Introduction
Heavy metals are referred to as those that have a high density of five times more than that of water. These metals include cadmium, mercury, arsenic, lead and the like. These metals, by binding to the protein of the cells, prevent the normal functioning of the cells and cause them to die. Some heavy metals accumulate in the body and trigger complications such as cancer, neurological, reproductive and growth disorders, kidney disease, antioxidant enzyme disorder, and so on. High levels of lead and mercury in the blood are associated with decreased intelligence, behavioral disorders, growth retardation and hearing impairment. At the global level, arsenic, mercury and lead are the most dangerous environmental toxins, and exposure to them can lead to significant increase in reactive oxygen species (ROS) (thiobarbituric acid reactive substances and Glutathione), reduction of superoxide dismutase (SOD) and catalase activity, and decrease in glutathione peroxidase (GPX) and glutathione disulfide levels [
1]. Mercury is an environmental and industrial pollutant depending on its different chemical forms; it causes intense toxic effects in the tissues of the body. Various compounds such as dietary protein, vitamins E, flavonoids, and rare elements (zinc, copper, etc.) modulate the induction of mercury-induced toxicity that is absorbed through breathing, digestion, even though skin. The central nervous system is a sensitive organ in contact with mercury vapors, but mercury mainly causes a variety of disorders, including impaired renal function, infertility, negative effects on the embryo and acute respiratory failure [
2] metallic fever and effects on the thyroid gland and gene toxicity [
3]. Mercury compounds are mainly metabolized in the liver or under reactions with glutathione. Hg
+2 is one of the most potent trace elements that can lead to increased free radicals that induce fat, protein, and DNA oxidation [
4].
The great threat to human health is consumption of fish from mercuric polluted water. Its entry into the human body causes Minamate disease (mercuric toxicity), and in acute cases, the death of the patient is followed [
3]. Exposure to methyl mercury can trigger oxidative stress that has direct and indirect outcomes: it directly catalyzes the direct effects of the production of ROS. The activity of antioxidant enzymes, namely catalase, superoxide dismutase, glutathione peroxidase in the liver and kidney, significantly decreases due to the oxidative stress produced by methyl mercury [
5]. Mercury chloride elevates the level of neoplastic thiol renal units (NPSH), and none of the therapies modulate the level of renal NPSH. The concentration of urea increases after exposure to HgCl
2 [
6]. Renal toxicity occurs when damaged or impaired renal function resulting from endogenous or exogenous toxins, decontamination and renal excretion are not correctly performed [
1,
5]. Renal toxicity has been associated with a slow increase in serum creatinine levels and increased urinary volume. Toxic compounds induce thickening of the glomerular base membrane, and effects on the blood flow of the kidneys and organs [
7]. Free radicals are the result of the failure of a bond in a molecule [
8]. Free radicals in living organs are produced by various functions such as cellular metabolism and cellular antioxidants, and can attack lipids, carbohydrates, and amino acids, oxidize them and intervene as an important factor at the onset of the disease [
9]. The family of free radicals can be classified into two groups: 1- free oxygen radicals such as superoxide ion, and 2- nitrogen free radicals [
10]. Free oxygen radicals play a physiological role in the transmission of signals, but they are destructive when produced in a large amount [
11] and by triggering the transmission of signals or oxidizing macromolecules in cells. Induced oxidative stress reduces the ability of antioxidants and enzyme function changes by attacking DNA or lipids [
12]. Free radicals are neutralized and exfoliated by antioxidant substances [
13]. Oxidative stress is the result of an imbalance between the production of oxidative precursors and the antioxidant defense capacity of the body and the rise beyond the ROS control [
12] and is the main cause of fetal defects, abortion, autism and cancers [
9]. In response to stress, the cell generates specific metabolites, compatibility and resistance to stressors. Antioxidant defense mechanisms exist in all living organisms and are classified into two; enzymatic and non-enzymatic categories
. Antioxidants include superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase [
14,
15]. Superoxide dismutase is a metal enzyme (metalloenzyme) and a defense that is produced under stress and decomposes superoxide ions into hydrogen peroxide and molecular oxygen. Superoxide is one of the main types of reactive oxygen in the cell. The peroxidase enzymes break down the hydrogen peroxide in the cell and prevent the production of ROS; therefore, with an increase in the activity levels of this enzyme, the cell becomes less invasive with ROS. Glutathione peroxidase can be regarded as a type of peroxidase defense enzyme [
14]. Selenium is one of the substances that has attracted the attention of many researchers. The most important effects of selenium are anti-cancer, reproductive performance and protection against oxidative damage [
16]. The use of selenium supplements in animal diets increases the ability of the immune system to respond to the disease. Selenium acts as the second line of defense for the body as part of the glutathione peroxidase enzyme and a direct correlation exists between selenium and the amount of this enzyme. Selenium prevents free radicals by destroying cell peroxides [
17]. There are two forms in nature: 1. Organic selenium adsorbed from the absorption of amino acids and stored in the body, so that organic human selenium can produce enriched foods for human consumption. In many cases it also plays an important role in antioxidant protection. 2. Non-organic selenium is found in selenite, selenide, and metal forms [
16]. Vitamin E is one of the lipid-soluble vitamins of plant origin that is essential for reproductive, muscular, nervous, and immune function. Its action mechanism is not clear; however, it has antioxidant properties and may be active in protecting the surface of the cell membrane [
18,
19]. Vitamin E is the most important soluble antioxidant in the cell membrane, which delays the oxidation process in meat. Animals are not able to synthesize vitamin E in their body. Adding high levels of vitamin E to the diet increases oxidative stability [
15]. The aim of this study is, modulating antioxidant enzymes by sodium selenide in laboratory animals exposed to mercuric chloride.
Materials and Methods
The animals used in this study, 36 adult Albino rats (weighing 250-300 g), were kept at 22±2˚C and sufficient light (12 hours of light and 12 hours of darkness) in order to adapt them to their new environment 10 days in the animal house of the Faculty of Pharmacy, Shahid Sadoughi University of Medical Sciences in Yazd; they were kept sterilized in polyethylene glycol in large cages. Mercury chloride (99% purity) and sodium selenide (99% purity) were obtained from Sigma Aldrich (Germany) and Vitamin E by Merck (Germany). Thirty-six male rats were divided into 6 groups each including 6. The first group was treated with 2.5 mg/kg mercuric chloride subcutaneously as the control group. The second received mercuric chloride (2.5 mg/kg) and sodium selenide (0.020 mg/kg) subcutaneously. The third was treated with mercuric chloride was 2.5 mg/kg and sodium selenide 0.04 mg/kg subcutaneously and the forth with 2.5 mg/kg mercuric chloride and sodium selenide 0.08 mg/kg subcutaneously. The fifth group received sodium selenide 0.04 mg/kg subcutaneously which was discontinued from day 25 but received 2.5 mg/kg of chloride subcutaneous injection instead. Group 6 was treated with 2.5 mg/kg of mercuric chloride subcutaneously, and vitamin E 100 mg/kg by gavage. The doses employed were based on previous studies. At the end of the day 28, experiments were carried out. After 12 hours of fasting, blood samples were prepared from animals being anesthetized with ketamine hydrochloride (60 mg/kg) and xylazine (10 mg/kg), and the kidney tissue was rapidly removed. Blood was taken from their hearts and centrifuged. The serum was removed and stored at -80˚C. To measure the enzymes desired by measuring kits, we placed the frozen serum sample at room temperature for 10 minutes and then centrifuged at 20 rpm at RPM2000 and, according to the instruction of each kit, the amount of the case. The need for the isolated solution was removed by the sampler and transferred to the house of each kit. After anesthetizing the animals, the kidney tissue was quickly removed for biochemical investigations and experiments, washed with mannitol and stored at -80˚C. To measure the enzymes desired by measuring kits, the frozen kidney tissue sample was placed at room temperature for a while, and 100 mg of it was weighed, 1 ml of phosphate buffer was poured onto the isolated tissue and homogenized with homogenizer. Homogenized tissue was centrifuged at 4000 RPM for 20 minutes, and according to the instructions of each kit, the required amount of supernatant was removed with sampler and transferred to the desired cell from each kit.
Measuring the biochemical factors of urea and creatinine
Serum urea and creatinine are the markers of kidney damage that increase in plasma renal insufficiency. In this project, after anesthetizing the animals, blood was taken from the heart of the rats. Following serum separation, serums were sent to the Yazd Diabetes Center laboratory for assessing blood urea and creatinine levels.
Measuring oxidative stress factors
Evaluation of glutathione peroxidase enzyme activity
Glutathione peroxidase activity in serum and kidney tissues using Antioxidant ZellBio kit.
GPX activity=(OD control- OD sample)/ (OD standard - OD Blank)*6000.
Evaluation of the activity of the enzyme superoxide dismutase
Superoxide dismutase enzyme activity in serum and kidney tissues using Antioxidant ZellBio kit
We measured the absorbance at zero and 2 minutes at 420 nm, and calculated the activity of superoxide dismutase by the following formula: SOD activity (U/mL)=(Vp-Vc) /(Vp)*100Vp=OD sample 2min-OD Blank 2minVc=OD sample 0min-OD blank 0 min
Evaluation of the activity of the catalase enzyme
Activity of catalase enzyme in serum and kidney tissues was performed using Antioxidant ZellBio kit. Catalase activity was measured by colorimetric method in 405 nm. Then catalase activity was calculated by the following formula: CAT activity (U/mL)=(OD blank-OD sample)*271*(1/60*Sample weigh). This study was approved by the Ethical Committee of Shahid Sadoughi university of Medical Sciences, Yazd, Iran.
Statistical analysis
The results were analyzed using SPSS Statistics 16. The results were analyzed by one-way ANOVA and reported as mean±standard error. Intermediate group comparisons were performed using Tukey test. A level below 5% was considered as meaningful.
Results
The activity of blood sample catalase in animals in the intervention and control groups is shown in the Fig.1. As can be seen, there was no significant difference in the activity of catalase in the treated blood sample compared to that of the control group. A significant difference in the activity of catalase enzyme in kidney tissue was identified as for the vitamin E-treated group compared with the control (p<0.01). There was no significant difference among other groups (Fig.2). As shown in the Fig.3, the highest level of superoxide dismutase enzyme activity is related to the second [HgCl2+NaSe (0.02)] and sixth [HgCl2+VitE (100)] mg/kg groups (p<0.05). No significant difference was observed in the activity of superoxide dismutase enzyme in kidney tissues of rats in different groups (Fig.4). Creatinine levels were significantly lower in the second [HgCl2+NaSe (0.02)], third [HgCl2+NaSe (0.04)], and sixth [HgCl2+VitE(100)] mg/kg, groups. The results are shown in Fig. 5. Significant differences at the level of blood urea in animals of the second [HgCl2+NaSe(0.02)], fifth [NaSe (0.04)+HgCl2] and sixth [HgCl2+VitE(100)] mg/kg groups compared to the control (p<0.01) (Fig.6). There was a significant difference as for the level of glutathione peroxidase in blood samples of animals in the fourth group [HgCl2+NaSe(0.08)] as the recipient of sodium selenide, and the sixth as recipient of vitamin E, compared to the control group (p<0.05, p<0.01 respectively). The results are shown in Fig.7. A significant difference was observed as to the level of glutathione peroxidase in the kidney samples in animals of the fifth [NaSe (0.04)+HgCl2] and sixth groups [HgCl2+VitE(100)] mg/kg compared to the control group (p<0.05) (Fig.8).
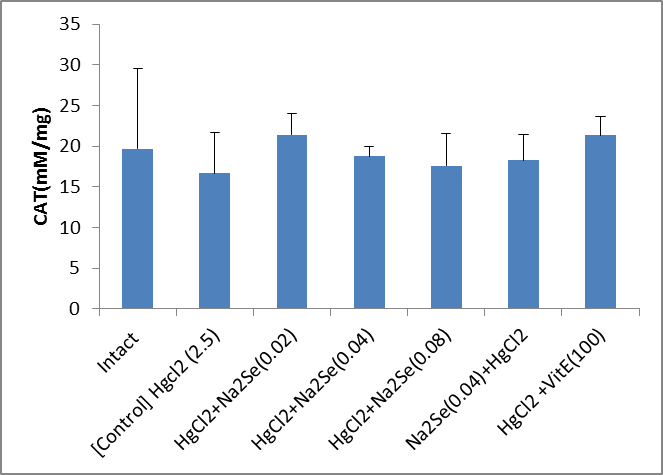
Fig.1. Comparison of the activity of catalase (CAT) of blood sample in animals in intervention (mg/kg) and control groups
 Fig.2.
Fig.2. Catalase activity (CAT) of kidney tissue samples in animals in the intervention groups (mg/kg) compared with the control (*p<0.01)
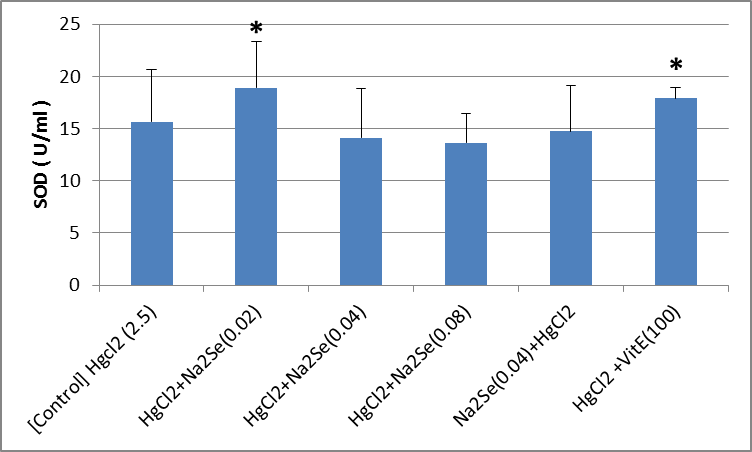 Fig.3.
Fig.3. Blood activity of superoxide dismutase (SOD) enzyme in animals of the interventional groups compared with control which only received HgCl2 2.5 mg/kg (*p<0.05)
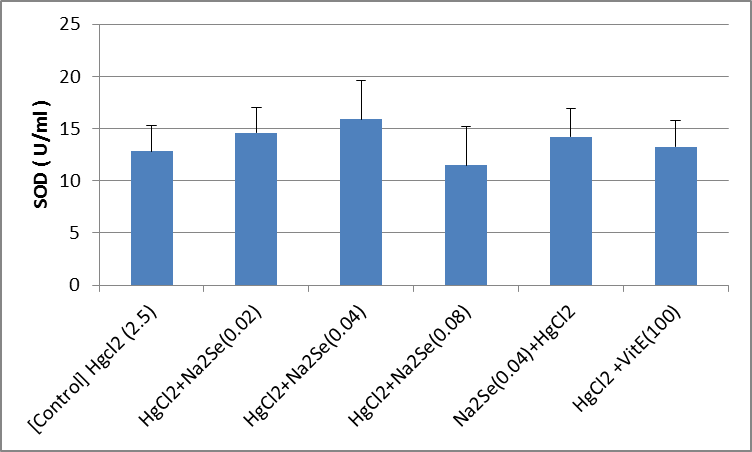 Fig.4.
Fig.4. Comparison of the activity of superoxide dismutase (SOD) enzyme in kidney tissue samples of animals in intervention and (mg/kg) control groups
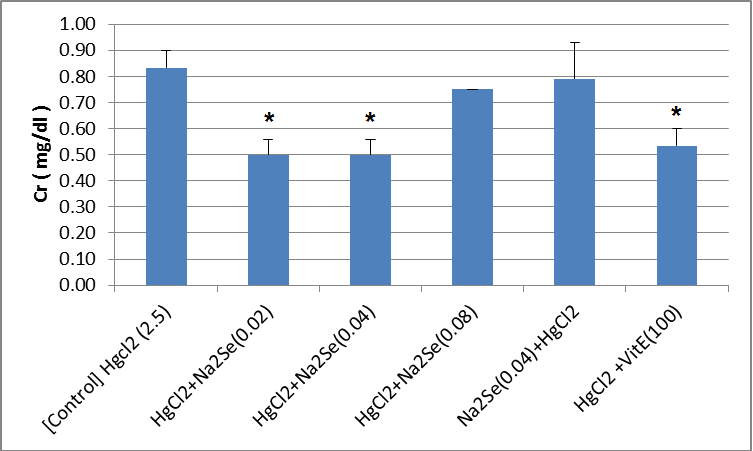 Fig.5.
Fig.5. Blood creatinine (Cr) level in animals of intervention (mg/kg) and control groups (*p<0. 05)
 Fig.6.
Fig.6. Comparison of blood urea in the treated (mg/kg) and control groups (*p<0.05)
 Fig.7.
Fig.7. Glutathione peroxide (GPX) activity of blood samples in animals in intervention groups (mg/kg) compared with the control (*p<0.05, **p<0.01)
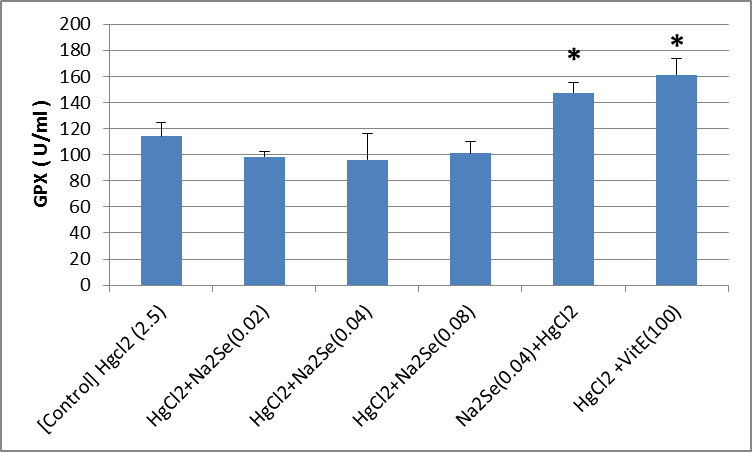 Fig.8.
Fig.8. Comparison of glutathione peroxide (GPX) enzyme activity in kidney samples of the animals in the control and treated groups (mg/kg)
*)p<0.05)
Discussion
As shown by the results, no significant difference was observed in the activity of catalase blood sample compared to the control group. For catalase activity of the kidneys, the highest values were observed in the group of rats treated with 2.5 mg/kg mercuric chloride and vitamin E 100 mg/kg by gavage (group 6). However, no significant difference was observed in other groups. This may indicate that sodium selenide has failed to raise the activity of this enzyme in any of other groups; however, vitamin E acting as a cogent antioxidant, affects these processes. In this case, due to the fact that sodium selenide is not a catalase enzyme cofactor, it does not bear the capacity to add enzymatic activity to this enzyme. Because catalase activity can be seen in the kidney of vitamin E-treated groups, it can be asserted that the kidney bears potential to increase the activity of catalase by vitamin E. The highest amount of activity of the superoxide dismutase enzyme of blood sample in the rat group treated with 2.5 mg/kg mercuric chloride was seen with sodium selenide 0.02 mg/kg compared to the control group. Additionally, there was a significant difference in the group of rats treated with mercuric chloride 2.5 mg/kg and vitamin E 100 mg/kg. So sodium selenide at a dose of 0.02 mg/kg and vitamin E as cofactors can increase SOD activity, also known as an antioxidant, bearing capacity to reduce oxidant effects against mercury chloride. No significant difference was found in the activity of the kidney superoxide dismutase enzyme in different groups of rat. As a result, sodium selenide and even vitamin E fail to compensate for the harmful effects of mercury chloride and have no significant effect on increasing the activity of SOD. It can be said that the kidney, even with the help of antioxidants such as vitamin E, is not able to cope with the oxidative effects of mercury chloride. The highest amount of glutathione peroxidase activity was seen in blood sample of animals treated with 2.5 mg/kg mercuric chloride and sodium selenide (0.08 mg/kg) subcutaneously (p<0.01). There was also a significant difference in the group of rat treated with mercuric chloride 2.5 mg/kg and subcutaneous vitamin E 100 mg/kg body weight (group 6) p<0.05. This increase in activity may indicate that sodium selenide at the highest dose (0.08 mg/kg) is able to increase the activity of this enzyme and prevent the effects of mercury-induced oxidative damage. If the group is compared to mercury chloride, it can be concluded that sodium selenide and even vitamin E can cope with the oxidant effects of mercury chloride. There is a significant difference at the level of glutathione peroxidase activity in the kidney samples in animals of groups 5 (sodium selenide 0.04mg/kg) and 6 (vitamin E 100 mg/kg) compared to the control group (p<0.05). The effect of selenide on the activity of GPX in the kidney has been shown to be protective in the long term in all sodium selenide and after 25 days when the animals are treated with sodium selenide, mercury chloride is able to produce its oxidant effects; this can be due to the fact that in the long term, sodium selenide significantly increases the GPX activity (p<0.05), which is comparable to the increase in GPX activity in the group treated with vitamin E. In general, sodium selenide, due to the presence of selenide in some groups, is able to cope with the harmful effects of mercury chloride, since sodium selenide is considered as a GPX cofactor. In the present study, creatinine levels in the control and rats treated with sodium selenide 0.02, 0.04 mg/kg and vitamin E 100 mg/kg subcutaneously showed significant differences. It means that sodium selenide appears to reduce the creatinine level, which is the index of kidney function, in the treated groups being comparable to that of receiving vitamin E, although the mechanism by which sodium selenide has the potential to reduce creatinine is not known. It can be said that sodium selenide, in addition to its antioxidant properties, has shown its capacity to reduce creatinine with an unknown mechanism. Blood urea levels decreased significantly in the rats of groups 2, 5, and 6; the group treated with 2.5 mg/kg mercuric chloride subcutaneously and sodium selenide 0.02 mg/kg for group 2, sodium selenide 0.04 mg/kg for 25 days for group 5, and vitamin E for group 6 subcutaneously. These effects were apart from their antioxidant effects, which reduced urea. In relation to urea in a group that received sodium selenide for 25 days and was subsequently exposed to mercury chloride, a significant decrease in urea levels was observed, which could indicate that sodium selenide is protective and can reduce the harmful effects of mercury chloride by reducing urea.
Zhang and his colleagues in 2016 asserted that mercury chloride exposure induces oxidative stress and bears an effect on the activity of antioxidant enzymes [
21]. Apaydin studied the effects of subacute exposure to low dose nitrous oxide and mercury chloride in rat kidneys and observed that mercury chloride causes severe histopathologic changes. It was also observed that simultaneous exposure to lead and mercury chloride nitrate significantly increases serum urea and levels of uric acid and creatinine thus generating significant changes in the activity of antioxidant enzymes (SOD, CAT, GPX and GST), and fat LDL peroxidation (MDA) in all groups and mercury chloride causing more harmful effects than lead nitrate in rats [
22]. In addition, Charab studied the protective effect of selenium on oxidative stress caused by hookah smoke on the lungs and liver of the mouse. In the group exposed to smoke, the glutathione peroxidase and catalase activity was significantly lower than that of the control. Selenium treatment of rats significantly modulated the abnormal levels of these parameters and administration of low doses of selenium could have protective effects against them [
23]. Thus, by increasing the antioxidant defense system, they can protect against the toxicity of mercury-induced liver toxicity in rats [
24]. Cruz conducted studies on the toxicity of mercury chloride and the results of oxidative stress assessment showed catalase activity and increased malondialdehyde levels [
25]. El-Sharaky conducted a study on the protective role of selenium against cadmium-induced renal toxicity in rats. The results revealed GPX activity and final lipid peroxidation activity significantly increasing in all cadmium exposed rats compared to the control group [
26]. All these findings accord with those of ours.
Conclusion
The oxidative stress caused by mercury chloride in the rat kidney triggers toxic effects. Sodium selenide likely increases the antioxidant enzymes activity by reducing the blood and renal toxicity of mercury chloride being comparable with vitamin E, although more research and further studies are needed in this regard.
Conflict of Interest
Authors declare that they have no conflicts of interest.
Acknowledgment
Authors would hereby like to appreciate Yazd University of Medical Sciences for funding the research.
References
[1]. Shin YJ, Kim JJ, Kim YJ, Kim WH, Park EY, Kim IY, et al. Protective effects of quercetin against HgCl2-induced nephrotoxicity in sprague-dawley rats. J Med Food 2015; 18(5): 524-34.
[2]. Orr SE, Bridges CC. Chronic kidney disease and exposure to nephrotoxic metals. Int J Mol Sci. 2017; 18(5): 1039.
[3]. Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int. 2011; 79(1): 33-45.
[4]. Pena C, Hernández Fonseca JP, Pedreanez A, Viera N, Mosquera J. Renal oxidative stress and renal CD8+T-cell infiltration in mercuric chloride-induced nephropathy in rats: role of angiotensin II. J immunotoxicol. 2016; 13(3): 324-34.
[5]. Sakhaee E, Emadi L, Azari O, Khanaman FS. Evaluation of the beneficial effects of Zataria multiflora Boiss in halothane-induced hepatotoxicity in rats. Adv Clin Experiment Med. 2011; 20: 23-9.
[6]. Al-Shabanah OA, Aleisa AM, Al-Yahya AA, Al-Rejaie SS, Bakheet SA, Fatani AG, et al. Increased urinary losses of carnitine and decreased intramitochondrial coenzyme A in gentamicin-induced acute renal failure in rats. Nephrol Dialysis Transplant. 2009; 25(1): 69-76.
[7]. Thyssen JP, Menné T. Metal allergy a review on exposures, penetration, genetics, prevalence, and clinical implications. Chem Res Toxicol. 2009; 23(2): 309-18.
[8]. Forte G, Petrucci F, Bocca B. Metal allergens of growing significance: epidemiology, immuno-toxicology, strategies for testing and prevention. Inflamm Allergy-Drug Target. 2008; 7(3): 145-62.
[9]. Klahr S. Urinary tract obstruction. In Seminars in nephrology. Elsevier. 2001; 21(2): 133-45.
[10]. García-Sevillano M, Rodríguez-Moro G, García-Barrera T, Navarro F, Gómez-Ariza J. Biological interactions between mercury and selenium in distribution and detoxification processes in mice under controlled exposure. Effects on selenoprotein. Chem-Biol Interac. 2015; 229(1): 82-90.
[11]. Eisler, R. Mercury hazards to living organisms. CRC Press: Taylor and Francis Group; 2006, p. 360.
[12]. Modi KS, Schreiner GF, Purkerson ML, Klahr S. Effects of probucol in renal function and structure in rats with subtotal kidney ablation. J Lab Clin Med. 1992; 120(2): 310-17.
[13]. Paitan CLT, Pino L. Mercury in the Everglades. Environmental Science. 2017.
[14]. Messaoudi I, El Heni J, Hammouda F, Saïd K, Kerkeni A. Protective effects of selenium, zinc, or their combination on cadmium-induced oxidative stress in rat kidney. Biol Trace Element Res. 2009; 130(2): 152-61.
[15]. Weydert CJ, Cullen JJ. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc. 2010; 5(1): 51-66.
[16]. Kara H, Cevik A, Konar V, Dayangac A, Servi K. Effects of Selenium with vitamin E and melatonin on cadmium-induced oxidative damage in rat liver and kidneys. Biol Trace Element Res. 2008; 125(3): 236.
[17]. Pierce S, Tappel AL. Glutathione peroxidase activities from rat liver. Biochimica et biophysica acta. 1978; 523(1): 27-36.
[18]. Ghlissi Z, Hakim A, Mnif H, Zeghal K, Rebai T, Boudawara T, et al. Combined use of Vitamins E and C improve nephrotoxicity induced by colistin in rats. Saudi J Kidney Dis Transplant. 2018; 29(3): 545-53.
[19]. Jaturakan O, Dissayabutra T, Chaiyabutr N, Kijtawornrat A, Tosukhowong P, Rungsipipat A, et al. Combination of vitamin E and vitamin C alleviates renal function in hyperoxaluric rats via antioxidant activity. J Veterin Med Sci. 2017; 79(5): 896-903.
[20]. Palm F, Cederberg J, Hansell P, Liss P, Carlsson PO. Reactive oxygen species cause diabetes-induced decrease in renal oxygen tension. Diabetologia 2003; 46(8): 1153-160.
[21]. Zhang QF, Li YW, Liu ZH, Chen QL. Exposure to mercuric chloride induces developmental damage, oxidative stress and immunotoxicity in zebrafish embryos-larvae. Aquatic Toxicol. 2016; 181: 76-85.
[22]. Apaydın FG, Baş H, Kalender S, Kalender Y. Subacute effects of low dose lead nitrate and mercury chloride exposure on kidney of rats. EnvironToxicol Pharmacol. 2016; 41: 219-24.
[23]. Charab MA, Abouzeinab NS, Moustafa ME. The protective effect of selenium on oxidative stress induced by waterpipe (narghile) smoke in lungs and liver of mice. Biologic Trace Element Res. 2016: 1-10.
[24]. Deng Y, Xu Z, Xu B, Liu W, Feng S, Yang T. Antioxidative effects of schidandrin B and green tea polyphenols against mercuric chloride-induced hepatotoxicity in rats. J Environ Pathol, Toxicol Oncol. 2014; 33(4): 349-61.
[25]. Cruz FF, Leite CE, Pereira TCB, Bogo MR, Bonan CD, Battastini AMO, et al. Assessment of mercury chloride-induced toxicity and the relevance of P2X7 receptor activation in zebrafish larvae. Comparative Biochemistry and Physiology Part C: Toxicol Pharmacol. 2013; 158(3): 159-64.
[26]. El-Sharaky A, Newairy A, Badreldeen M, Eweda S, Sheweita S. Protective role of selenium against renal toxicity induced by cadmium in rats. Toxicol. 2007; 235(3): 185-93.
















































