In most parts of the world, medicinal plants are used to treat many infectious diseases [1]. It is estimated that about 80% of people living in developed countries rely on traditional treatments for primary health care [2]. Some of these herbs have been shown to accelerate the healing process of the wound or have shown antimicrobial, antifungal, or antiviral activity, while others have been useful in general wound healing [3]. The Arnebia euchroma (A. euchroma), an Abu Ghulsa or aerobic plant, is used to treat burn wounds.
Studies have shown that the roots obtained from the species of the bivalve family, including the naphthoquinone, are rich in naphthoquinone [4]. These substances, as well as their derivatives (Alkannin) and alkanes, have intrinsic properties with a wide range of biological properties such as wound healing, antifungal [5-8], antiviral (such as influenza and HIV) [9, 10], and anti-cancer activities. Scientific research has also shown that this plant has antimicrobial and
anti-inflammatory effects. Shikonin is the major active constituent among these naphthoquinones [11-14]. Shikonin and Shikonin derivatives have strong anti-tumor activities, can inhibit cell proliferation, and induce apoptosis in several human malignancies, such as gastric cancer [15]. Additionally, they have exhibited antiviral, antioxidation, anti-inflammatory, anti-fertility, and other pharmacological effects and can also be used in food additives and cosmetics [16, 17].
The amniotic membrane (AM) is the innermost layer of the placenta that has been successfully used in the surgical field in recent decades. Using amnion for treating foot ulcers and eye and ear surgery in recent years has opened a new window in medical science. The AM acts as a basement membrane, facilitating the migration of epithelial cells, enhancing adhesion to the basal epithelium, stimulating epithelial differentiation, and delaying epithelial apoptosis. The AM produces several growth factors stimulating the growth of epithelial cells [18]. On the other hand, it accelerates the differentiation of cells and prevents apoptosis. The basement membrane acts as a healthy substrate for epithelial growth. [19]. The AM secretes nutritional and defense factors that can resist the microorganisms [20]. In this regard, binding the wound bed with collagen to the AM leads to the development of traps for trapping bacteria on the wound's surface, and existing fibrin substances stimulate phagocytic activity. Through this mechanism, the AM reduces bacterial growth even in infected wounds [21]. Since the cost of burn treatment is high and routine treatments have side effects for patients, and on the other hand, people are now inclined to use naturally occurring medicines, research on the use of medicinal plants is also of interest due to the therapeutic effects of these herbs.
Antiviral chemicals currently have significant disadvantages, such as high toxicity and adverse effects in humans, the emergence of drug-resistant viral strains, low numbers, and limited diversity. Therefore, it is necessary to evaluate new phytochemicals to obtain new therapeutic methods. Accordingly, the present study was carried out to evaluate the antiviral activity of AE extract, AM, a mixture of A. euchroma extract and AM, and carrier (sesame and ostrich) against herpes simplex virus type 1 (HSV-1), influenza A, and rotavirus in-vitro.
Materials and Methods
Preparation of A. euchroma extract
10 g of A. euchroma root was carefully washed under running tap water, dried for two days at room temperature, then treated to a fine powder and stored in sealed containers. Extracts were prepared by soaking plant material in 150 mL EtOH 67% under sonication (combined in two separate containers, then every three times under low-intensity sonication) at room temperature [22]. The extract was filtered through Whatman No. 1 filter paper, and then the filtrates were pooled up successively and concentrated under vacuum by a Rotary evaporator (Buchi® Rotavap R-210). Then, it was powdered in a special mill and was used to mix dry extract into the 5 ml ointment (sesame and ostrich oil) and was stored at 37 ˚C for 73 hours in a special tank in the dark. It is prepared at the above temperature for half an hour for consumption. The final product was sterilized by gamma irradiation kGy [23].
Preparation of AM
Due to the contamination of the AM with vaginal microbial flora in vaginal delivery, in most cases, the cesarean section is a preferred method for the removal of the fetal membrane. In this regard, the cesarean section should be elective, and the gestation period should be complete. After selecting the donor, the patient's history should be carefully evaluated regarding high-risk sexual behaviors, including injectable drugs, tattooing, blood transfusions, and malignancies. All mothers with (AM) fluid should be tested for hepatitis C, B, and human immunodeficiency virus. Serologic tests were performed again to ensure the umbilical cord blood (AM) attached to hepatitis and acquired immunodeficiency syndrome, and syphilis. The (AM) was dried several times after washing with a normal solution of saline and sodium hypochlorite of 0.05% and after sterilization using radio imaging of the anamnesis of the Gamma-Ray tube (25 kGy). A careful examination needs to be performed by a responsible physician. Upon confirmation of the donor's eligibility, written consent will be obtained from the donor. After processing, the amnion is placed in special containers and stored at a controlled temperature of -80 ˚C. Frozen tissue was transferred into glass lyophilizer flasks and lyophilized before cry milling. To prepare (AM) extract, the experiments mentioned in the article have been used [24].
Cell culture and virus
Vero, MDCK, and MA104 cell lines were used to propagate HSV-1, Influenza A, and rotavirus, respectively. Dulbecco's Modified Eagle's medium (DMEM) (Invitrogen, USA) medium containing 10% fetal bovine serum (FBS; Invitrogen, USA) and 1% penicillin/streptomycin (Bioidea, Iran) was used for cell culture.
Cellular cytotoxicity test
MTT assay was used to determine the toxicity effect of compounds on Vero, MDCK, and MA-104 cells. In this method, Vero, MDCK, and MA104 cell suspension was prepared at a concentration of 1.5 × 105 cells/ml and was added to each well of 96-well microplates (SPL Life Science, South Korea) using 100 μl of this solution (this was done for each cell line separately). 96-well plates were incubated at 37 ˚C for 24 hours to form a cellular monolayer at the bottom of each well. When a cellular monolayer was formed, the medium in each well was removed, and six different concentrations of four compounds were prepared and added to each well. From carrier, concentrations of 96.25, 48.125, 24.06, 12.03, 6.015 and 3.00 μg / ml, from A. euchroma, concentrations of 144.15, 72.07, 36.038, 18.018, 9.009 and 4.504 μg /ml, from the material AM + A. euchroma, concentrations of 11.788, 5.894, 2.947, 1.473, 0.736, and 0.368 μg /ml, and from AM, concentrations of 11.5, 5.75, 2.875, 1.437, 0.718, and 0.359 μg /ml were prepared. Wells containing only DMEM medium were also considered as cellular controls. Plates were incubated at 37 ˚C for 48 hours. Afterward, the media were removed, and the cells were washed twice with phosphate-buffered saline (PBS, Bioidea, Iran). Then, 100µl of RPMI without phenol red (Bioidea, Iran) was added to all wells, and then 10 µl of MTT solution (5 mg/ml) (Bioidea, Iran) was added. Plates were incubated again at 37 ˚C for 3 hours. Then, the contents of each well were removed, and purple formazan crystals in the cells were dissolved by adding 50 μl of dimethyl sulfoxide (DMSO) (Bioidea, Iran). Plates were incubated for 10 minutes at 37 ˚C and placed on the shaker for 15 minutes. Finally, light absorption was read at 550 nm by enzyme-linked immunosorbent assay (ELISA) Reader (Hiperion MPR 4+, Germany) on each well. Cell viability was calculated for each compound concentration compared to the results obtained from cell control wells.
Evaluation of antiviral activity by TCID50 method
Initially, monolayers of Vero, MDCK, and MA104 cells were prepared in 96-well plates (separately to investigate the antiviral effect of different compounds on Rota, Influenza A, and HSV-1 viruses). Then, the cells were washed once with PBS, and 100 μl of 100TCID50 virus suspension was added to all wells. Plates were incubated at 37 ˚C for one hour. Then, the plates were removed, and cells were washed three times with PBS to remove untreated viruses from wells. Then, 100 μl of the highest concentration of the four compounds in the non-toxic range was added to the wells. Wells were also considered as controls where DMEM medium was added with 2% FBS instead of suspension containing desired compounds. After the formation of cytopathic effects on the contents of each well, the TCID50 assay was performed. The Ethical Committee of Iran University of Medical Sciences, Tehran, Iran, where the study was conducted, approved the study design (approval number: 95-02-206-28800). This study was conducted by the principles of the Helsinki Declaration.
Statistical analysis
All experiments were done in triplicate independently. To compare the means at different concentrations, the first one-way analysis of variance was used, and then if the result of the analysis of variance test was significant, the Dant test was used to compare the mean of different viral groups with virus control.
Results
Cytotoxicity test
Toxicity test results showed that a concentration of 48.125 μg/ml of the carrier, a concentration of 36.038 μg/ml of A. euchroma, a concentration of 2.947 μg/ml of AM + A. euchroma, and a concentration of 1.437 μg/ml of AM could maintain up to 90% of cell viability on all three cell lines and therefore these concentrations have been used in subsequent antiviral tests.
Determination of antiviral activity by TCID50 assay
Antiviral activity against HSV-1
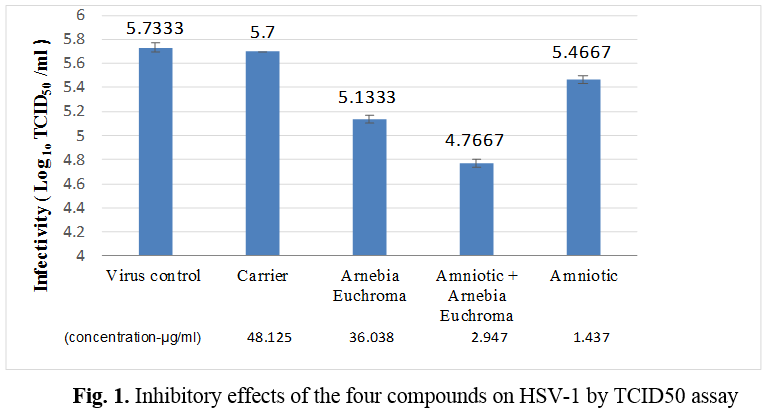
Among four compounds, a mixture of AM + A. euchroma at the concentrations of 2.947 μg/ml could exert the highest antiviral activity against HSV-1 and led to 1 log10 TCID50 reduction in virus titer when compared to the virus control, respectively (p < 0.0001). Also, exposure to HSV-1 with A. euchroma (36.038 μg/ml) and AM (1.437 μg/ml) could be led to 0.6 and 0.3 log10 TCID50 reductions in virus titer when compared to the virus control, respectively (p < 0.0001) (Table 1, Figure 1).
Antiviral activity against Influenza virus
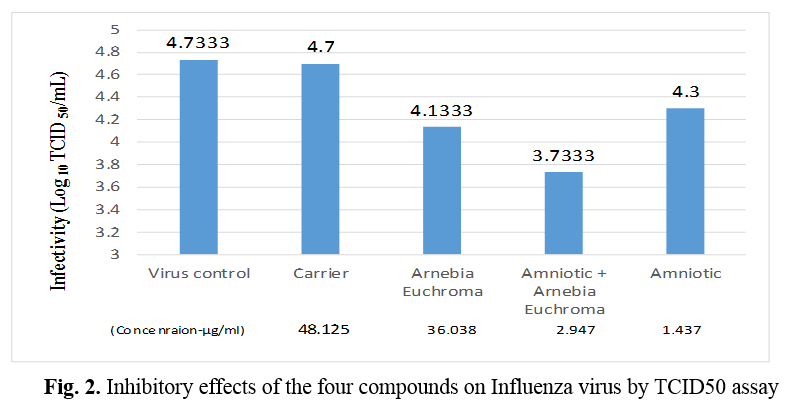
Similar to HSV-1, a mixture of AM + AE at the highest non-toxic concentration (2.947 μg/ml) showed the highest antiviral activity against the Influenza virus and led to 1 log10 TCID50 reduction in virus titer when compared to the virus control, respectively (p < 0.0001). Likewise, exposure to Influenza virus with AE (36.038 μg/ml) and AM (1.437 μg/ml) could be led to 0.6 and 0.4 log10 TCID50 reductions in virus titer when compared to the virus control, respectively (p < 0.0001) (Table 2, Figure 2).
Antiviral activity against rotavirus
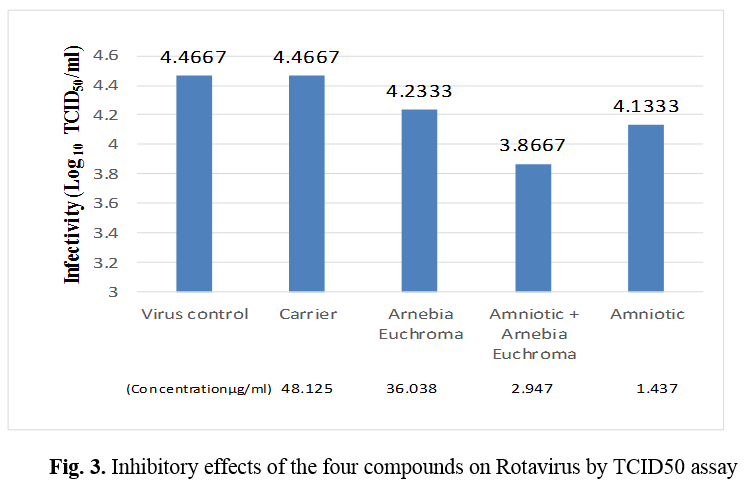
Regarding the rotavirus, the highest non-toxic concentration of a mixture of AM + AE (2.947 μg/ml) exerted the highest antiviral activity, leading to 0.6 log10 TCID50 reductions in virus titer when compared to the virus control, respectively (p < 0.0001). Exposure of rotavirus with AM at the concentration of 1.437 μg/ml and AE at the concentration of 36.038 μg/ml could be led to 0.3 (p < 0.0001) and 0.2 (p =0.002) log10 TCID50 reduction in virus titer when compared to the virus control, respectively (Table 3, Figure 3).
Table 1. Antiviral effect of the studied compounds against HSV-1 by TCID50 assay
| Test Groups |
Concentration
(µg/ml) |
Mean log10 TCID50/ml |
The P-value for the difference with virus control |
| Virus Control |
- |
5.7333 |
- |
| Carrier |
48.125 |
5.7000 |
0.8 |
| A. euchroma |
36.038 |
5.1333 |
<0.0001 |
| Amniotic + A. euchroma |
2.947 |
4.7667 |
<0.0001 |
| Amniotic |
1.437 |
5.4667 |
<0.0001 |
Table 2. Antiviral effect of the studied compounds against Influenza virus by TCID50 assay
| Test groups |
Concentration
(µg/ml) |
Mean Log10 TCID50/ml
Test |
The P-value for the difference with virus control |
| Virus control |
- |
4.7333 |
- |
| Carrier |
48.125 |
4.7000 |
0.9 |
| A. euchroma |
36.038 |
4.1333 |
<0.001 |
| Amniotic + A. euchroma |
2.947 |
3.7333 |
<0.001 |
| Amniotic |
1.437 |
4.3000 |
<0.001 |
Table 3. Antiviral effect of the studied compounds against Rotavirus by TCID50 assay
| Test Groups |
Concentration
(µg/ml) |
Mean Log TCID50/ml
Test |
The P-value for the difference with virus control |
| Virus Control |
- |
4.4667 |
- |
| Carrier |
48.125 |
4.4667 |
0.9 |
| A. euchroma |
36.038 |
4.2333 |
0.002 |
| Amniotic + A. euchroma |
2.947 |
3.8667 |
<0.0001 |
| Amniotic |
1.437 |
4.1333 |
<0.0001 |
Discussion
Recently, the limited therapeutic effect of antiviral chemicals is considered a concern with high toxicity and side effects, as well as the emergence of drug resistance. In treating viral diseases, it should be noted that viruses proliferate cellularly, so new antiviral compounds must be capable of differentiating viral and host activity with a high point of specificity to minimize host cell damage. In this regard, the use of plants in recent years has received special attention. In most regions of the world, herbs are used to treat many infectious diseases, such as lemongrass extract, which is employed to treat influenza and HSV. It likewise receives an effective inhibitory effect against HSV.
A. euchroma is considered an aerobic and herbaceous plant, which is utilized as one of the traditional plants in treating burn injuries. Surveys have proven that the roots of dicotyledonous species of the bivalve family are rich in naphthoquinone [25]. These substances, their derivatives (alkanes), and their alkanes have intrinsic properties with a wide range of biological properties such as wound healing [7, 26], antifungal [27], antiviral (such as influenza AIDS) [28, 29], and anti-cancer activities [11-13]. Research has also shown that this plant has antimicrobial [29] and anti-inflammatory [13] effects. Therefore, the main focus of the present study was to evaluate the antiviral activity of the extract A. euchroma [23, 30]. Initially, the toxicity of the extract, as described in the experimental section on Vero, MDCK, and MA-104 cells at 36.038 μg / ml, was determined to be non-toxic. However, it caused cell death at higher concentrations.
AM can be used as a proliferative agent in wounds because they contain fibronectin, elastin, fibrinogen, collagen, elastin, and hyaluronic acid [31, 32]. It also produces B-defensin, a large group of antimicrobial and antiviral peptides [33]. These peptides protect the epithelial surface against microbial colonization. Besides, Elafin and proteinase-inhibiting leukocytes, both of which have antimicrobial activity, are also secreted from the amniotic fluid. These agents protect the wound surface against HSV and varicella-zoster [34, 35]. Kejargard (2001) concluded that the presence of cysteine E in (AM), which is an analog of cysteine protease inhibitors, has antiviral properties [34]. In a study, Tehrani et al. examined synthetic membranes' antibacterial (AM) effect on E-coli, Pseudomonas aeruginosa, and strains of Proteus mirabilis in vitro. They also examined the ratio of bacterial growth below the surface of embryonic membranes to amniotic secretions [36].
In the last century, with the advancement of virology and the discovery of the viral cause of many diseases, the need for research on antiviral therapy has become increasingly apparent, as antiviral chemical drugs, which have limited therapeutic, toxic, and side effects, are now available. Effects and emergence of drug resistance concerns have increased. Open chemotherapy against viruses always has interested researchers [37]. However, some limitations prevent the development of chemotherapy for viruses. Examples of these barriers are viruses that multiply within the cell. Thus, antiviral compounds are useful for distinguishing between viral and host activities with a high degree of specificity to differentiate the least damage to host cells. In recent studies to expand new therapeutic research for antiviral substances to reduce the unwanted side effects of resistant viruses, plants are given special attention [38, 39] as an example of herpes simplex virus type I infections, the most common infection. It is a virus that is more common in humans than any other virus.
The primary infection occurs early in life, and gradually, the body produces antibodies against the virus. However, this antibody's production eventually leads to the removal of the virus [40, 41], and the virus is hidden in the sensory ganglia that lead to hosts; the person carries the virus for a lifetime and sometimes causes an infection. It reappears in the person, and the carrier is active at the time of infection and easily transmits the virus through daily contact. Acyclovir, which acts on DNA polymerase to prevent virus replication, is the treatment of choice for herpes simplex.
In current years, concerns about treatment with acyclovir due to the report of drug-resistant mutations, on the one hand, oral and injectable administration with several side effects, on the other hand, this drug only reduces the duration of the disease and the ability to prevent It does not transmit viral infections to others and prevents recurrence of infections. According to the above, we must try to find new ways to treat herpes infections. The results of the documentation of the use of AM in combination with antiviral drugs in treating some diseases allowed us to seriously pursue this research despite the limitations [42-44]. Double research is required to understand exactly how these combinations' mechanisms of synergistic effects work. This research shows that with additional tests, especially on animal and human models, we can have therapeutic interventions against infections caused by these viruses in various forms of medicine.
Given the satisfactory results of our experiments, but due to the research limitations, the subsequent in vivo steps must be carefully examined and additional diagnostic tests performed. Parts of the extract identified and isolated the effect of antiviral activity, but ultimately its effect on altering the synthesis of proteins and various viral agents must be determined.
Considering the antimicrobial effect of A. euchroma and AM, it can be concluded that the Amniotic compound + A. euchroma has the greatest inhibitory effect on HSV-1, influenza A, and rotavirus. It probably has the greatest antiviral effect on the absorption or early
stages of proliferation. This theory is reinforced by the presence of Shikonin and naphtho-quinone in the plant. A. euchroma roots contain important chemical components such as naphthoquinones, Shikonin, alkanes, and their derivatives extracted with organic solvents. Shikonin is a naphthoquinone pigment that has been documented to study its antiviral properties [11, 17].
This study has several limitations. First, we could not determine the mechanism of antiviral activity of compounds, and more studies are needed to clarify this. The second limitation is that we examined compounds' cytotoxicity and antiviral activity on the animal cell lines, and future studies can perform on human cell lines.
Conclusions
The AM is the innermost adjacent to the amniotic fluid and the fetus. This membrane is rich in growth factors and has antimicrobial and antiviral properties, reducing infection, anti-inflammatory properties, and pain. The unique properties of the AM have led to its widespread use in medicine, the exact mechanism of which is not yet known. The AM contains cystatin-E, an analog of cysteine proteinase inhibitors with antiviral properties. The efficacy of drug compounds resulting from the combination of two drugs with antiviral properties can synergistically alter the virus sensitivity to the drug and the effectiveness of the drug. Confirmation of such compounds' antimicrobial and antiviral properties certainly requires further studies. There is also a need to present the results of long-term animal and clinical studies.
Conflict of Interest
The authors have no conflict of interest.
Acknowledgments
We would like to thank Dr. Fazel Gorjipour (Biotechnology Research Center of Iran University of Medical Sciences), Mohammad Taghi Rahnama (Medical Laboratory Expert and Officials of the Maternity ward), the pharmaceutical care department of Firozabadi Hospital, and the Virology department of Iran University of Medical Sciences, for their great cooperation during this research project. This study was financially supported by a research grant from the Iran University of Medical Sciences, Grant Numbers 95-02-206-28800 and 95-02-206-28802.
References
- Aschale Y, Wubetu M, Abebaw A, Yirga T, Minwuyelet A, Toru M. A systematic review on traditional medicinal plants used for the treatment of viral and fungal infections in Ethiopia. J Exp Pharmacol. 2021; 13(5): 807.
- Ashkani-Esfahani S, Emami Y, Esmaeilzadeh E, Bagheri F, Namazi M. Glucosamine enhances tissue regeneration in the process of wound healing in rats as animal model: a stereological study. J Cytol Histol. 2012; 3(1): 150.
- Chen X, Yang L, Zhang N, Turpin JA, Buckheit RW, Osterling C, et al. Shikonin, a component of Chinese herbal medicine, inhibits chemokine receptor function and suppresses human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2003; 47(9): 2810-816.
- Pirbalouti A, Azizi S, Koohpayeh A, Golparvar A. Researches Centre of Medicinal Plants & Ethnoveterinary, Islamic Azad University-Shahrekord, Iran. Int J Biosci Biochem Bioinforma. 2011; 5(1): 144.
- Sasaki K, Abe H, Yoshizaki F. In vitro antifungal activity of naphthoquinone derivatives. Biol Pharm Bull. 2002; 25(5): 669-70.
- Shen CC, Syu WJ, Li SY, Lin CH, Lee GH, Sun CM. Antimicrobial activities of naphthazarins from arnebia e uchroma. J Nat Prod. 2002; 65(12): 1857-862.
- Annan K, Houghton PJ. Antibacterial, antioxidant and fibroblast growth stimulation of aqueous extracts of Ficus asperifolia Miq. And Gossypium arboreum L., wound-healing plants of Ghana. J Ethnopharmacol. 2008; 119(1): 141-44.
- Bali YY, Yilanci S, Yuzbasioglu M, Unlu RE, Orhan E, Guvenalp Z, et al. The evaluation of wound healing potential of acetyl alkannin isolated from Arnebia purpurea. Planta Med. 2015; 81(16): 14.
- Zhang Y, Han H, Sun L, Qiu H, Lin H, Yu L, et al. Antiviral activity of shikonin ester derivative PMM-034 against enterovirus 71 in vitro. Braz J Med Biol Res. 2017; 50(10): 6586.
- Nuorani M. Encyclopedia of Medicine. Volume III Tehran: Miras Maktub Pub, 2005.
- Papageorgiou VP, Assimopoulou AN, Couladouros EA, Hepworth D, Nicolaou K. The chemistry and biology of alkannin, shikonin, and related naphthazarin natural products. Angew Chem Int Ed. 1999; 38(3): 270-301.
- You YJ, Kim Y, Song GY, Ahn BZ. (E)-6-(1-alkyloxyiminoalkyl)-5, 8-dimethoxy-1, 4-naphthoquinones: synthesis, cytotoxic activity and anti-tumor activity. Bioorg Med Chem Lett. 2000; 10(20): 2301-303.
- Singh B, Sharma M, Meghwal P, Sahu P, Singh S. Anti-inflammatory activity of shikonin derivatives from Arnebia hispidissima. Phytomedicine 2003; 10(5): 375-80.
- Malik S, Bhushan S, Sharma M, Ahuja PS. Biotechnological approaches to the production of shikonins: a critical review with recent updates. Crit Rev Biotechnol. 2016; 36(2): 327-40.
- Liang W, Cai A, Chen G, Xi H, Wu X, Cui J, et al. Shikonin induces mitochondria-mediated apoptosis and enhances chemotherapeutic sensitivity of gastric cancer through reactive oxygen species. Sci Rep. 2016; 6(1): 1-2.
- Huang G, Zhao HR, Meng QQ, Zhang QJ, Dong JY, Zhu BQ, et al. synthesis and biological evaluation of sulfur-containing shikonin oxime derivatives as potential antineoplastic agents. Eur J Med Chem. 2018; 143(1): 166-81.
- Gao H, Liu L, Qu ZY, Wei FX, Wang SQ, Chen G, et al. Anti-adenovirus activities of shikonin, a component of Chinese herbal medicine in vitro. Biol Pharm Bull. 2011; 34(2): 197-202.
- Kim JS, Kim JC, Na BK, Jeong JM, Song CY. Amniotic membrane patching promotes healing and inhibits proteinase activity on wound healing following acute corneal alkali burn. Exp Eye Res. 2000; 70(3): 329-37.
- Calvin SE, Oyen ML. Microstructure and mechanics of the chorioamnion membrane with an emphasis on fracture properties. Ann N Y Acad Sci. 2007; 1101(1): 166-85.
- Meller D, Pires RT, Mack RJ, Figueiredo F, Heiligenhaus A, Park WC, et al. Amniotic membrane transplantation for acute chemical or thermal burns. Ophthalmology 2000; 107(5): 980-89.
- Ye X, Berg M, Fossum C, Wallgren P, Blomström AL. Detection and genetic characterisation of porcine circovirus 3 from pigs in Sweden. Virus Genes 2018; 54(3): 466-69.
- Liu T, Ma C, Yang L, Wang W, Sui X, Zhao C, et al. Optimization of shikonin homogenate extraction from arnebia euchroma using response surface methodology. Molecules 2013; 18(1): 466-81.
- Gatera M, Bhatt S, Ngabo F, Utamuliza M, Sibomana H, Karema C, et al. Successive introduction of four new vaccines in Rwanda: High coverage and rapid scale up of Rwanda's expanded immunization program from 2009 to 2013. Vaccine 2016; 34(29): 3420-426.
- Mahbod M, Shahhoseini S, Khabazkhoob M, Beheshtnejad AH, Bakhshandeh H, Atyabi F, et al. Amniotic membrane extract preparation: what is the best method? J Ophthalmic Vis Res. 2014; 9(3): 314.
- Muñoz LS, Barreras P, Pardo CA. Zika virus–associated neurological disease in the adult: Guillain–Barré syndrome, encephalitis, and myelitis. Semin Reprod Med. 2016; 34(05): 273-79.
- Parashar UD, Gibson CJ, Bresee JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006; 12(2): 304.
- Glass RI, Bresee JS, Parashar UD, Jiang B, Gentsch J. The future of Rotavirus vaccines: a major setback leads to new opportunities. Lancet 2004; 363(9420): 1547-550.
- Guerrant RL, Carneiro-Filho BA, Dillingham RA. Cholera, diarrhea, and oral rehydration therapy: triumph and indictment. Clin Infect Dis. 2003; 37(3): 398-405.
- Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe Rotavirus gastroenteritis. N Engl J Med. 2006; 354(1): 11-22.
- Sanford C, Langley JM, Halperin SA, Zelman M, Maritime Universal Rotavirus Vaccination Program MU. A universal infant Rotavirus vaccine program in two delivery models: Effectiveness and adverse events following immunization. Hum Vaccin Immunother. 2015; 11(4): 870-74.
- Lavie G, Valentine F, Levin B, Mazur Y, Gallo G, Lavie D, et al. Studies of the mechanisms of action of the antiretroviral agents hypericin and pseudohypericin. Proc Natl Acad Sci. 1989; 86(15): 5963-967.
- Malhotra C, Jain AK. Human Amniotic Membrane transplantation: different modalities of its use in ophthalmology. World J Transplant. 2014: 4(2): 111.
- Miki T. Amnion-derived stem cells: in quest of clinical applications. Stem Cell Res Ther. 2011; 2(3): 25.
- Kjaergaard N, Hein M, Hyttel L, Helmig R, Schønheyder H, Uldbjerg N, et al. Antibacterial properties of human amnion and chorion in vitro. Eur J Obstet Gynecol Reprod Biol. 2001; 94(2): 224-29.
- Shimazaki J, Yang HY, Tsubota K. Amniotic Membrane transplantation for ocular surface reconstruction in patients with chemical and thermal burns. Ophthalmology 1997; 104(12): 2068-2076.
- Tehrani FA, Modaresifar K, Azizian S, Niknejad H. Induction of antimicrobial peptides secretion by IL-1β enhances human amniotic membrane for regenerative medicine. Sci Rep. 2017; 7(1): 1-7.
- Field AK, Biron KK. The end of innocerice revisited: resistance of herpesvirus to antiviral drugs. Clin Microbiol Rev. 1994; 7: 1-13.
- Vlietinck AJ, Vanden Berghe DA. Can ethnopharmacology contribute to the development of antiviral drugs? J Ethnopharmacol. 1991; 32(1): 141-53.
- Cragg GM, Newman DJ, Snader KM. Natural products in drug discovery and development. J Nat Prod. 1997; 60(1): 52-60.
- David M, Peter M, Hawley. Fields Virology. New York: Williams & Wilkins, 2001; p. 2399-460.
- Collier L, Balows A, Sussman M. Microbiology and microbial infections. Around USA, Collier L,(eds) 9th ed WB. Saunders Company; 1998.
- Shi W, Chen M, Xie L. Amniotic membrane transplantation combined with antiviral and steroid therapy for herpes necrotizing stromal keratitis. Ophthalmology 2007; 114(8): 1476-481.
- Cheng AM, Tseng SC. Self-retained amniotic membrane combined with antiviral therapy for herpetic epithelial keratitis. Cornea 2017; 36(11): 1383.
- Mencucci R, Paladini I, Menchini U, Gicquel JJ, Dei R. Inhibition of viral replication in vitro by antiviral-treated amniotic membrane. Possible use of amniotic membrane as drug-delivering tool. Br J Ophthalmol. 2011; 95(1): 28-31.