Wed, Feb 4, 2026
[Archive]
Volume 7, Issue 3 (August 2020)
IJML 2020, 7(3): 168-178 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Dangana A, Emeribe A U, Musa S, Moses L, Ogar C, Ugwu C, et al . Plasma Iron Indices in Pregnant Women Referred to the University of Abuja Teaching Hospital, Nigeria. IJML 2020; 7 (3) :168-178
URL: http://ijml.ssu.ac.ir/article-1-359-en.html
URL: http://ijml.ssu.ac.ir/article-1-359-en.html
Plasma Iron Indices in Pregnant Women Referred to the University of Abuja Teaching Hospital, Nigeria
Amos Dangana 
 , Anthony Uchenna Emeribe
, Anthony Uchenna Emeribe 
 , Sanusi Musa
, Sanusi Musa 
 , Lugos Moses
, Lugos Moses 
 , Christopher Ogar
, Christopher Ogar 
 , Charles Ugwu
, Charles Ugwu 
 , Chibueze Obi-George
, Chibueze Obi-George 
 , Jessy Medugu
, Jessy Medugu 
 , Olasoji Matthew Billyrose
, Olasoji Matthew Billyrose 
 , Idris Nasir Abdullahi *
, Idris Nasir Abdullahi * 



 , Anthony Uchenna Emeribe
, Anthony Uchenna Emeribe 
 , Sanusi Musa
, Sanusi Musa 
 , Lugos Moses
, Lugos Moses 
 , Christopher Ogar
, Christopher Ogar 
 , Charles Ugwu
, Charles Ugwu 
 , Chibueze Obi-George
, Chibueze Obi-George 
 , Jessy Medugu
, Jessy Medugu 
 , Olasoji Matthew Billyrose
, Olasoji Matthew Billyrose 
 , Idris Nasir Abdullahi *
, Idris Nasir Abdullahi * 


Department of Medical Laboratory Science, Faculty of Allied Health Sciences, Ahmadu Bello University, Zaria, Nigeria,
Full-Text [PDF 229 kb]
(799 Downloads)
| Abstract (HTML) (1962 Views)
Introduction
With about one-gram requirement for the expansion of maternal red cell mass, placental, and fetal development, iron becomes essential for a healthy pregnancy [1]. Increasingly, severe iron deficiency and iron-deficiency anemia in pregnancy have been linked to higher chances of adverse maternal and fetal outcomes, including maternal mortality, perinatal death, preterm birth, low birth weight, impaired immune function, and long-term cognitive defects in newborns and infants [2]. In a bid to forestall these adverse outcomes, the World Health Organization (WHO) has recommended daily iron supplementation for all women during pregnancy [3]. On the other hand, the implications of high maternal iron levels in pregnancy are still poorly understood [4].
References
Full-Text: (2346 Views)
Introduction
Iron in excess can lead to generating harmful reactive oxygen species (ROS) that are deleterious to proteins, lipids, and nucleic acids [5], as well as increased susceptibility to infection [6]. Maternal plasma ferritin, which is a marker of body iron stores, is linked to adverse pregnancy outcomes in a U-shaped manner [7]. Increased levels of plasma ferritin, however, are not only pointing to increased iron stores but additionally to inflammation. Besides, the exact contribution of excess iron is still needed to be elucidated. Despite its importance, efforts are still ongoing to accurately discern the regulation of maternal and fetal iron homeostasis in pregnancy, as well as the relative contribution of the maternal, placental, and fetal signals.
The cut-off marks for establishing anemia in pregnancy are hemoglobin concentration levels lower than 11.0 g/dL during the first and third trimester of gestation or lower than 10.5 g/dL in the second trimester of pregnancy [8]. The above slight differences in values stem from the plasma volume expansion of 40 to 50% exceeding the 20 to 25% increase in red cell mass, leading to physiological haemo-dilution [9]. This physiologic plasma expansion has been associated with favorable pregnancy outcomes [10]. The reason for the adoption of the 10.0 g/dL cut-off level in developing countries was because adverse feto-maternal outcomes are not usually found ˃10.0 g/dL hemoglobin level in these countries [8]. The estimated global prevalence of anemia in pregnancy is 41.8% [11]. With its high incidence, it becomes pertinent to assay the level of iron metabolism in pregnant women regularly. It means that when the mother becomes anemic, this transfer reduces, and the fetus will be at risk of deficiency [12].
At the apical surface of the syncytiotrophoblast, transferrin receptor 1 (TFR1) takes up iron-transferrin (Fe-Tf) from the maternal circulation [13]. The complex of Fe-Tf and TFR1 is endocytosed, and iron is released in the endosome and subsequently will be exported on the basal side of the placental syncytiotrophoblast through the iron transporter ferroportin (FPN) into the fetal circulation [5]. Both TFR1 and FPN are thought to be critical for iron transport across the placenta because the global deletion of either transporter leads to embryonic death. About 75% of all cases of non-physiologic anemia in pregnancy roots in iron deficiency [2].
In the laboratory, complete cell blood count provides the basis for the diagnosis of iron deficiency anemia. Other useful tests include the determination of the levels of plasma iron, total iron-binding capacity, and transferrin [14]. Iron deficiency anemia (IDA) has increasingly been associated with high chances of preterm delivery, postpartum hemorrhage, low birth weight, and delayed psychomotor development in infancy [14-16]. On the other hand, other studies have revealed conflicting conclusions regarding specific outcome measures [17, 18]. Indeed, several studies across Nigeria have investigated IDA in pregnancy. However, little or none have comprehensively evaluated the iron metabolism indices. In this regard, there is every need to assess the current plasma iron metabolism indices as a measure of iron deficiency anemia among pregnant women attending the University of Abuja Teaching Hospital, Gwagwalada, Abuja, Nigeria. It will help to inform policies on preventive strategies and optimization of maternal and fetal outcomes during and after pregnancy.
Materials and Methods
The current study was a hospital-based cross-sectional study conducted on pregnant women who consented to participate in the study at the antenatal clinic at the University of Abuja Teaching Hospital (UATH), Gwagwalada, Abuja, Nigeria.
This study was carried out at UATH, which is a tertiary referral hospital situated in Gwagwalada, a suburb from the Federal Capital Territory (FCT) of Nigeria. The University of Abuja Teaching Hospital has an average of 3,000 deliveries annually.
The minimum sample size for this study was determined from a previous cross-sectional study done by Ajepe et al. [19], which reported 12.3% iron deficiency among pregnant women in Nigeria. The calculated size at a 95% confidence interval was 170 individuals. However, a total of 176 subjects, which comprised of 118 pregnant women and 58 non-pregnant, were recruited.
The present study involved a total of one hundred and eighteen pregnant and fifty-eight non-pregnant women. Among the aged-matched pregnant women, 22 were on their first trimester, 52 and 44 were in their second and third trimester, respectively. They were selected based on having no history of surgical operation, malaria, HIV and significant inflammation. Besides, non-pregnant women were not on menstruation. All participants voluntarily consented to be enrolled in this study, and self-reported adherence with routine oral iron supplementation and completion of intermittent malarial prophylaxis at least for four weeks interval after the first trimester were considered as the inclusion criteria. In Nigeria, the routine iron supplementation program exists during antenatal clinic visits. However, some women (especially rural residents) do not adhere to their appropriate consumption due to ignorance and certain sociocultural beliefs. Ethical approval was obtained from the Human Research Ethical Committee (HREC) of the UATH. Written consent was also obtained from all participants. This study was conducted following the standards of human experimentation described in the Helsinki Declaration of 1975 (as revised in 2000).
Because of the circadian fluctuation of iron, three millilitres (3mL) of whole fasting blood samples were collected from all participants and carefully dispensed into lithium heparin anticoagulant container. It was spun at 10,000 g for 5 minutes to ensure plasma separation, and the harvested plasma samples were used for the quantification of the analytes of interest. Samples were analyzed in batches within 1 hour of collection.
Laboratory analytical protocol
Data generated were analyzed by Statistical Package for Social Science (SPSS version 22). Statistical differences between mean and standard deviation were determined using the student’s t-test (for two groups) and ANOVA (for three groups or more). The association between iron deficiency (ID) and pregnancy status of participants was determined by univariate logistic regression. P values<0.05 at 95% confidence interval were considered as statistically significant.
Results
The mean±SEM age (years) of these women were 25.53±0.70, 31.05±1.03, 30.9±0.61, 29.32±0.60, for the non-pregnant women, those in their first trimester, second and third trimester, respectively. The overall prevalence of ID in pregnant women was 33.1%. The prevalence of ID was 29.3%, 22.7%, 34.6%, and 36.4% among non-pregnant women, women in their first trimester, second and third trimester, respectively (Table 1). There was no significant association between the prevalence of ID and the trimester of pregnant women (p>0.05).
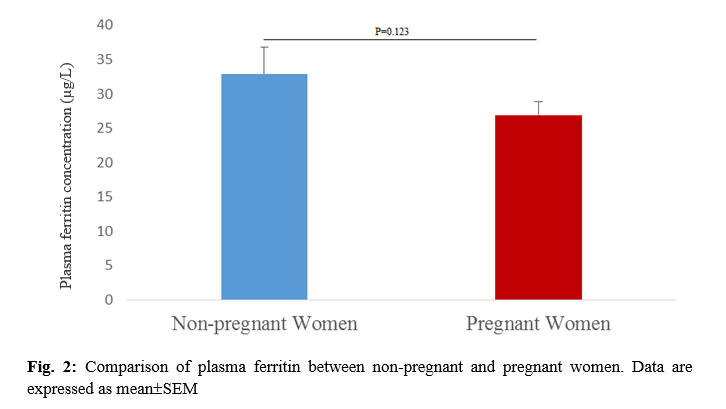
The mean±SEM iron levels among pregnant women were 13.65±0.29 µmol/L, which was significantly higher than that of non-pregnant women with 12.09±0.48 µmol/L (p=0.004) (Fig. 1). The mean±SEM ferritin level among the pregnant women was lower, 26.88±1.99 µg/L when compared to the non-pregnant women, 32.93±3.81 µg/L. However, there was no significant difference in the plasma levels of ferritin between pregnant women compared to their non-pregnant counterparts (p=0.123) (Fig. 2).
There was a significantly lower mean±SEM unsaturated iron-binding capacity (UIBC) level in pregnant women, 53.87±1.19 µmol/L in comparison with the non-pregnant women, 65.55±2.20 µmol/L (p<0.001) (Fig. 1). The TIBC was lower, but it was not significantly different among pregnant women, 68.23±3.53 µmol/L as compared to non-pregnant women, 77.71±2.61 µmol/L (p=0.079) (Fig. 1). The mean±SEM transferrin level among the pregnant women (17.62±0.69 µmol/L) was higher, but it was not significantly different compared to their non-pregnant counterparts (16.25±0.90 µmol/L) (p=0.239) (Fig. 1).
After the categorization of the trimester of pregnant women, the mean±SEM plasma levels of iron, ferritin, and UIBC significantly differed from one another (p˂0.05). Nevertheless, there was no significant difference between the mean±SEM plasma levels of TIBC and transferrin of all the four categories of the participants (p>0.05) (Table 2). The mean±SEM plasma level (12.09±0.48 µmol/L) of iron among the non-pregnant women was significantly lower than that of pregnant women in their second trimester, 13.92±0.48 µmol/L (p=0.005) and third trimester, 13.64±0.42 µmol/L (p=0.021). However, the mean±SEM plasma iron level among the non-pregnant women did not significantly differ from that of pregnant women in first trimester, 13.01±0.65 µmol/L (p=0.270) (Table 3).
The mean±SEM plasma ferritin level among the non-pregnant women, 32.93±3.81 µg/L, was not significantly lower than that of pregnant women in their first trimester, 38.50±5.50 µg/L (p=0.356), but higher than those in the second trimester, 24.19±2.52 µg/L (p=0.059) and third trimester, 24.25±3.29 µg/L (p=0.073) (Table 3). The mean±SEM plasma UIBC level among the non-pregnant women was 65.54±2.20 µmol/L, which was not significantly higher than those in the first trimester (p=0.086). However, it was significantly higher compared to those in the second trimester, 51.86±1.66 µmol/L (p˂0.001) and those in the third trimester, 53.47±2.15 µmol/L, respectively (p=0.000) (Table 3).
Discussion
The development of iron storage deficiency and anemia has been reported in various categories of people. Primarily, ID is widespread, especially among pregnant women in developing countries. Indeed, most cases of non-physiological anemia in pregnancy are due to ID [2].
As a result of iron aggregation, mobilization, or haemo-dilution, iron status seems to decline with increasing gestational periods [20]. Therefore, several cut-offs values suggestive of iron deficiency across the trimesters of pregnancy have been proposed [21]. Using plasma to evaluate iron homeostasis, this present study evaluated the iron metabolic indices of pregnant women, and take into consideration their various gestational periods. Generally, based on the gestational age in the assessment and methods of assay and study population, it is quite informative to note that the prevalence of ID in pregnant women differs across the world [22-24].
From this study, ID was more widespread among pregnant women when compared to their non-pregnant counterparts. Although no significant association was observed, the prevalence of ID among pregnant women increases as the gestation age increases. This result is in agreement with similar studies conducted in Singapore, which reported a very high (74%) prevalence of ID among pregnant women at 26-28 weeks than at the early stage of their gestation [24]. It could be attributed to the low intake of iron-rich diets since the most typical form of anemia is nutritional and mostly linked to ID and other dietary elements such as vitamin C, folic acid and vitamin B12. Besides, it could be mentioned that most of these pregnant women had inadequate knowledge about and adherence to good diets recommended for pregnant women. Perhaps, most of these women are among low-income earners who do not have the financial ability to purchase or cook good meals rich in iron. All these could be possible reasons for the ID reported among pregnant women [25, 26, 27]. These studies claimed that knowledge and awareness of ID during pregnancy, in addition to iron supplements, are of utmost significance during antenatal visits [21, 28].
The prevalence of ID reported in this study is higher than 12.3%, 15.7%, and 3.5% prevalence reported by Ejepe [19], Okafor et al. [29] and Erhabor [30] conducted in Cross River, Lagos and the Sokoto States of Nigeria, respectively. This difference in prevalence could be due to the duration of using iron supplements, which is a useful predictor of reduced risk for ID [19]. Besides, this variance may be attributed to the differences in the geographical location of the study populations.
In the current study, a significant difference was found in the mean iron concentration between pregnant and non-pregnant women. Although it is slightly elevated in pregnant women compared to their non-pregnant counterparts, this change could be attributed to the increase in the demand for iron during the first and second trimesters and possibly it is a result of increased placental and fetal demand [31]. This finding corroborated with the report by Amah-Tariah et al. [32], who recorded significant changes in the plasma iron concentration of pregnant participants. However, they reported a consistent decline in plasma iron levels with an increase in gestational age.
The findings of this study revealed a significant difference in the plasma ferritin across three categories of pregnant women, which indicates that plasma ferritin concentration decreases considerably with the progression of the gestational periods, probably as a result of iron depletion [33, 34]. This observation is supported by a study conducted among pregnant women in Scotland. They reported a high proportion of participants with significantly depleted iron stores associated with increased gestational age, leading to a decline in ferritin concentration [24].
The values of the unsaturated iron-binding capacity from this study showed significant differences between non-pregnant women and their pregnant counterparts. These values declined as the gestational age advanced, which is contrary to the findings of Amah-Tariah et al., who reported plasma elevation in UIBC in their participants with increasing gestational age. It could have been a mechanism to ensure a fetal adequate iron delivery based on the decreasing iron concentration with gestation [32]. Furthermore, these values are lower in pregnant women compared to non-pregnant women in the study, and unsaturated iron-binding capacity has been used as a diagnostic indicator of genetic hemochromatosis. Hence, its values remain clinically significant in the management of pregnant women with presumed iron metabolic disorders [35]. Another study also reported a significant increase in UIBC as the age of gestation progressed [36].
Although maternal transferrin production steadily increases during pregnancy [37], which may function to increase iron delivery to the placenta, the plasma transferrin level of pregnant women showed no significant difference compared to those of non-pregnant women. Nevertheless, there was an increased level of transferrin during the second trimester, which is contrary to the findings of Chaudhari et al. [38], who reported an increased level of serum transferrin concentration in the third trimester. The increase in transferrin concentration is likely to be due to elevated steroid levels during pregnancy. Also, a study in Port-Harcourt, Nigeria, reported a consistent increase in plasma transferrin and TIBC with an increase in gestational age. It suggests a protective mechanism of ensuring proper iron delivery to the developing fetus [32, 39].
Conclusion
This study revealed a high prevalence of ID during pregnancy, which increases with the gestation of pregnant women. It also highlighted the potential importance of routine monitoring and screening for ID at several time points during pregnancy for the timely commencement of ID management.
Iron deficiency has been linked to increased risk of preterm delivery, postpartum bleeding, low birth weight and delayed sensorineural development in infants. Hence, it is essential to periodically evaluate the prevalence of maternal ID and its associated outcomes because it helps to make empirical policies on ID preventive measures and optimization of the outcomes of maternal and fetal wellbeing. Thus, healthcare interventional measures that could address this nutritional and metabolic disorder are recommended.
Conflict of Interest
None declared.
Acknowledgment
None declared.
The cut-off marks for establishing anemia in pregnancy are hemoglobin concentration levels lower than 11.0 g/dL during the first and third trimester of gestation or lower than 10.5 g/dL in the second trimester of pregnancy [8]. The above slight differences in values stem from the plasma volume expansion of 40 to 50% exceeding the 20 to 25% increase in red cell mass, leading to physiological haemo-dilution [9]. This physiologic plasma expansion has been associated with favorable pregnancy outcomes [10]. The reason for the adoption of the 10.0 g/dL cut-off level in developing countries was because adverse feto-maternal outcomes are not usually found ˃10.0 g/dL hemoglobin level in these countries [8]. The estimated global prevalence of anemia in pregnancy is 41.8% [11]. With its high incidence, it becomes pertinent to assay the level of iron metabolism in pregnant women regularly. It means that when the mother becomes anemic, this transfer reduces, and the fetus will be at risk of deficiency [12].
At the apical surface of the syncytiotrophoblast, transferrin receptor 1 (TFR1) takes up iron-transferrin (Fe-Tf) from the maternal circulation [13]. The complex of Fe-Tf and TFR1 is endocytosed, and iron is released in the endosome and subsequently will be exported on the basal side of the placental syncytiotrophoblast through the iron transporter ferroportin (FPN) into the fetal circulation [5]. Both TFR1 and FPN are thought to be critical for iron transport across the placenta because the global deletion of either transporter leads to embryonic death. About 75% of all cases of non-physiologic anemia in pregnancy roots in iron deficiency [2].
In the laboratory, complete cell blood count provides the basis for the diagnosis of iron deficiency anemia. Other useful tests include the determination of the levels of plasma iron, total iron-binding capacity, and transferrin [14]. Iron deficiency anemia (IDA) has increasingly been associated with high chances of preterm delivery, postpartum hemorrhage, low birth weight, and delayed psychomotor development in infancy [14-16]. On the other hand, other studies have revealed conflicting conclusions regarding specific outcome measures [17, 18]. Indeed, several studies across Nigeria have investigated IDA in pregnancy. However, little or none have comprehensively evaluated the iron metabolism indices. In this regard, there is every need to assess the current plasma iron metabolism indices as a measure of iron deficiency anemia among pregnant women attending the University of Abuja Teaching Hospital, Gwagwalada, Abuja, Nigeria. It will help to inform policies on preventive strategies and optimization of maternal and fetal outcomes during and after pregnancy.
Materials and Methods
The current study was a hospital-based cross-sectional study conducted on pregnant women who consented to participate in the study at the antenatal clinic at the University of Abuja Teaching Hospital (UATH), Gwagwalada, Abuja, Nigeria.
This study was carried out at UATH, which is a tertiary referral hospital situated in Gwagwalada, a suburb from the Federal Capital Territory (FCT) of Nigeria. The University of Abuja Teaching Hospital has an average of 3,000 deliveries annually.
The minimum sample size for this study was determined from a previous cross-sectional study done by Ajepe et al. [19], which reported 12.3% iron deficiency among pregnant women in Nigeria. The calculated size at a 95% confidence interval was 170 individuals. However, a total of 176 subjects, which comprised of 118 pregnant women and 58 non-pregnant, were recruited.
The present study involved a total of one hundred and eighteen pregnant and fifty-eight non-pregnant women. Among the aged-matched pregnant women, 22 were on their first trimester, 52 and 44 were in their second and third trimester, respectively. They were selected based on having no history of surgical operation, malaria, HIV and significant inflammation. Besides, non-pregnant women were not on menstruation. All participants voluntarily consented to be enrolled in this study, and self-reported adherence with routine oral iron supplementation and completion of intermittent malarial prophylaxis at least for four weeks interval after the first trimester were considered as the inclusion criteria. In Nigeria, the routine iron supplementation program exists during antenatal clinic visits. However, some women (especially rural residents) do not adhere to their appropriate consumption due to ignorance and certain sociocultural beliefs. Ethical approval was obtained from the Human Research Ethical Committee (HREC) of the UATH. Written consent was also obtained from all participants. This study was conducted following the standards of human experimentation described in the Helsinki Declaration of 1975 (as revised in 2000).
Because of the circadian fluctuation of iron, three millilitres (3mL) of whole fasting blood samples were collected from all participants and carefully dispensed into lithium heparin anticoagulant container. It was spun at 10,000 g for 5 minutes to ensure plasma separation, and the harvested plasma samples were used for the quantification of the analytes of interest. Samples were analyzed in batches within 1 hour of collection.
Laboratory analytical protocol
- Quantification of plasma ferritin by sandwich Chemiluminescence Assay (CLIA). This study was assayed using Maglumi ® (Snibe Co., Ltd, China), and Ferritin CLIA Kit (Catalog No. 130201001M). The assay was done based on the kits’ instruction manual.
- Total iron-binding capacity (TIBC) and plasma iron assays were done using an Abcam® (UK) enzyme-linked immunosorbent assay (ELISA) Kit (Catalogue No. ab239715). It provided a simple, sensitive and high-throughput adaptable approach to quantify TIBC and plasma iron concentration. The assay was done, and the results were interpreted based on kits manufacturer’s instructions.
- The unsaturated iron-binding capacity (UIBC) was calculated by subtracting the TIBC concentration from plasma iron concentration.
- The plasma transferrin concertation was determined by using Human Transferrin Simple Step Abcam® (UK) ELISA Kit (Catalogue No. ab187391). The assay was completed, and the results were read based on the kit manufacturer’s instructions.
Data generated were analyzed by Statistical Package for Social Science (SPSS version 22). Statistical differences between mean and standard deviation were determined using the student’s t-test (for two groups) and ANOVA (for three groups or more). The association between iron deficiency (ID) and pregnancy status of participants was determined by univariate logistic regression. P values<0.05 at 95% confidence interval were considered as statistically significant.
Results
The mean±SEM age (years) of these women were 25.53±0.70, 31.05±1.03, 30.9±0.61, 29.32±0.60, for the non-pregnant women, those in their first trimester, second and third trimester, respectively. The overall prevalence of ID in pregnant women was 33.1%. The prevalence of ID was 29.3%, 22.7%, 34.6%, and 36.4% among non-pregnant women, women in their first trimester, second and third trimester, respectively (Table 1). There was no significant association between the prevalence of ID and the trimester of pregnant women (p>0.05).
The mean±SEM iron levels among pregnant women were 13.65±0.29 µmol/L, which was significantly higher than that of non-pregnant women with 12.09±0.48 µmol/L (p=0.004) (Fig. 1). The mean±SEM ferritin level among the pregnant women was lower, 26.88±1.99 µg/L when compared to the non-pregnant women, 32.93±3.81 µg/L. However, there was no significant difference in the plasma levels of ferritin between pregnant women compared to their non-pregnant counterparts (p=0.123) (Fig. 2).
There was a significantly lower mean±SEM unsaturated iron-binding capacity (UIBC) level in pregnant women, 53.87±1.19 µmol/L in comparison with the non-pregnant women, 65.55±2.20 µmol/L (p<0.001) (Fig. 1). The TIBC was lower, but it was not significantly different among pregnant women, 68.23±3.53 µmol/L as compared to non-pregnant women, 77.71±2.61 µmol/L (p=0.079) (Fig. 1). The mean±SEM transferrin level among the pregnant women (17.62±0.69 µmol/L) was higher, but it was not significantly different compared to their non-pregnant counterparts (16.25±0.90 µmol/L) (p=0.239) (Fig. 1).


After the categorization of the trimester of pregnant women, the mean±SEM plasma levels of iron, ferritin, and UIBC significantly differed from one another (p˂0.05). Nevertheless, there was no significant difference between the mean±SEM plasma levels of TIBC and transferrin of all the four categories of the participants (p>0.05) (Table 2). The mean±SEM plasma level (12.09±0.48 µmol/L) of iron among the non-pregnant women was significantly lower than that of pregnant women in their second trimester, 13.92±0.48 µmol/L (p=0.005) and third trimester, 13.64±0.42 µmol/L (p=0.021). However, the mean±SEM plasma iron level among the non-pregnant women did not significantly differ from that of pregnant women in first trimester, 13.01±0.65 µmol/L (p=0.270) (Table 3).
The mean±SEM plasma ferritin level among the non-pregnant women, 32.93±3.81 µg/L, was not significantly lower than that of pregnant women in their first trimester, 38.50±5.50 µg/L (p=0.356), but higher than those in the second trimester, 24.19±2.52 µg/L (p=0.059) and third trimester, 24.25±3.29 µg/L (p=0.073) (Table 3). The mean±SEM plasma UIBC level among the non-pregnant women was 65.54±2.20 µmol/L, which was not significantly higher than those in the first trimester (p=0.086). However, it was significantly higher compared to those in the second trimester, 51.86±1.66 µmol/L (p˂0.001) and those in the third trimester, 53.47±2.15 µmol/L, respectively (p=0.000) (Table 3).
Table 1. The prevalence of iron deficiency by pregnancy status of participants
| Pregnancy status No. of participants Iron Deficiency (%) Odd Ratio (95% CI) P-value | ||||
| Non-pregnant | 58 | 17 (29.3) | Referent | |
| First trimester | 22 | 5 (22.7) | 0.71 (0.23-2.23) | 0.557 |
| Second trimester | 52 | 18 (34.6) | 1.28 (0.57-2.85) | 0.551 |
| Third trimester | 44 | 16 (36.4) | 1.37 (0.59-3.18) | 0.451 |
Significant association determined by univariate logistic regression
Table 2. Comparison of Iron Indices between non-pregnant and the various trimesters of pregnant women
*Significant difference determined by one-way ANOVA
| Iron indices | Non-pregnant state (n=58) | First trimester (n=22) | Second trimester (n=52) | Third trimester (n=44) | F value | P-value |
| Iron (µmol/L) | 12.09±0.48 | 13.01±0.65 | 13.92±0.48 | 13.64±0.42 | 3.206 | 0.025* |
| Ferritin (µg/L) | 32.93±3.81 | 38.50±5.50 | 24.19±2.52 | 24.25±3.29 | 2.928 | 0.035* |
| Unsaturated iron-binding Capacity (µmol/L) | 65.55±2.20 | 59.41±2.37 | 51.86±1.66 | 53.47±2.15 | 10.227 | 0.000* |
| Total iron binding Capacity (µmol/L) | 77.71±2.61 | 71.71±2.55 | 63.89±1.90 | 71.62±9.14 | 1.556 | 0.202 |
| Transferrin (µmol/L) | 16.25±0.90 | 17.20±1.50 | 18.19±1.04 | 17.17±1.18 | 0.650 | 0.584 |
Table 3. Comparison of the mean of iron, ferritin, and UIBC levels between non-pregnant and the various trimesters of pregnant women using the least significant difference (LSD) post hoc.
| Parameter | Groups | Mean Difference | P-value | |
| Non-pregnant state | First trimester | |||
| Iron (µmol/L) | 12.09±0.48 | 13.01±0.65 | -0.93±0.17 | 0.270 |
| Ferritin (µg/L) | 32.93±3.81 | 38.50±5.50 | -5.57±1.69 | 0.356 |
| Unsaturated iron-binding capacity (µmol/L) | 65.55±2.20 | 59.41±2.37 | 6.14±0.17 | 0.086 |
| Non-pregnant status | Second trimester | |||
| Iron (µmol/L) | 12.09±0.48 | 13.92±0.48 | -1.83±0.00 | 0.005* |
| Ferritin (µg/L) | 32.93±3.81 | 24.19±2.52 | 8.74±1.29 | 0.059 |
| Unsaturated iron-binding capacity (µmol/L) | 65.55±2.20 | 51.86±1.66 | 13.69±0.54 | 0.000* |
| Non-pregnant status | Third trimester | |||
| Iron (µmol/L) | 12.09±0.48 | 13.64±0.42 | -1.56±0.06 | 0.021* |
| Ferritin (µg/L) | 32.93±3.81 | 24.25±3.29 | 8.68±0.52 | 0.073 |
| Unsaturated iron-binding capacity (µmol/L) | 65.55±2.20 | 53.47±2.15 | 12.08±0.05 | 0.000* |
*Significant difference determined by participant’s T-test
Discussion
The development of iron storage deficiency and anemia has been reported in various categories of people. Primarily, ID is widespread, especially among pregnant women in developing countries. Indeed, most cases of non-physiological anemia in pregnancy are due to ID [2].
As a result of iron aggregation, mobilization, or haemo-dilution, iron status seems to decline with increasing gestational periods [20]. Therefore, several cut-offs values suggestive of iron deficiency across the trimesters of pregnancy have been proposed [21]. Using plasma to evaluate iron homeostasis, this present study evaluated the iron metabolic indices of pregnant women, and take into consideration their various gestational periods. Generally, based on the gestational age in the assessment and methods of assay and study population, it is quite informative to note that the prevalence of ID in pregnant women differs across the world [22-24].
From this study, ID was more widespread among pregnant women when compared to their non-pregnant counterparts. Although no significant association was observed, the prevalence of ID among pregnant women increases as the gestation age increases. This result is in agreement with similar studies conducted in Singapore, which reported a very high (74%) prevalence of ID among pregnant women at 26-28 weeks than at the early stage of their gestation [24]. It could be attributed to the low intake of iron-rich diets since the most typical form of anemia is nutritional and mostly linked to ID and other dietary elements such as vitamin C, folic acid and vitamin B12. Besides, it could be mentioned that most of these pregnant women had inadequate knowledge about and adherence to good diets recommended for pregnant women. Perhaps, most of these women are among low-income earners who do not have the financial ability to purchase or cook good meals rich in iron. All these could be possible reasons for the ID reported among pregnant women [25, 26, 27]. These studies claimed that knowledge and awareness of ID during pregnancy, in addition to iron supplements, are of utmost significance during antenatal visits [21, 28].
The prevalence of ID reported in this study is higher than 12.3%, 15.7%, and 3.5% prevalence reported by Ejepe [19], Okafor et al. [29] and Erhabor [30] conducted in Cross River, Lagos and the Sokoto States of Nigeria, respectively. This difference in prevalence could be due to the duration of using iron supplements, which is a useful predictor of reduced risk for ID [19]. Besides, this variance may be attributed to the differences in the geographical location of the study populations.
In the current study, a significant difference was found in the mean iron concentration between pregnant and non-pregnant women. Although it is slightly elevated in pregnant women compared to their non-pregnant counterparts, this change could be attributed to the increase in the demand for iron during the first and second trimesters and possibly it is a result of increased placental and fetal demand [31]. This finding corroborated with the report by Amah-Tariah et al. [32], who recorded significant changes in the plasma iron concentration of pregnant participants. However, they reported a consistent decline in plasma iron levels with an increase in gestational age.
The findings of this study revealed a significant difference in the plasma ferritin across three categories of pregnant women, which indicates that plasma ferritin concentration decreases considerably with the progression of the gestational periods, probably as a result of iron depletion [33, 34]. This observation is supported by a study conducted among pregnant women in Scotland. They reported a high proportion of participants with significantly depleted iron stores associated with increased gestational age, leading to a decline in ferritin concentration [24].
The values of the unsaturated iron-binding capacity from this study showed significant differences between non-pregnant women and their pregnant counterparts. These values declined as the gestational age advanced, which is contrary to the findings of Amah-Tariah et al., who reported plasma elevation in UIBC in their participants with increasing gestational age. It could have been a mechanism to ensure a fetal adequate iron delivery based on the decreasing iron concentration with gestation [32]. Furthermore, these values are lower in pregnant women compared to non-pregnant women in the study, and unsaturated iron-binding capacity has been used as a diagnostic indicator of genetic hemochromatosis. Hence, its values remain clinically significant in the management of pregnant women with presumed iron metabolic disorders [35]. Another study also reported a significant increase in UIBC as the age of gestation progressed [36].
Although maternal transferrin production steadily increases during pregnancy [37], which may function to increase iron delivery to the placenta, the plasma transferrin level of pregnant women showed no significant difference compared to those of non-pregnant women. Nevertheless, there was an increased level of transferrin during the second trimester, which is contrary to the findings of Chaudhari et al. [38], who reported an increased level of serum transferrin concentration in the third trimester. The increase in transferrin concentration is likely to be due to elevated steroid levels during pregnancy. Also, a study in Port-Harcourt, Nigeria, reported a consistent increase in plasma transferrin and TIBC with an increase in gestational age. It suggests a protective mechanism of ensuring proper iron delivery to the developing fetus [32, 39].
Conclusion
This study revealed a high prevalence of ID during pregnancy, which increases with the gestation of pregnant women. It also highlighted the potential importance of routine monitoring and screening for ID at several time points during pregnancy for the timely commencement of ID management.
Iron deficiency has been linked to increased risk of preterm delivery, postpartum bleeding, low birth weight and delayed sensorineural development in infants. Hence, it is essential to periodically evaluate the prevalence of maternal ID and its associated outcomes because it helps to make empirical policies on ID preventive measures and optimization of the outcomes of maternal and fetal wellbeing. Thus, healthcare interventional measures that could address this nutritional and metabolic disorder are recommended.
Conflict of Interest
None declared.
Acknowledgment
None declared.
References
- Fisher AL, Nemeth E. Iron homeostasis during pregnancy. Am J Clin Nutr. 2017; 106(S 6): 1567-574.
- Daru J, Zamora J, Fernández-Félix BM, Vogel J, Oladapo OT, Morisaki N, et al. Risk of maternal mortality in women with severe anaemia during pregnancy and postpartum: a multilevel analysis. Lancet Glob Health 2018; 6(5): 548-54.
- World Health Organization. Guideline: Daily iron and folic acid supplementation in pregnant women. Geneva, Switzerland: World Health Organization; 2012.
- Taylor CL, Brannon PM. Introduction to workshop on iron screening and supplementation in iron-replete pregnant women and young children. Am J Clin Nutr. 2017; 106(S 6): 1547-1554.
- Sangkhae V, Fisher AL, Wong S, Koenig MD, Tussing-Humphreys L, Chu A, Lelic M, et al. Effects ofmaternal iron status on placental and fetal iron homeostasis. J Clin Invest. 2020; 130(2): 625-40.
- Ganz T. Iron and infection. Int J Hematol. 2018; 107(1): 7-15.
- Dewey KG, Oaks BM. U-shaped curve for risk associated with maternal hemoglobin, ironstatus, or iron supplementation. Am J Clin Nutr. 2017; 106(S 6): 1694-702.
- Balarajan Y, Ramakrishnan U, Ozaltin E, Shankar AH, Subramanian SV. Anaemia in low-income and middle-income countries. Lancet 2011; 378(9809): 2123-135.
- Afolabi BB, Oladipo OO, Akanmu AS, Abudu OO, Sofola OA, Broughton Pipkin F. Volume regulatory hormones and plasma volume in pregnant women with sickle cell disorder. J Renin Angiotensin Aldosterone Syst. 2016; 17(3): 1-9.
- Steer PJ. Maternal hemoglobin concentration and birth weight. The American Journal of Clinical Nutrition 2000; 71(5): 1285-287.
- Reveiz L, Gyte GM, Cuervo LG, Casasbuenas A. Treatments for iron-deficiency anaemia in pregnancy. Cochrane Database Syst Rev. 2011; 5(10): 3094.
- Fall C. Iron requirements and iron status during infancy. In: Report of the 2004 Symposium. Iron deficiency in early life: challenges and progress. Lima: International Nutritional Anemia Consultative Group; 2004. p. 13-6.
- Bastin J, Drakesmith H, Rees M, Sargent I,Townsend A. Localisation of proteins of iron metabolism in the human placenta and liver. Br J Haematol. 2006;134(5): 532-43.
- Al-Khaffaf A, Frattini F, Gaiardoni R, Mimiola E, Sissa C, Franchini M. Diagnosis of anemia in pregnancy. J Lab Precis Med. 2020; 5(9): 1-5.
- Neave L, Scully M. Microangiopathic hemolytic anemia in pregnancy. Transfus Med Rev. 2018; 32: 230-36.
- Horowitz KM, Ingardia CJ, Borgida AF. Anemia in pregnancy. Clin Lab Med. 2013; 33: 28191.
- Nair M, Choudhury MK, Choudhury SS, Kakoty SD, Sarma UC, Webster P, et al On behalf of the IndOSS-Assam steering committee. Association between maternal anaemia and pregnancy outcomes: a cohort study in Assam, India. BMJ Global Health 2016; 1(1): 26.
- Aimakhu CO, Olayemi O. Maternal haematocrit and pregnancy outcome in Nigerian women. West Afr J Med. 2003; 22(1): 18-21.
- Ajepe AA, Okunade KS, Sekumade AI, Daramola ES, Beke MO, Ijasan O, et al. Prevalence and foetomaternal effects of iron deficiency anaemia among pregnant women in Lagos, Nigeria. PLoS One 2020; 15(1): 227965.
- Scholl TO. Maternal iron status: relation to fetal growth, length of gestation and the neonate’s iron endowment. Nutr Rev. 2011; 69(S 1): 23-9.
- Milman N, Taylor CL, Merkel J, Brannon PM. Iron status in pregnant women and women of reproductive age in Europe. Am J Clin Nutr. 2017; 106(S 1): 1655-662.
- Khambalia AZ, Collins CE, Roberts CL, Morris JM, Powell KL, Tasevski V. Iron deficiency in early pregnancy using plasma ferritin and soluble transferrin receptor concentrations are associated with pregnancy and birth outcomes. Eur J Clin Nutr. 2015; 70(4): 358-63.
- Alwan NA, Cade JE, McArdle HJ, Greenwood DC, Hayes HE, Simpson NA. Maternal iron status in early pregnancy and birth outcomes: insights from the Baby’s vascular health and Iron in pregnancy study. Br J Nutr. 2015; 113(1): 1985-992.
- da Costa AG, Vargas S, Clode N, Graca LM. Prevalence and risk factors for Iron deficiency anemia and iron depletion during pregnancy: a prospective study. Acta Medica Port. 2016; 29(1): 514-18
- Health Promotion Board. Report of the National Nutrition Survey 2019, Singapore. https://www. hpb.gov.sg/docs/default-source/pdf/nns-2019-report.pdf?sfvrsn=18e3f172_2 . [Accessed 20 Apr 2020]
- Adams C, Costello A, Flynn S. Iron deficiency anaemia in Ecuador: does education matter. 2018. https://pdfs.semanticscholar.org/cf26/89806194d67bf893d3615f398dc7e8f096db.pdf. [Accessed 5 Feb 2020]
- Schuepbach RA, Bestmann L, Bechir M, Fehr J, Bachli EB. High prevalence of Iron deficiency among educated hospital employees in Switzerland. Int J Biomed Sci. 2011; 7(1): 150-57.
- Rohner F, Namaste SML, Larson LM, Addo OY, Mei Z, Suchdev PS, et al. Adjusting soluble transferrin receptor concentrations for inflammation: biomarkers reflecting inflammation and nutritional determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017; 106(S 1): 372-82.
- Okafor IM, Asemota EA, Antai AB, Usanga EA. Prevalence of iron deficiency anaemia among pregnant women in Calabar, Cross River State Nigeria. IOSR Journal of Pharmacy and Biological Sciences 2013; 7(2): 60-64.
- Erhabor O, Isaac IZ, Isah A, Udomah FP. Iron deficiency anaemia among antenatal women in Sokoto, Nigeria. Brit J Med Health Sci. 2013; 1(4): 47-57.
- Africa KMC, Aghana LE, Alday A, Bautista GG, Bautista MJ, Carbigon R, et al. A preliminary determination of plasma transferrin levels in normal Filipino pregnant women in the three trimesters of pregnancy. Acta Medica Philippina 1997; 202(90): 120-26.
- Amah-Tariah FG, Ojeka SO, Dapper DV. Haematology values in pregnant women in port Harcourt, Nigeria; Nigeria Journal of Physiological Science 2011; 26(12): 172-78.
- Mei Z, Cogswell ME, Looker AC, Pfeiffer CM, Cusick SE, Lacher DA, et al. Assessment of iron status in US pregnant women from the national health and nutrition examination survey (NHANES), 1999-2006. Am J Clin Nutr. 2011; 93(3): 1312-320.
- Rawal S, Hinkle SN, Bao W, Zhu Y, Grewal J, Albert PS, et al. A longitudinal study of iron status during pregnancy and the risk of gestational diabetes: findings from a prospective, multiracial cohort. Diabetologia. 2017; 60(1): 249-57.
- Dacie JV, Lewis SM. Practical Hematology, 7th Ed. Edinburgh: Churchill Livingstone; 2001.
- Dapper DV, Ibe CJ, Nwauche CA. Haematological values in pregnant women in Port Harcourt, Nigeria; Nig J Phy Sci. 2006;15 (3): 237-40.
- Bauer KA. Hematologic changes in pregnancy. In: Post TW, editor. Waltham (MA): UpToDate; 2016
- Chaudhari H, Dixit R, Jadeja JM. Serum level of iron and transferrin in normal and anemic pregnant women. Int J Basic Appl Physiol. 2015; 2 (1); 123-26.
- Akesson A, Bjellerup P, Berglund M, Bremme K, Vahter M. Serum transferrin receptor: a specific marker of iron deficiency in pregnancy. The American Journal of Clinical Nutrition 1998; 68(6): 1241-246.
Type of Study: Research |
Subject:
Hematology & Blood Banking
Received: 2020/04/26 | Accepted: 2020/08/16 | Published: 2020/08/27
Received: 2020/04/26 | Accepted: 2020/08/16 | Published: 2020/08/27
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |