Introduction
Formaldehyde (FA) is widely used in scientific laboratories, hospitals, and industrial facilities [1, 2]. Many harmful effects of airborne FA have been described in studies of the respiratory system and carcinomas, and nasal squamous cell mutations [3, 4]. In testicular tissues, FA exposures reduced the numbers of Leydig cells and sperm quality [1]. FA exposures also led to decreased testicular weights, numbers, motilities, viabilities, and morphologies of sperm cells and induced apoptosis in testicular spermatogenesis cells [2, 3].
By increasing free oxygen radicals, FA causes damage in the cell and oxidative stress in many tissues such as testis [4]. Under normal cellular conditions, excess reactive oxygen species (ROS) are neutralized by antioxidants. Hence, oxidative stress occurs when antioxidant activity is reduced or increased by ROS production [5]. Several antioxidant preparations are currently used to reduce oxidative damage. Curcumin (diferuloylmethane) is the main curcuminoid of turmeric, which is a known spice. It has been shown that curcuminoids are polyphenols and are responsible for the yellow-orange color of turmeric. Noorafshan and colleagues previously showed that curcumin protects the Leydig cells against metronidazole treatments [6]. In another study, curcumin reduced testicular damage in diabetic rats by reducing oxidative stress [7].
Moreover, Farombi et al. used curcumin to prevent oxidative changes and enhance sperm motility and reduce sperm abnormalities [8]. Histological examinations of testicle tissues are core to histopathological studies in humans and laboratory models. Although histopathological analyses of testicle tissues are usually limited to qualitative or semi-quantitative parameters, statistical comparisons of normal and experimental or pathologic samples have been performed. Such stereological methods improve biological studies’ efficiency based on shapes, sizes, and orientations of cells. Stereology also provides quantitative morphological data based on the most critical features of testicles. These include total volumes of testicles, seminal tubes, germinal and epithelial tissues, lengths and diameters of interstitial tissues, and numbers of Sertoli, Leydig, myoid, spermatogonia, spermatocytes, and spermatids in tubular sections [9]. This study aimed to assess the protective effects of curcumin on sperm and stereological parameters in testes from formaldehyde-exposed NMRI mice.
Materials and Methods
In this experimental study, 24 male NMRI mine aged 6-8 weeks and weighing 30-35 gr were studied in 4 groups (n = 6). Group I (control): no injection, group ΙΙ: FA (10mg/kg) [10], group ΙΙΙ: FA (10 mg/kg)+curcumin (100 mg/kg) [11], and group ΙV received curcumin solvent (DMSO, 2ml/day) [7], intraperitoneally for 35 days.
The animals were obtained from the animal house of Yazd Reproductive Science Institute animal house and fed in standard animal chow and water. They were kept under a 12-hour light/ dark cycle at 20 to 22˚C.
Epididymal sperm aspiration and sperm analysis
After 35 days, mice were killed by dislocation of cervical vertebrae, and caudal epididymis tissues were dissected and placed in 1-ml aliquots of Ham’s F10 culture medium that had been balanced in an incubator containing CO2. Sperms were removed from tissues and placed in an incubator containing 5% CO2 at 37˚C for 20 min. Sperm viability was evaluated using Eosin staining. Besides, motility and sperm concentrations were determined using a Mackler chamber and an optical microscope at ×100 magnification (Olympus Co. Tokyo, Japan) [12]. Motility was graded as progressive, non-progressive, or immotile. Papanicolaou staining was used to evaluate normal morphology and 100 spermatozoa were counted using a light microscope at ×100 magnification [13]. FA and curcumin were bought from Merck and Sigma (Germany), respectively.
Lipid peroxidation
Using malondialdehyde (MDA) kits manufactured in Germany according to the Zell bio-protocol [14]. So, the R2 and R3 solutions and chromogenic reagents were prepared as described by the manufacturer. All reagents except biological samples were stored at ambient temperature. We added 50-μL aliquots of reagent R4 to 50-μL samples in tubes heated until being clear. Subsequently, 1 ml aliquots of prepared chromogen solution were added to each sample. Sample mixtures were placed in a boiling bin for one hour and then centrifuged at 3000–4000 rpm for 10 min. Finally, 200-μl aliquots of pink supernatant solution were added, and sample absorbance was determined using enzyme-linked immunosorbent assays (ELISA) at 535 nm. MDA concentrations in samples were determined according to the absorbance of standards [14].
Stereological study
After initial fixation in buffer solution, sections of 5 μm were cut using a microtome machine. Single sections were randomly selected from the first 10, and were placed in sets with the 10th sections and the sections before and after. Selected sections were stained with Hematoxylin-Eosin. In each section, three microscopic fields were uniformly and randomly selected for quantitative analysis of cells. The numerical density of Leydig cells and spermatogonia were estimated using the physical disector method.
Nv = ∑ Q/N (dis)×V (dis)
Where ∑Q is the number of cells in each disector frame of sampled sections, N(dis) is the sum of all counted disector frames, V (dis) is the volume of disector frame: V (dis)=A (frame) × h
Where A (frame) is the known area associated with each disector frame and h is the height of the section and was equal to the section thickness.
We used counting points that hit the tubule for quantifying the areal fraction of the tubule, dividing by points hitting the section. For this purpose, ten systematic sections and a total point of 200 points were counted in each testis.
V=(p tubules)/ (p testicle (tubule+interstitial tissue)) [15] .
All experimental protocols were approved by the Ethics Committee of Shahid Sadoughi University of Medical Sciences, Yazd, Iran
(IR.SSU.MEDICINE.REC.1396.103).
The Institutional Animal Care and Use Committee protocols were followed during handling, maintenance, treatment, and euthanasia of animals.
Statistical analysis
All data were analyzed by SPSS version 20.0 software (SPSS Inc., Chicago, IL). One-way ANOVA was applied to evaluate the data, and an LSD post-test was performed to determine the difference between the two groups. Two-sided p<0.05 indicated a statistically significant difference between sperm evaluations.
Results
Sperm parameters
In Table 1, according to sperm analysis, sperm parameters were presented in terms of cell counts (A), progressive (B), non-progressive (C), abolished motility (D), viability (E), and morphology (F). Significant differences in sperm counts, motility, morphology, and viability were found between groups I and II (p=0.001). These parameters also differed significantly between the groups I and III (p=0.018, 0.002, 0.001, 0.001, 0.001, and 0.001, respectively). There were significant differences in counts, motility, viability, and morphology, which were identified between the Groups ΙΙ and ΙII (p≤0001). There were no significant differences in morphology, non-progressive motility, or viability between Groups Ι and ΙV (Table 1).
Oxidative stress assessments
MDA concentrations were significantly lower in the control group than in groups II and III (p≤0.001). MDA concentrations were significantly increased in group II compared with group I, III, and IV (p≤0.001). MDA
levels were also significantly increased in group III compared with groups I, IV and II (p=0.001, p=0.976, p=0.001). MDA levels were significantly lower in group IV than groups II and III (p≤0.001) (Table 1).
Leydig cell numbers
Mean numbers of Leydig cells did not differ between control and curcumin-treated groups (p=0.003, p≤0.001) or between control and sham groups. However, mean numbers of Leydig cells in groups ΙΙ and ΙΙΙ differed significantly from those in the control group. Besides, mean numbers of Leydig cells were significantly higher in group ΙV than in groups ΙΙ and ΙΙΙ (p≤0.001, p=0.006) (Table 2).
Spermatogonia cell numbers
Mean numbers of spermatogonia cells differed significantly between the control group and groups ΙΙ and ΙΙΙ (p ≤ 0.001), but no differences were identified between the groups Ι and ΙV.
In comparing two groups, mean numbers of spermatogonia cells were significantly different between group ΙΙ and all other groups (p ≤ 0.001) (Table 2).
Surface to volume ratios of seminiferous tubules (v)
Surface to volume ratios of seminiferous tubules (v) was significantly higher in the control group than in groups ΙΙ and ΙΙΙ (p≤0.001). Pairwise comparisons also indicated significant differences between group ΙΙ and the other groups (Figure 1) (Table 2).
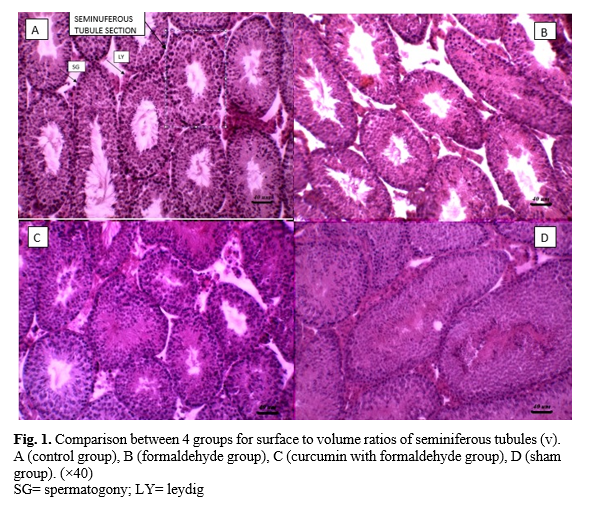
Table 1. The results of sperm parameter analysis and malondialdehyde in the study group (n=4)
| Variable |
Group Ι |
Group ΙΙ |
Group ΙΙΙ |
Group ΙV |
P-value |
| Sperm count (106) |
35.37 ± 1.68 |
15.30 ± 4.07 |
31.83 ± 1.42 |
35.16 ± 1.12 |
≤ 0/001a,d,e
0/018 b
0/882 c
0/025 f |
| Progressive motility (%) |
57.00 ± 3.68 |
21.50 ± 2.58 |
47.33 ± 7.99 |
57.16 ± 2.85 |
≤ 0/001a ,d ,e
0/002 b
0/953 c
0/002 f |
| Non-progressive motility (%) |
23.00 ± 2.75 |
50.50 ± 3.67 |
30.16 ± 3.31 |
24.33 ± 3.44 |
≤ 0/001a,b,d,e
0/494 c
0/006 f |
| Immotile (%) |
7.00 ± 0.89 |
39.16 ± 5.30 |
17.66 ± 2.73 |
9.66 ± 1.63 |
≤ 0/001a,b,d,e,f
0/155 c |
| Viability (%) |
86.83 ± 3.97 |
51.33 ± 4.71 |
69.33 ± 4.58 |
80.33 ± 5.12 |
≤ 0/001a,b,d,e
0/024 c |
| Morphology (%) |
60.50 ± 3.08 |
29.83 ± 3.65 |
47.83 ± 3.54 |
58.16 ± 1.94 |
≤ 0/001a,b,d,e,f
0/211 c |
| Malondialdehyde (%) |
6.67 ± 0.50 |
16.10 ± 0.58 |
9.37 ± 1.01 |
6.66 ± 0.46 |
≤ 0/001a,b,d,e,f
0/976 c |
To analyze data, we used a standard one-way ANOVA test. The mean difference was significant at the 0.05 level. All data are presented as mean ± SD
a: Significant difference between group Ι and II
b: Significant difference between group Ι and III
c: Significant difference between group Ι and IV
d: Significant difference between group ΙI and III
e: Significant difference between group II and IV
f: Significant difference between group III and IV
Table 2. The results of stereological indices in the study group (n=8)
| Variable |
Group Ι |
Group ΙΙ |
Group ΙΙΙ |
Group ΙV |
P-value |
Leydig cell
number |
1048.00 ± 109.10 |
523.33 ± 56.19 |
869.66 ± 73.72 |
1033.00 ± 113.54 |
≤ 0.001a,b,d,e,f
0.490 c |
| Spermatogony cell number |
4997.16 ± 210.97 |
3093 ± 204.60 |
4227.50 ± 96.59 |
4789.66 ± 339.58 |
≤ 0.001a,b,d,e,f
0.133 c |
| V |
86.50 ± 1.51 |
70.00 ± 1.54 |
77.33 ± 2.87 |
85.66 ± 1.96 |
0.001a,b,d,e,f
0.490 c |
V: Surface-to-volume ratios of seminiferous tubule
To analyze data, we used the standard one-way ANOVA test. The mean difference was significant at the 0.05 level. All data are presented as mean ± SD
a: Significant difference between group Ι and II
b: Significant difference between group Ι and III
c: Significant difference between group Ι and IV
d: Significant difference between group ΙI and III
e: Significant difference between group II and IV
f: Significant difference between group III and IV
Discussion
In the present experiment, curcumin, as an antioxidant reduced FA-induced damage in sperm parameters, including count, moility, viability, and stereological indices such as Leydig and spermatogonia cell number. Also, the surface to volume ratios of seminiferous tubules in mice testis improve MDA levels and morphology.
FA induces free radical production in testicular structures and negatively influences sperm production and sperm parameters [1, 2, 16]. Naghdi and co-workers performed a 14-day-experiment with 10-mg/kg FA injections in 25 male rats and observed reduced Gonado Somatic Index (GSI) values and increased percentages of immotile sperm compared with the control group [4]. Similarly, Tootian et al. observed decreases in body weights and GSI index values in rats after 40-day treatments with FA at 0.5, 2.5, 5, and 7.5 mg/kg doses [10]. In a study by Tajaddini and co-workers, 10 mg FA injections reduced testicular weights and sperm cell number, mobility, survival, and morphology after 14 days in 20 male mice [17]. In this study, FA reduced sperm count, viability, and progressive motility. It also had more morphological effects than other treatment groups. It has been shown that various doses of FA cause atrophy of testicular tubules and abnormal sperm production [18]. Vosoughi and colleagues studied the toxic effects of FA in 36 adult male mice over 35 days and showed damaging effects on testicular tissues, with decreased sperm count, viability, motility, and altered morphology, which agrees with the present findings [19]. The numbers of Sertoli, Leydig, Myoid, Spermatogonia, Spermatocytes and Spermatids can also be estimated using stereological methods, which provide additional information related to tissues’ spatial arrangements cells, and organs in testes [9]. Askaripour and co-workers found that FA affects the rat reproductive system, with decreased serum testosterone levels, seminal tube diameters, and sperm parameters, such as life span, movement, numbers, and morphologies of sperm cells, and numbers of Leydig cells [20]. Our results confirmed the inhibition of spermatogenesis and sperm characteristics in the FA-treated group. Multiple studies show that FA increases the production of ROS in non-testicular tissues [17, 20]. Although higher ROS concentrations in testis have been shown to increase germ cell apoptosis [21], the mechanisms by which FA damages sperm profiles remain poorly understood. Nonetheless, high ROS concentrations were associated with lipid peroxidation in sperm outer membranes, leading to loss of motility [17]. Antioxidants neutralize cellular ROS under physiological conditions, but oxidative stress occurs when antioxidant activity is reduced or when ROS levels are increased [5]. Curcumin is an antioxidant that may reduce oxidative stress by reducing ROS production [7]. Curcumin is the main active ingredient of turmeric, obtained from stems of turmeric plants [22]. ROS levels were evaluated in seminal fluids by performing MDA assays in the present studied groups. These experiments confirmed that FA treatments increase ROS production and that concomitant curcumin treatments moderate these effects. In semen taken from healthy men, significant antioxidant contents have been demonstrated, but they do not prevent malignant and excessive lipid peroxidation. However, endogenous antioxidants have been shown to prevent excessive formation of peroxide [23]. With conflicting data between studies, several assumptions exist about ROS functions in reproductive health and sperm. Increased ROS production inhibits phosphorylation of oxonic proteins, resulting in defective sperm motility [24]. Therefore, the present study’s changes may be due to the oxidative damage caused by FA. Sudjarwo et al. examined the protective effects of curcumin in 40 male mice after 35-day treatments with 35 mg/kg lead acetate. They concluded that curcumin improves sperm parameters (count, motility, viability, and morphology) by reducing MDA levels, similar to our results [25]. Mahmoudi et al. also examined the effects of curcumin on rats exposed to sodium metabisulfite. They showed that curcumin prevented structural damage of testicles and improved morphology and numbers of sperm cells [26]. In a study done on aflatoxin toxicity, Mathuria and co-workers showed that curcumin limits lipid peroxidation in mice, particularly in testicles which are in agreement with the current study [27]. Moreover, Kanter and co-workers showed that curcumin reduced the risk of testicular damage in 24 diabetic rats after 8 weeks of intravenous injections of streptozotocin, presumably by reducing oxidative stress [7]. Rashid and co-workers also showed that injections of 100-mg/kg curcumin for 8 weeks protected against cell damage in testicular cells from diabetic rats [28]. Also, Karbalayi-doust et al. showed improved sperm parameters in metronidazole-treated mice after 16 days with 165 and 500 mg/kg/day doses of curcumin [29]. Moreover, Zha et al. found that curcumin ameliorates testicular damage, oxidative stress, and apoptosis in type 2 diabetic rats [30]. Noorafshan and colleagues observed changes in structural parameters of tubes and numbers of Leydig cells following treatments with curcumin, similar to this study’s findings [31]. In this research, the improvement of histopathological changes in testis and sperm parameters by curcumin in the formaldehyde-treated group is probably related to its antioxidant effect, even though further studies are needed [7].
Conclusion
This study showed that curcumin could reduce formaldehyde-induced damage to the testis structure and sperm parameters, possibly by inhibiting oxygen free radicals’ production. These results suggest that curcumin is a potential therapeutic agent on spermatogenesis caused by a testicular injury triggered by FA in mice. However, further studies are required to investigate the precise mechanism of curcumin’s function.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This research was financially supported by the Department of Biology and Anatomy of Shahid Sadoughi University of Medical Sciences, Yazd, Iran. The authors would like to thank the staff who helped with experiments and data collection.
References
- Vosoughi S, Khavanin A, Salehnia M, Mahabadi HA, Shahverdi A, Esmaeili V. Adverse effects of formaldehyde vapor on mouse sperm parameters and testicular tissue. Int J Fertil Steril. 2013; 6(4): 250.
- Zhou DX, Qiu SD, Zhang J, Tian H, Wang HX. The protective effect of vitamin E against oxidative damage caused by formaldehyde in the testes of adult rats. Asian J Androl. 2006; 8(5): 584-88.
- Zhou D, Qiu S, Zhang J, Wang Z. Reproductive toxicity of formaldehyde to adult male rats and the functional mechanism concerned. Sichuan da xue xue bao Yi xue ban= J Sichuan Univ Med Sci. 2006; 37(4): 566-9.
- Naghdi M, Maghbool M, Seifalah-Zade M, Mahaldashtian M, Makoolati Z, Kouhpayeh SA, et al. Effects of common fig (ficus carica) leaf extracts on sperm parameters and testis of mice intoxicated with formaldehyde. Evidence-Based Complemen Alt Med. 2016; 2539127: 1-9.
- Khosrowbaki A. The role of oxidative stress in male infertility: A review. Arak Med Univ J. 2013; 15(9): 94-103.
- Noorafshan A, Karbalay-Doust S. Curcumin protects the seminal vesicles from metronidazole-induced reduction of secretion in mice. Acta Med(Hradec Králové). 2012; 55(1): 32-6.
- Kanter M, Aktas C, Erboga M. Curcumin attenuates testicular damage, apoptotic germ cell death, and oxidative stress in streptozotocin‐induced diabetic rats. Mol Nutr Food Res. 2013; 57(9): 1578- 585.
- Farombi EO, Abarikwu SO, Adedara IA, Oyeyemi MO. Curcumin and kolaviron ameliorate di‐n‐butylphthalate‐induced testicular damage in rats. Basic Clin Pharmacol Toxicol. 2007; 100(1): 43-8.
- Noorafshan A. Stereology as a valuable tool in the toolbox of testicular research. Annals of Anatomy-Anatomischer Anzeiger 2014; 196(1): 57-66.
- Tootian Z, Tajik P, Fazelipour S, Taghva M. Effect of formaldehyde injection in mice on testis function. Int J Pharmacol. 2007; 3(5): 421-24.
- Momeni HR, Soleimani Mehranjani M, Eskandari N, Hemayatkhah Jahromi V. Protective effect of curcumin on testis histopathology in sodium arsenite-treated adult mice. J Arak Univ Med Sci. 2014; 17(3): 73-81.
- Bandegi L, Anvari M, Vakili M, Khoradmehr A, Mirjalili A, Talebi AR. Effects of antidepressants on parameters, melondiadehyde, and diphenyl-2-picryl-hydrazyl levels in mice spermatozoa. Int J Reprod BioMed. 2018; 16(6): 365.
- Talebi AR, Khalili MA, Nahangi H, Abbasi A, Anvari M. Evaluation of epididymal necrospermia following experimental chronic spinal cord injury in rat. International Journal of Reproductive BioMedicine 2007; 5(5): 171-76.
- Kamali FS, Shahrooz R, Najafi G, Razi M. Ameliorative effects of crocin on paraquat-induced oxidative stress in testis of adult mice: An experimental study. International Journal of Reproductive BioMedicine 2019; 17(11): 807.
- Moridian M, Khorsandi L, Talebi A. Morphometric and stereological assessment of the effects of zinc oxide nanoparticles on the mouse testicular tissue. Bratislavske lekarske listy. 2015; 116(5): 321-25.
- Razi M, Malekinejad H, Sayrafi R, Hosseinchi MR, Feyzi S, Moshtagion SM, et al., editors. Adverse effects of long-time exposure to formaldehyde vapour on testicular tissue and sperm parameters in rats. Veterinary Res Forum: Int Quart J. 2013; 4(4): 213-19.
- Tajaddini S, Ebrahimi S, Behnam B, Bakhtiyari M, Joghataei M, Abbasi M, et al. Antioxidant effect of manganese on the testis structure and sperm parameters of formalin‐treated mice. Andrologia. 2014; 46(3): 246-53.
- Köse E, Sarsılmaz M, Taş U, Kavaklı A, Türk G, Özlem Dabak D, et al. Rose oil inhalation protects against formaldehyde‐induced testicular damage in rats. Andrologia 2012; 44(4): 342-48.
- Vosoughi S, Khavanin A, Salehnia M, Mahabadi HA, Shahverdi A, Esmaeili V. Adverse effects of formaldehyde vapor on mouse sperm parameters and testicular tissue. Int J Fertil Steril. 2013; 6(4): 1-8.
- Askaripour M, Hasanpour A, Hosseini F, Moshrefi M, Moshtaghi G, Hasannejad M, et al. The effect of aqueous extract of Rosa damascena on formaldehyde-induced toxicity in mice testes. Pharmaceut Biol. 2018; 56(1): 12-7.
- Gules O, Eren U. The effect of xylene and formaldehyde inhalation on testicular tissue in rats. Asian-Australasian J Animal Sci. 2010; 23(11): 1412-420.
- Drake RL, Vogl W, Mitchell AW. Gray’s Anatomy: anatomy of the Human Body. Inggris: Elsevier 2014: 228-30.
- Palermo GD, Neri QV, Cozzubbo T, Cheung S, Pereira N, Rosenwaks Z. Shedding light on the nature of seminal round cells. PloS One 2016; 11(3): 151640.
- Löfgren E, Tapanainen JS, Koivunen R, Pakarinen A, Isojärvi JI. Effects of carbamazepine and oxcarbazepine on the reproductive endocrine function in women with epilepsy. Epilepsia 2006; 47(9): 1441-446.
- Sudjarwo SA, Giftania Wardani Sudjarwo K. Protective effect of curcumin on lead acetate-induced testicular toxicity in Wistar rats. Res Pharmaceut Sci. 2017; 12(5): 381.
- Mahmoudi R, Honarmand Z, Karbalay-Doust S, Jafari-Barmak M, Nikseresht M, Noorafshan A. Using curcumin to prevent structural impairments of testicles in rats induced by sodium metabisulfite. EXCLI J. 2017; 16: 583-92.
- Mathuria N, Verma RJ. Curcumin ameliorates aflatoxin-induced lipid peroxidation in liver, kidney and testis of mice-an in vitro study. Acta Pol Pharm. 2007; 64(5): 413-16.
- Rashid K, Sil PC. Curcumin ameliorates testicular damage in diabetic rats by suppressing cellular stress-mediated mitochondria and endoplasmic reticulum-dependent apoptotic death. Biochimica et biophysica acta (BBA)-molecular basis of disease 2015; 1852: 70-82.
- Karbalay-Doust S, Noorafshan A. Ameliorative effects of curcumin on the spermatozoon tail length, count, motility and testosterone serum level in metronidazole-treated mice. Prague Med Rep. 2011; 112(4): 288-97.
- Zha W, Bai Y, Xu L, Liu Y, Yang Z, Gao H, et al. Curcumin attenuates testicular injury in rats with streptozotocin-induced diabetes. BioMed Res Int. 2018; 7468019: 1-10.
- Noorafshan A, Karbalay-Doust S, Valizadeh A, Aliabadi E. Ameliorative effects of curcumin on the structural parameters of seminiferous tubules and Leydig cells in metronidazole-treated mice: a stereological approach. Exper Toxicol Pathol. 2011; 63(7-8): 627-33.









































