |
11.30±(0.8) |
13.17±(2) |
14.01±(2) |
| The number of fibroblast cells** |
1 |
21.16±(110) |
22.12±(1.09) |
23.45±(2.15) |
| 14 |
18.14±(1.1) |
33.10±(1.15)* |
30.22±(2)* |
| 28 |
15±(1.36) |
19.26±(1.26) |
20.21±(1.12) |
| Area of wound closure (%) |
7 |
96. 32±11) |
98. 12±(1.31) |
92. 20 ±(4.18) |
| 14 |
76. 45±(2.50) |
66. 13±(1.34)* |
59. 67±(1.46)* |
| 21 |
70. 14±( .87) |
38. 01±(4.11)* |
31. 12±(3.21) * |
| 28 |
14. 90±(1.21) |
8. 42±( .96)* |
10. 14±(2.01)* |
Data is presented as mean ± SD; * Significance with negative control; **Significance with negative control
Further examination in the negative control group (on the 14th day) indicated that the skin tissue (dermis area) with
strands of
graft collagen still have not any regular normal microscopic structure. Nevertheless, in the other positive control group (Silver Sulfa Diazine ointment) and the experimental groups (1% and 3%of RJ) natural skin structure with mature collagen fibers were observed (figure 11). Morphometric properties comprising the data from wound healing, epidermal thickness, number of small blood vessels, and fibroblasts have been investigated and presented in figure 11.
Discussion
Various natural products apply in wound healing therapeutic strategies [1, 10, 12]. In this regards RJ as a bee product has numerous pharmacological activities on experimental animals [10]. Therefore, in this study we evaluate the effects of RJ on the induction of wound healing response in Wistar rats. The present study confirmed that in burned-induced wounds of Wistar rats, RJ induce the wound healing and increases the collagen content indicating the triggering the reconstruction and thickening of the epithelium in treated groups.
As an effective common histopathological examination for tissue change after therapeutic intervention in animal models, Hematoxylin and Eosin staining [13, 14] and also Mason's Trichrome staining were conducted. According to our results, RJ improved angiogenesis during wound healing process versus to the control group. The therapeutic effects of RJ confirmed
by reduced wound's surface area after RJ exposures.
The present data also revealed that in RJ treated cases, the time required for complete healing of the burn- lesion, was shorter than the control. Accordingly, RJ may be effective for increasing the healing activity in definite pathway. Commonly, wound healing takes an extended time in some patients. Generally, anti-inflammatory agents delay wound healing process by decreasing cell proliferation that is an unfavorable properties of these drugs. Nevertheless, RJ acted as an anti-inflammatory drug, although these activities, RJ reduced the time required for wound healing. These activities might be related to one of the numerous components in RJ, such as skin respiratory factor. Nevertheless, another related factor has not yet been recognized [15, 16].
In this regards,
Fujii et al., showed some anti-inflammatory activity of RJ. They also indicate that, RJ shortened the period of wound healing and is able to enhance wound healing [16]. In comparable survey, which conducted by Siavash et al. [17], RJ dressing applied as effective technique for treating diabetic foot ulcers. They indicate that this treatment option may promote wound healing as an effective intervention. Also, other study in animal model shown that RJ could promote wound healing [18] which is in accordance to our data.
In study by Rashidi et al, the impacts of
topical usage of mixture of RJ, olive oil, honey, and propolis extract were evaluated on cutaneous wound healing. Overall estimation of wound healing progress of this mixture including RJ was enhanced than those of the control group [19]. Certainly, previous reports indicate that RJ increases wound-healing activity. In both in vitro and in vivo wound-healing models, in presence of RJ, human fibroblasts were capable to migrate and also RJ increases the levels of sphingolipids. Therefore, RJ shortened the curing time of desquamated skin lesions [20, 21].
Another survey on the RJ administration have also displayed protective action on human skin against ultraviolet B-induced photoaging by inducing collagen production [22]. RJ wound dressing exerted vasodilation impacts nearby the wound, that can help to dilate the blood vessels to increase blood flow [17]. Based on author knowledge the burn-wound healing activity of RJ has not yet been reported. Consequently, based on our findings, RJ induce wound healing effects and might be considered as potential treatment option to improve the quality and quantity of burn wound healing.
Conclusion
The present study focused on the potential wound healing effects of RJ. This product is extremely rich in active ingredients that have biological actions in promoting good health. Accordingly, reconstruction and thickening of the epithelium in RJ treated groups confirmed therapeutic effects. In addition, RJ increased angiogenesis compared to the control group. In addition, fibroblast cell proliferation increased in the
groups receiving RJ compared to the control. Thus, this product could be applied as potent wound healing agent. However, it is crucial to conduct further researches to define the main mechanisms that is associated with pharmacological activities of RJ and the suitable amounts that could be administered to obtain favorable health incomes.
Conflict of Interests
The authors declared no conflict of interest.
Acknowledgments
Thanks to Dr. Mojtaba Abbasi for helpful comments on the article and help with preparing the manuscript and formatting of tables, figures and the entire article. There is no financial support for this work.
References
- Shirzad M, Yousofi M, Zamanzad B, Sedaghat A, Hosseini M, Shahinfard N, et al. Effects of royal jelly on sterile skin cut repair. J HerbMed Pharmacol. 2014; 3(2): 97-100.
- Wu SC, Marston W, Armstrong DG. Wound care: the role of advanced wound-healing technologies. J Am Podiatr Med Assoc. 2010; 100(5): 385-94.
- Gupta S, Andersen C, Black J, de Leon J, Fife C, Lantis Ii JC, et al. Management of chronic wounds: diagnosis, preparation, treatment, and follow-up. Wounds: a compendium of clinical research and practice 2017; 29(9): 19-36.
- Peck M, Molnar J, Swart D. A global plan for burn prevention and care. Bull World Health Organ. 2009; 87(10): 802-803.
- World Health Organization. A WHO plan for burn prevention and care; 2008.
- Ahuja RB, Bhattacharya S. Burns in the developing world and burn disasters. BMJ 2004; 329(4): 447-49.
- Rybarczyk MM, Schafer JM, Elm CM, Sarvepalli S, Vaswani PA, Balhara KS, et al. A systematic review of burn injuries in low-and middle-income countries: epidemiology in the WHO-defined African Region. Afr J Emerg Med. 2017; 7(1): 30-7.
- Barzegari AA, Hashemzaei M, Majdani R, Alihemmati AR. Effectsof topical treatment of second-degree burn wounds with Lactobacillus acidophiluson the wound healing process in male rats. Pharm Biomed Res. 2017; 3(3): 23-30.
- Cho AR. Effect of silver sulfadiazine on the skin cell proliferation and wound healing process in hairless mouse 2nd degree burn model. J Pharm Investig. 2002; 32(1): 113-37.
- Shirzad H, Sedaghat A, Ghasemi S, Shirzad M. Effect of Royal Jelly on sterile wound healing in Balb/C mice. Armaghane danesh. 2010; 15(1): 38-46.
- Albus U. Guide for the Care and Use of Laboratory Animals, 8th ed. SAGE Publications Sage UK: London, England; 2012.
- Dashtpeima AR, Moshfe AA, Manzouri L, Arefkhah N, Shahryari S, Mohseni M, et al. The Effects of Matricaria chamomilla on Leishmania major ulcers in Balb/c mice. Armaghane-danesh 2015; 20(2): 127-37.
- Abbasi M, Namjoo AR, Khamesipour F. Ethanol effects on histobiochemical parameters of suckling pups borned from alcoholic rat mothers. Comp Clin Pathol. 2016; 25(4): 833-39.
- Abbasi M, Namjoo AR. Low dose effects of ethanol on suckling rats: Enzymes activity, histological alterations and growth parameters. J Shahrekord Univ Med Sci. 2014; 15(6): 54-64.
- Goodson W, Horn D, Hunt TK, Leung DYK. Augmentation of some aspects of wound healing by a skin respiratory factor. J Surg Res. 1976; 21(1): 125-29.
- Fujii A, Kobayashi S, Kuboyama N, Furukawa Y, Kaneko Y, Ishihama S, et al. Augmentation of wound healing by royal jelly (RJ) in streptozotocin-diabetic rats. Jpn J Pharmacol. 1990; 53(3): 331-37.
- Siavash M, Shokri S, Haghighi S, Mohammadi M, Shahtalebi MA, Farajzadehgan Z. The efficacy of topical Royal Jelly on diabetic foot ulcers healing: A case series. J Res Med Sci. 2011; 16(7): 904-909.
- Gayar MH, Aboshanab KM. Aboulwafa MM, Hassouna NA. Antivirulence and wound healing effects of royal jelly and garlic extract for the control of MRSA skin infections. Wound Medicine 2016;13(1): 18-27.
- Rashidi MK, Mirazi N, Hosseini A. Effect of topical mixture of honey, royal jelly and olive oil-propolis extract on skin wound healing in diabetic rats. Wound Medicine 2016; 12(1): 6-9.
- Pasupuleti VR, Sammugam L, Ramesh N, Gan SH. Honey, propolis, and royal jelly: A comprehensive review of their biological actions and health benefits. Oxid Med Cell Longev. 2017; 2017(3): 1259510.
- Kim J, Kim Y, Yun H, Park H, Kim SY, Lee KG, et al. Royal jelly enhances migration of human dermal fibroblasts and alters the levels of cholesterol and sphinganine in an in vitro wound healing model. Nutrition Research and Practice 2010; 4(5); 362-68.
- Park HM, Hwang E, Lee KG, Han SM, Cho Y, Kim SY, et al. Royal jelly protects against ultraviolet B-induced photoaging in human skin fibroblasts via enhancing collagen production. Journal of Medicinal Food 2011; 14(9); 899-906.










































 , Fereshteh Amiri
, Fereshteh Amiri 

 , Zohreh Mohammadi
, Zohreh Mohammadi 

 , Parviz Farzadinia
, Parviz Farzadinia 

 , Fahimeh Safizadeh
, Fahimeh Safizadeh 
 , Zahra Zare
, Zahra Zare 
 , Rahimeh Rahimi
, Rahimeh Rahimi 

 , Zahra Dehghani
, Zahra Dehghani 

 , Fariba Mohammadi Tahroodi *
, Fariba Mohammadi Tahroodi * 


.png)

.png)

.png)

