Introduction
Hepatoxicity is defined as a liver injury caused by exposure to a drug or another non-infectious substance [1, 2]. Drug-induced liver injury (DILI) is considered a rare but drug-related severe adverse effect [3]. Several studies have illustrated that oxidative stress plays a significant role in DILI development [4-6]. Oxidative stress is a broad term used to define cells or tissues’ challenge to elevated levels of highly reactive molecules such as reactive oxygen species [7, 8].
Antibiotics, also known as antibacterials, are a pharmacological group most often associated with DILI [7, 9, 10]. Amoxicillin / clavulanic acid (Augmentin®), also known as co-amoxiclav (CoA), is an antibacterial drug combination consisting of amoxicillin and clavulanate potassium [11]. Amoxicillin, a semisynthetic penicillin derivative, is a p-hydroxy analog of ampicillin posing the same broad-spectrum of bactericidal activity ampicillin against many gram-positive and also some gram-negative bacteria [12]. The clavulanic acid component, also known by its potassium salt form clavulanate, has a β-lactamase-inhibiting property that will allow the β-lactam antibiotic work uninhibited [13, 14]. CoA has become one of the most commonly prescribed antibiotics and is now widely used as first-line therapy for community-acquired respiratory tract infections [15]. Throughout the years, different combinations of amoxicillin to clavulanate have been introduced worldwide to enhance the dosing convenience, prescribing needs, and therapy recommendations of more severe infections or those caused by drug-resistant bacteria [4].
Despite the useful applications assigned to this drug, CoA-induced hepatotoxicity has been indicated in multiple studies [16, 17]. Conversely, CoA is considered as one of the leading causes of hospitalization for hepatic adverse drug reactions [18, 19]. The liver injury associated with CoA is related mainly to the clavulanic acid component due to the low occurrence of hepatic reactions with amoxicillin alone [20, 21]. Studies have shown that CoA-induced liver injury is frequently associated with cholestasis features resulting from hypersensitivity drug allergy, which may progress to hepatocellular damage [20, 22, 23]. Although the exact cause of CoA-induced hepatotoxicity remains unknown [24, 25], oxidative stress has been suggested as one of the possible mechanisms involved in the hepatotoxic effect of CoA [11, 26].
On the other hand, natural products and their derivatives represent almost half of all the agents used in liver therapy and [27, 28]; therefore, there have been a lot of efforts to develop new herbal medicines in order to reduce DILI [29]. Thymol, chemically known as 2-isopropyl-5-methyl phenol, is the main constituent of the thyme (Thymus vulgaris L., Lamiaceae) essential oil [30]. Thymol, in addition to its antimicrobial property, implies potent anti-inflammatory and antioxidant properties [31]. Despite the fact that thymol can exhibit antioxidant activity in both cellular and in vitro models [30, 32], there is a lack of evidence about the effectiveness of the thymol in DILI. Thus, the present study was conducted to explore the thymol anti-hepatotoxic effect against the CoA-induced hepatotoxicity in rats.
Materials and Methods
Chemicals
CoA was available in the form of 200 mg/ 28.5 mg/ 5 ml oral suspension that contained 200 mg amoxicillin (as amoxicillin trihydrate) and 28.5 mg clavulanic acid (as potassium clavulanate) per 5 ml. It was obtained from Cosar Pharmaceutical Co, Tehran, Iran. Thymol was purchased from the Sigma Chemical Corporation (St Louis, MO, USA). Corn oil was obtained from the local market. Thymol solution was prepared separately by dissolving various amounts in 0.5 ml of corn oil. All other reagents were of analytical grade.
Animals and treatments
Thirty male albino rats (180-200 g) were obtained from the animal house of Shahid Sadoughi University of Medical Sciences, Yazd, Iran. The rats were housed at a temperature of 20-22°C under artificial light for a 12-h light/dark cycle with access to water and standard rodent chow ad libitum. All the experiments reported here were carried out under protocols approved by the local Institutional Animal Ethics Committee of Shahid Sadoughi University of Medical Sciences. After one week of acclimatization, the animals were divided into five groups of six rats each. Finally, the groups were arranged as follows: Group I was used as a control and received corn oil (0.25 ml/100 g of body weight) once daily by gastric tube. Group II was given only CoA in doses of 10 mg/kg daily by gastric tube. Group III, IV, and V orally received CoA at the same dose as the second group along with thymol at daily doses of 50, 150, and 300 mg/kg, respectively. Doses used in the present study were selected based on previous studies conducted on CoA induced liver injury and thymol effects on drug-induced hepatoxicity [26, 33]. Thymol was given within 1 hour following CoA administration. All groups were treated over 7 consecutive days. At the termination of the treatment, the rats were anesthetized through a slight diethyl ether exposure. Blood samples were obtained by cardiac puncture procedure using sterile and disposable syringes and needles. Blood samples were collected from each rat into heparin and serum separator tubes simultaneously. Rats were then sacrificed, and serum separator tubes were transported to the Danesh Medical Laboratory (Yazd, Iran) immediately on ice to determine the activity of serum alanine transaminase, aspartate transaminase (AST), alkaline phosphatase (ALP), and measure serum total bilirubin and conjugated bilirubin values. Heparinized plasma was prepared by centrifugation at 3000 rpm for 10 min and stored, protected from light, at -80°C for later analysis of Glutathione-S-transferase (GST) activity.
Assay of serum biochemical parameters
The activity of liver function enzymes, including serum AST, alanine transaminase (ALT), and ALP alongside total bilirubin and conjugated bilirubin values, were determined using the photometric method provided by the diagnostic kits (Man company, Iran).
Measurement of GST activity
GST activity was determined calorimetrically (412 nm) using a ZellBio GmbH assay kit obtained from ZellBio, ULM, Germany. The assay was performed according to the instruction manuals supplied with the kit. The research was approved by the Research Deputy and Ethics Committee of Shahid Sadoughi University of Medical Science, Yazd, Iran. (Approval Number: IR.SSU.MEDICINE. REC.1398.128).
Statistical analysis
The obtained data were analyzed with SPSS Ver. 25.0 software. Data were expressed as mean values ± standard error of the mean (SEM). For the comparison of the group mean values, one-way analysis of variance (ANOVA), and for the determination of the intergroup differences, Tukey test was used. The value with P<0.05 was considered significant.
Results
The serum ALT activity
According to the results shown in Figure 1, there was a statistically significant difference between
the activity of serum ALT enzyme in animals of CoA group and control group (p<0.001). Also, the activity of the ALT in the animals’ blood serum of CoA group was significantly different from the activity of this enzyme in animals’ blood serum of CoA+ thymol 150 (CoA+T150) and CoA+ thymol 300 (CoA+T300) groups (p<0.01 and p<0.05, respectively). On the other hand, no significant difference was observed in the activity of ALT in the animals’ blood serum of CoA group comparing the CoA+ thymol 50 (CoA+T50) group (p>0.05).
The serum AST activity
According to the results shown in Figure 2, there was a statistically significant difference in the serum AST activity between the control group and CoA group (p<0.001). There was also a significant difference in AST activity between animals in CoA+T150 group and CoA+T300 group compared to CoA group (p<0.05). At the same time, there was no significant difference between the AST activity in the blood serum of animals of CoA group and CoA+T50 group (p>0.05).
The serum ALP activity
According to the results shown in Figure 3, a statically significant difference was noticed in the activity of ALP in the blood serum of the control group comparing the CoA group (p<0.001). There was also a significant difference in the activity of ALP among animals in CoA+T150 group and CoA group (p<0.05). Besides, ALP activity in the blood serum of animals of the CoA+T300 group had a significant decrease comparing the CoA group (p<0.01). However, no significant decrease was observed in the activity of ALP in the blood serum of the CoA+50T group comparing the CoA group (p>0.05).
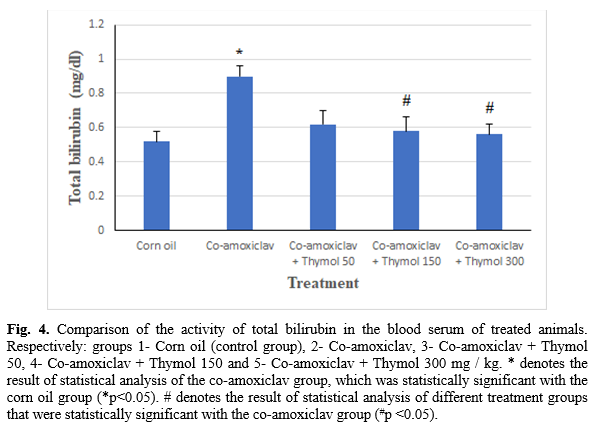
The serum total bilirubin level
According to the results shown in Figure 4, there was a significant difference in the amount of total bilirubin in the animals’ blood serum of the control group compared to the CoA group (p<0.05). A significant reduction was also noticed in the amount of total bilirubin of CoA+T150 and CoA+T300 groups comparing CoA group (p<0.05). There was no significant difference in the total serum bilirubin levels of animals in the CoA+T50 group compared to the CoA group (p>0.05).
The serum conjugated bilirubin level
According to the results shown in Figure 5, there was a significant difference in the amount of conjugated bilirubin in the animals’ blood serum of the control group (p<0.01) and the CoA+T300 group comparing CoA group (p<0.05). Conversely, there was no significant difference in this index’s level in the blood serum of animals of the CoA group comparing CoA+T50 and CoA+T150 groups (p>0.05).

The plasma GST activity
According to the results shown in Figure 6, there was a significant difference in glutathione S-transferase enzyme activity in the blood plasma of the control group comparing the CoA group (p<0.001). There was also a significant difference in the activity of glutathione S-transferase enzyme in the blood plasma of animals of CoA+T150 group (p<0.01) and CoA+T300 group comparing CoA group (p <0.001).
Discussion
The results’ assessment shows a significant difference in ALT, AST, and ALP activity in the blood serum of animals treated with CoA alone (CoA group) compared with the control group. This event is in line with previous studies’ results, which have indicated an increase in liver function enzymes’ values in liver damage caused by CoA [9, 11]. The concomitant administration of thymol at the dose level of 50, 150, and 300 mg/kg of body weight with CoA resulted in decreased ALT activity, AST, and ALP in the blood serum of the studied animals. Notably, there was a significant reduction in the activity of ALT, AST, and ALP in the treated animals with thymol at the dose level of 150 and 300 mg/kg (CoA+T150 and CoA+T300 groups) compared to the animals in the treated group with CoA alone (CoA group). The treatment of rats with thymol at the dose level of 50 mg/kg (CoA+T50 group) has partially reduced the activity of the transaminases and ALP in the blood serum of animals; however, these reductions were not significantly different compared to the activity level of these enzymes in the blood serum of animals that were treated with CoA alone. In general, by comparing the results, it can be noticed that the most significant decrease in the activity of ALP has been observed in the blood serum of animals treated with thymol at a dose of 300 mg/kg. Subsequently, the gradual increase in thymol has significantly reduced ALT, AST, and ALP activity compared to the group administered CoA alone. The results are consistent with previous studies on the protective effect of thymol on liver damage [16, 34]. We also observed a significant increase in total bilirubin levels and direct bilirubin levels in the blood serum of animals treated with CoA alone compared to the control group. This event could be due to the liver damage caused by CoA. Several studies have demonstrated an increase in bilirubin levels due to CoA administration in rats [9, 11]. In the present study, the administration of thymol at a dose level of 50 mg/kg reduced the amount of total bilirubin and conjugated in the blood serum of animals compared to the treated group CoA alone; however, the difference was not statistically significant (p>0.5). It is important to note that following the administration of increasing doses of thymol (CoA+T150 and CoA+T300 groups), a significant decrease in total bilirubin levels was observed in the blood serum of animals compared to animals treated with CoA alone. On the other hand, the level of conjugated bilirubin in the blood serum of animals treated with CoA alone was only significantly different from those treated with the thymol at the dose level of 300 mg/kg. These results once again demonstrate the dose-dependent hepato-protective effect of thymol against CoA induced hepatotoxicity. Although little research has been conducted on the effect of thymol on bilirubin indicators in liver damage, studies on carvacrol (another important component of thyme essential oil phenol which, like thymol, have antioxidant effects) [35, 36]; has demonstrated the effect of the carvacrol on a significant reduction in the rate of bilirubin indicators in liver damage [37]. In the present study, the assessment of animal blood serum indicators revealed ALT/ALP ratio less than 2 that a hyperbilirubinemia has accompanied in animals treated with CoA alone, which can be signs of cholestatic liver damage [26]. This result is consistent with the findings of previous studies on the type of liver damage caused by CoA [17, 38]. Additionally, prescribing CoA significantly reduced glutathione S-transferase activity in the blood plasma of animals in the treated group with CoA alone compared to the control group. This incident can be due to the oxidative effect of CoA that can cause the depletion of reduced glutathione. glutathione is an important hydrophilic antioxidant that protects cells against exogenous and endogenous toxins. GST activity can be decreased by the depletion of glutathione [4, 11]. In addition, administration of thymol at daily doses of 150, and 300mg/kg resulted in a statically significant elevation of GST activity.
Conclusion
In the present study, the administration of CoA developed hepatotoxicity in rats. We propose that thymol's concomitant administration with CoA can exert a hepatoprotective effect attributed to the thymol’s antioxidant activity. The assessment of the results also revealed that the hepatoprotective activity of thymol was dose-dependent, suggesting that it can be more effective at higher doses.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Acknowledgment
The authors declare no acknowledgment.
References
- Bahirwani R, Reddy KR, editors. Drug-induced liver injury due to cancer chemotherapeutic agents. Seminars in Liver Disease Thieme Medical Publishers 2014; 34(2): 162-71.
- Lee WM. Drug-induced hepatotoxicity. New Eng J Med. 2003; 349(5): 474-85.
- Andrade RJ, Robles M, Fernández-Castañer A, López-Ortega S, López-Vega MC, Lucena MI. Assessment of drug-induced hepatotoxicity in clinical practice: a challenge for gastroentero-logists. World J Gastroenterol. 2007; 13(3): 329.
- El-Hosseiny L, Alqurashy N, Sheweita S. Oxidative stress alleviation by sage essential oil in co-amoxiclav induced hepatotoxicity in rats. Int J Biomed Sci. 2016; 12(2): 71.
- Lin Y, Li Y, Hu X, Liu Z, Chen J, Lu Y, et al. The hepatoprotective role of reduced glutathione and its underlying mechanism in oxaliplatin-induced acute liver injury. Oncol Lett. 2018; 15(2): 2266-272.
- Mahmoud AM, Alexander MY, Tutar Y, Wilkinson FL, Venditti A. Oxidative stress in metabolic disorders and drug-induced injury: the potential role of Nrf2 and PPARs activators. Hindawi 2017; 2508909: 1-4.
- Ferrajolo C, Verhamme KM, Trifirò G, W‘t Jong G, Giaquinto C, Picelli G, et al. Idiopathic acute liver injury in paediatric outpatients: incidence and signal detection in two European countries. Drug Safety 2013; 36(10): 1007-1016.
- Halliwell B, Gutteridge JM. Free radicals in biology and medicine. 5th ed, USA: Oxford University Press; 2015.
- Meier Y, Cavallaro M, Roos M, Pauli-Magnus C, Folkers G, Meier PJ, et al. Incidence of drug-induced liver injury in medical inpatients. Euro J Clin Pharmacol. 2005; 61(2): 135-43.
- Robles M, Toscano E, Cotta J, Isabel Lucena M, Andrade RJ. Antibiotic-induced liver toxicity: mechanisms, clinical features and causality assessment. Curr Drug Safety 2010; 5(3): 212-22.
- Olayinka ET, Olukowade I, Oyediran O. Amoxycillin/clavulanic acid combinations (Augmentin 375 and 625 tablets) induce-oxidative stress, and renal and hepatic damage in rats. Afr J Pharmacy Pharmacol. 2012; 6(33): 2441-449.
- Tallarida RJ. TOP 200: a compendium of pharmacologic and therapeutic information on the most widely prescribed drugs in America: Springer; 1982.
- Drawz SM, Bonomo RA. Three decades of β-lactamase inhibitors. Clin Microbiol Rev. 2010; 23(1): 160-201.
- Gilbert N. Four stories of antibacterial breakthroughs. Nature 2018; 555(7695).
- White AR, Kaye C, Poupard J, Pypstra R, Woodnutt G, Wynne B. Augmentin® (amoxicillin/clavulanate) in the treatment of community-acquired respiratory tract infection: a review of the continuing development of an innovative antimicrobial agent. J Antimicrob Chemother. 2004; 53(S1): 3-20.
- Beraldo DO, Melo JF, Bonfim AV, Teixeira AA, Teixeira RA, Duarte AL. Acute cholestatic hepatitis caused by amoxicillin/clavulanate. World J Gastroenterol. 2013; 19(46): 8789.
- Jordan T, Gonzalez M, Casado M, Suarez J, Pulido F, Guerrero E, et al. Amoxicillin-clavulanic acid induced hepatotoxicity with progression to cirrhosis. Gastroenterologia y hepatologia 2002; 25(4): 240.
- Leise MD, Poterucha JJ, Talwalkar JA. Drug-induced liver injury. Mayo Clin Proc. 2014; 89(1): 95-106.
- Salvo F, Polimeni G, Moretti U, Conforti A, Leone R, Leoni O, et al. Adverse drug reactions related to amoxicillin alone and in association with clavulanic acid: data from spontaneous reporting in Italy. J Antimicrob Chemotherapy 2007; 60(1): 121-26.
- Bolzan H, Spatola J, Castelletto R, Curciarello J. Intrahepatic cholestasis induced by amoxicillin alone. Gastroenterologia y Hepatologia 2000; 23(5): 237-39.
- De Abajo FJ, Montero D, Madurga M, Rodríguez LAG. Acute and clinically relevant drug‐induced liver injury: a population based case‐control study. Br J Clin Pharmacol. 2004; 58(1): 71-80.
- Berg P, Hahn EG. Hepatotoxic reactions induced by beta-lactamase inhibitors. European J Med Res. 2001; 6(12): 535-42.
- Chaabane NB, Safer L, Njim L, Zakhama A, Saffar H. Cholestatic hepatitis related to amoxicillin. Drug Chem Toxicol. 2011; 34(4): 357-58.
- Health NIo. LiverTox: clinical and research information on drug-induced liver injury. [electronic database]. Available from: https://livertox.nih.gov; 2017.
- Stephens C, López-Nevot MÁ, Ruiz-Cabello F, Ulzurrun E, Soriano G, Romero-Gómez M, et al. HLA alleles influence the clinical signature of amoxicillin-clavulanate hepato-toxicity. PloS one 2013; 8(7): 68111.
- El-Sherbiny GA, Taye A, Abdel-Raheem IT. Role of ursodeoxycholic acid in prevention of hepatotoxicity caused by amoxicillin-clavulanic acid in rats. Ann Hepatol. 2009; 8(2): 134-40.
- Xiong F, Guan Y-S. Cautiously using natural medicine to treat liver problems. World J Gastroenterol. 2017; 23(19): 3388.
- Zhang A, Sun H, Wang X. Recent advances in natural products from plants for treatment of liver diseases. Euro J Med Chem. 2013; 63(4): 570-77.
- Madkour FF, Khalil WF, Dessouki AA. Protective effect of ethanol extract of Sargassum dentifolium (Phaeophyceae) in carbon tetrachloride-induced hepatitis in rats. Int J Pharm Pharm Sci. 2012; 4(5): 637-41.
- Amiri H. Essential oils composition and antioxidant properties of three thymus species. Evidence-Based Complementary and Alternative Medicine 2012; 728065: 1-8.
- Mishra RK, Baker MT. Seizure prevention by the naturally occurring phenols, carvacrol and thymol in a partial seizure-psychomotor model. Bioorgan Med Chem Lett. 2014; 24(23): 5446-449.
- Ündeğer Ü, Başaran A, Degen G, Başaran N. Antioxidant activities of major thyme ingredients and lack of (oxidative) DNA damage in V79 Chinese hamster lung fibroblast cells at low levels of carvacrol and thymol. Food Chemical Toxicol. 2009; 47(8): 2037-2043.
- Alam K, Nagi M, Badary O, Al-Shabanah O, Al-Rikabi A, Al-Bekairi A. The protective action of thymol against carbon tetrachloride hepatotoxicity in mice. Pharmacol Res. 1999; 40(2): 159-63.
- Aboelwafa HR, Yousef HN. The ameliorative effect of thymol against hydrocortisone-induced hepatic oxidative stress injury in adult male rats. Biochem Cell Biol. 2015; 93(4): 282-89.
- Mastelic J, Jerkovic I, Blažević I, Poljak-Blaži M, Borović S, Ivančić-Baće I, et al. Comparative study on the antioxidant and biological activities of carvacrol, thymol, and eugenol derivatives. J Agricul Food Chem. 2008; 56(11): 3989-996.
- Yanishlieva NV, Marinova EM, Gordon MH, Raneva VG. Antioxidant activity and mechanism of action of thymol and carvacrol in two lipid systems. Food Chemistry 1999; 64(1): 59-66.
- Aristatile B, Al-Numair K, Veeramania C, Pugalendi KV. Antihyperlipidemic effect of carvacrol on D-galactosamine induced hepatotoxic rats. J Basic Clin Physiol Pharmacol. 2009; 20(1): 15-28.
- O’donohue J, Oien K, Donaldson P, Underhill J, Clare M, MacSween R, et al. Co-amoxiclav jaundice: clinical and histological features and HLA class II association. Gut. 2000; 47(5): 717-20.