Heart hypertrophy is the growth of the heart muscle, the increase in wall thickness, and the heart's rheumatoid arthritis. There is no new cell in hypertrophy, and only the size of the cells becomes larger than normal, which is due to the increase in the number of organs, the synthesis of building proteins, the size of the myocytes, and changes in changes metabolism. Left ventricular hypertrophy is an enlargement of the muscles of the heart's left ventricle that occurs due to high blood pressure or narrowing of the aortic valve [
1]. Studies over the past decade have shown that cardiac hypertrophy is a complex process caused by hemodynamic, genetic, neurological, neurological changes, growth factors, and cytokines. Cardiac fibers change without changing the number of heart cells [
2,
3]. Cardiac deformation is associated with the growth and alteration of cardiac myocytes and interstitial matrix, which involves the accumulation of fibroblasts and macromolecules such as collagen, elastin, and fibronectin in the extracellular space. It also causes loss of myofilament and myocyte apoptosis. These changes are associated with abnormalities in extracellular calcium regulation, inflammatory processes initiation, initiation of complex changes in the expression of cardiac coding genes in ion channels, growth factors, and metabolic enzymes. These improper changes cause disproportionate growth of the heart muscle.
Bcl-2 family of anti-apoptotic proteins has great potential in the treatment of heart disease. Researchers have found that transgenic mice with high
Bcl-2 expression in their hearts reduce ischemia in these transgenic mice [
4]. Although many medications are used to treat myocardial hypertrophy, the prevalence of heart failure indicates the inability to treat the disease. Various human and animal studies have shown that plant compounds can play an important role in increasing cardiac resistance to cardiovascular disease. Monoterpenes such as thymol are suitable candidates for these compounds, whose cardiovascular effects have been considered recently. Despite the importance of
Bcl-2 family proteins in myocardial hypertrophy pathogenesis, no study has been performed on thymol-induced protein expression in the heart, so in the present study, we examined this issue [
5,
6].
Materials and Methods
Chemicals
Ketamine, Xylazine, Thymol (Sigma-Aldrich), Master mix RT High Rox, Dnase free Rnase free 1.5 microtubes (Cinagen, Iran), Dnase free Rnase free 0.5 microtube (Cinagen, Iran), Dnase free Rnase free 0.2 microtube (Cinagen, Iran), DNA extraction solution (Cinagen, Iran), DNA synthesis kit (Cinagen, Iran), DNaxi Lyophilized powder (Cinagen, Iran) were used in this study.
Animals
The healthy male rats of the Wistar breed weighing 220-170 gr were used as the animals in the present study. The animal shelter was equipped with light control, ventilation, and temperature control systems. The animals were kept in day and night conditions for 12 hours of light and 12 hours of darkness, a temperature
of 22.2 °C, free access to water and food. All ethical principles were implemented in accordance with the principles of working
with approved laboratory animals at Shahid Sadoughi University of Medical Sciences in Yazd. These animals were kept in the animal wards of the Department of Physiology, Yazd University of Medical Sciences.
Animal grouping
For this study, 30 male Wistar rats were divided into six groups (5n = in each group). 1- Control group (ctl): The experiment in this group aimed to record the blood pressure of intact animals, measure HW / BW, and collect left ventricular blood samples for molecular examination of
Bcl-2 family gene expression and comparison with other groups. 2- Hypertrophic group (H): In this group, the animals underwent surgery and narrowing of the abdominal aorta, and four weeks later, without any drug intervention, samples were collected in them.3- Thymol receiving group with a dose of 25 mg/kg /day + hypertrophy (Thym 25 + H). 4- Timol receiving group with a dose of 50 mg/kg/day + hypertrophy (Thym 50 + H).
Induction of hypertrophic model
Anesthesia was induced by intramuscular injection of ketamine-xylazine. The dose was 70 mg/kg ketamine and 10 mg/kg xylazine. Left side hair, the distance between the last rib and the femur shaved, and the animal was placed on a surgical bed with a thermal pad on it. The skin is cut near the spine with a surgical razor, then using scissors and pliers. The areas under the skin loosened, and the layer under the skin was cut with scissors in the same direction as before. Using the tilted head pliers, we removed the fat, found the abdominal aortic artery, passed the thread under it and around the artery, and tied the needle. After ensuring that the artery is tight and not completely blocked, we removed the excess threads and the needle, then returned all the fat to its place. Then, using a Rando needle and absorbable thread, we removed the layer under the skin, sutured the catheter, and non-absorbable thread to the skin as a single suture, using a tetracycline spray at the site of the incision, and returned the rat to the cage. Each animal was kept in a separate cage. After two weeks, we used a hypertrophic model for our treatments [
7].
Collecting tissues
The rats were weighed and recorded, then anesthetized and fixed their body on the surgical screen. We measured blood pressure through cannulation of the carotid artery. Then, using a pair of pliers, we cut the ends of the ribs and cut them with special scissors. After cutting the skin with a zippered clamp, we cut the diaphragm using scissors and cut the internal visceral connections with the chest wall. It was cut with scissors to make the heart fully accessible. We fixed the xiphoid, then removed the heart from the chest, removed the tissue from the left ventricle, and transferred it into liquid nitrogen and then to the 80-freezer for molecular studies. Target gene expression changes were evaluated by real time polymerase chain reaction (RT-PCR).
Homogeneous tissue and cDNA synthesis
According to the instructions of the RNA extraction kit, the extraction solution -RNAxplus (RNA Cinagen, Iran) was extracted. A certain dilution of the sample was read using a BioPhotometer at a wavelength of 260 nm to calculate the RNA concentration in the spectrophotometry method. cDNA synthesis was performed according to the instructions in the Vivantis kit.
Primers PCR
It was done before the RT-PCR by inserting 1 micrometer of the forward primer, 1 microliter of the reverse primer, 10 microliters of the red master, 6 microliters of water, and 2 microliters of the cDNA into a 2. (200 microliters) microtubule. A temperature gradient was performed to obtain the optimum temperature. After this step, 5 microliters of PCR product were placed in the agar gel wells immersed in the TBE buffer, and after 45 minutes, the results were observed under the UV device, resulting in 56
o C for BCL2. PCR images of different primers can be seen in Table 1. All experimental procedures were approved
by the Ethics Committee of Shahid
Sadoughi University of Medical Sciences, Yazd, Iran, with the Approval Number: IR.SSU.MEDICINE.REC.1397.022.
Statistical analysis
Rotor-Gene Q Series Software was used to analyze Real-time PCR data. Comparative quantification analysis was used to analyze the raw data, and the CTs of each of the studied genes were extracted in different groups. Next, to analyze the information related to the expression of the target genes, we analyzed the Ct results from the Real-time PCR result by the 2 2 (- ∆∆ct) method. This method is used to study changes in the relative expression of a gene between a basic state, a calibrator, and a state altered in the cell, tissue, or animal conditions. For this purpose, the CT obtained for the treatment state is divided into the CT obtained for the baseline state (negative control - without the treatment), and the obtained result indicates the increase or decrease of gene expression in these two cases. Since the efficiency of the sample purification process and reverse transcription is not the same for both cell and base modes (negative-no-treatment control) and treatment, it is necessary to remove this heterogeneity from a gene which expression in the cell is the same under any circumstances. It can be used as a reference.
Table 1. PCR images of different primers
| Primer sequences |
Gene |
5′- GGAGCATCGTTCAGCAGCAG- 3′
5′- CCATCCCTTCATCTTCCTCAGTC -3′ |
F |
BAD |
| R |
5′- GCTGGTGGTTGACTTTCTCTCC- 3'
5′- GGCTTCAGTCCTGTTCTCTTCG- 3′ |
F |
Bcl-xl |
| R |
5′ -ATACCTGGGCCACAAGTGAG- 3′
5′ -TGATTTGACCATTTGCCTGA- 3′ |
F |
Bcl2 |
| R |
5′ -CGAGCTGATCAGAACCATCA- 3′
5′ -CTCAGCCCATCTTCTTCCAG- 3′ |
F |
Bax |
| R |
5′-CTGACGTCCACCCTGACT-3′
5′-GGCAGCTATGTGAGAGCC-3′ |
F |
GAPDH |
| R |
The gene should be selected from the House of Keeping gene family, so GAPDH was used in this study, and at the end of the final data extracted from Real-time PCR, it was analyzed using the Graph Pad Prism program, and its graphs were plotted. Changes in the mRNA levels of different groups were assessed by a two-way ANOVA test and subsequent appropriate test.
Results
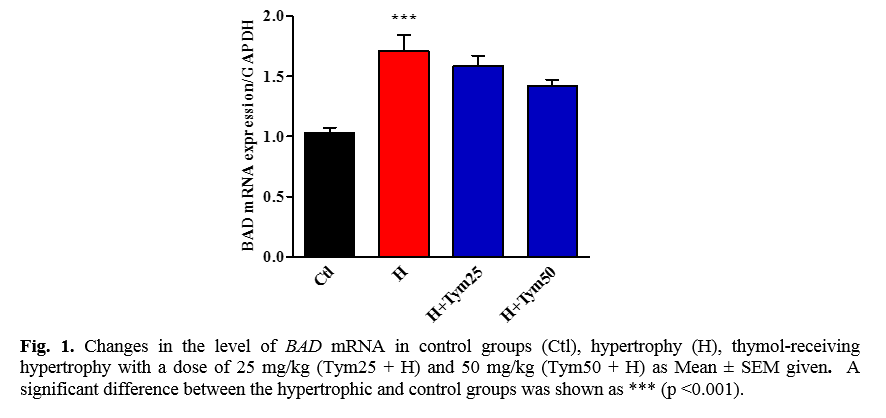
The effect of thymol on the expression of the BAD proapoptotic gene in the hypertrophied tissue of myocardial infarction
As shown in Figure 1. increasing the level of BAD mRNA in a hypertrophic group compared to control is significant. Although the level of BAD mRNA in the hypertrophic group receiving thymol doses of 25 and 50 mg/kg has increased compared to the control group, this increase is not significant.
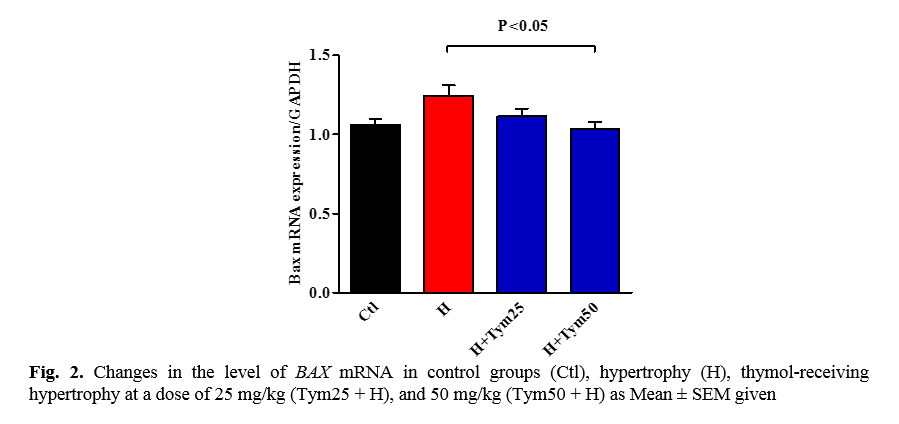
The effect of thymol on the expression of the proapoptotic BAX gene in the hypertrophied tissue of the myocardium
The results of this study can be seen in Figure 2. The mRNA BAX level in the hypertrophic and hypertrophic groups receiving thymol increased by 25 mg/kg compared to the control group, which is insignificant. According to Figure 2, a decrease in the expression of the BAX mRNA gene in the hypertrophic group receiving 50 mg/kg thymol is significant compared to the hypertrophic group (p ˂ 0.05).
The effect of thymol on the expression of Bcl-2 anticoagulant gene in myocardial hypertrophied tissue
The results of this part of the study explained that the level of mRNA Bcl-2 in the hypertrophic group (p < 0.01) and hypertrophic groups receiving 25 and 50 mg/kg thymol (p < 0.001) has a significant increase compared to the control group. The difference between the level of mRNA Bcl-2 in the hypertrophic group receiving 50 mg/kg thymol is significant compared to the hypertrophic and hypertrophic groups receiving 25 mg/kg thymol (p < 0.01) (Fig. 3).
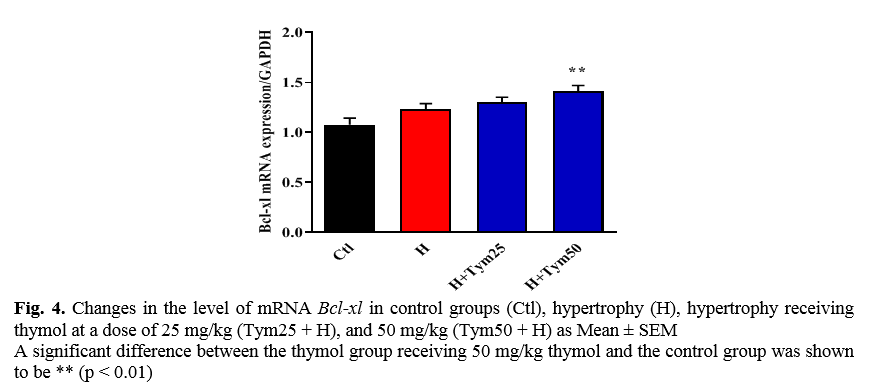
The effect of thymol on the expression of the Bcl-xl antiapoptotic gene in myocardial hypertrophied tissue
As shown in Figure 4, the level of mRNA Bcl-xl in the hypertrophic and hypertrophic groups receiving 25 and 50 mg/kg thymol increased compared to the control group. The increase is significant, but only in the hypertrophic group receiving 50 mg/kg thymol.
Discussion
The present study results showed that the mRNA
BAD level increases significantly compared to the control group in the hypertrophic group. However, in none of the groups receiving thymol, this significantly increased. Regarding the level of another pro-apoptotic factor,
BAX, the level of mRNA
BAX in the hypertrophic group and the hypertrophic group receiving 25 mg / kg of thymol increased compared to the control group, which is not significant. On the other hand, the level of mRNA
BAX in the hypertrophic group receiving 50 mg per kilogram thymol has been significantly reduced compared to the hypertrophic group. The hypertrophic group and the hypertrophic groups receiving thymol increased significantly compared to the control group. Also, in the dose-receiving group of 50 thymol, the mRNA level of
Bcl-2 is significant compared to the untreated hypertrophic groups and the 25-thymol dose-receiving hypertrophy. The expression of another factor, Bcl-xl, also increased significantly in the 50-thymol dosing group compared with the control group.
The first part of this study looked at the effects of thymol on the expression of
BAD and
BAX pro-apoptotic genes in myocardial hypertrophied tissue, which eventually revealed that the expression of these genes decreased in thymol-receiving groups [
8]. The second part of our study examined the effects of thymol on
Bcl-2 and Bcl-xl anti-apoptotic genes expressed in myocardial hypertrophied tissue, which was eventually observed to increase these genes' expression in thymol-receiving groups. There is limited information on the cardiovascular effects of thymol and similar monoterpenes; for example, Magyar et al. studied thymol monoterpenes in the dog's heart, indicating that thymol edema can be counteracted by inhibiting potassium and calcium flows. Apply erythema [9]. The research team also demonstrated the effect of thymol on inhibition of L-type calcium flow in cardiomyocytes isolated from the human ventricle by a patch-clamp technique in 2004 [
9]. Continuation of the group's research also revealed that this monoterpene affects the calcium content of the sarcoplasmic reticulum and inhibits the membrane pump of the heart cell, affecting the function of heart cells [
10]. Peixoto and colleagues conducted another study in 2010 on aorta isolated from mice that showed that thymol relaxed the vessels by the non-endothelium-dependent method. Introducing calcium from the cell membrane is one of the most important mechanisms proposed for these effects [
11].
Another finding from this study suggests that the rate of mRNA in apoptotic genes (
BAX, BAD) has increased following abdominal aortic stenosis. Past studies have shown that the mortality rate of cardiomyocytes due to apoptosis increases during hypertrophy. Yu W and colleagues (2013) showed that the rate of apoptosis in hypertrophy increased significantly [
12]. Studies by Teiger and colleagues have shown that apoptosis can be an important regulatory mechanism involved in the adaptive response of the heart to overload pressure. That concept could serve a new purpose in responding to the treatment of heart failure [
13].
Conclusion
This study showed that thymol can protect hypertrophied hearts from damage caused by hypertrophy by increasing the expression of anti-apoptotic factors and reducing the expression of pro-apoptotic factors.
Conflicts of Interest
The authors declare that there is no conflict of interest. The authors alone are responsible for the content of the paper.
Acknowledgment
The authors gratefully acknowledge all collegues assisting in this research.
References
[1]. Polyakova V, Richter M, Ganceva N, Lautze HJ, Kamata S, Pöling J, et al. Distinct structural
and molecular features of the myocardial extracellular matrix remodeling in compensated and decompensated cardiac hypertrophy due to aortic stenosis. IJC Heart Vessels 2014; 4(2): 145-60.
[2]. Morisco C, Sadoshima J, Trimarco B, Arora R, Vatner DE, Vatner SF. Is treating cardiac hypertrophy salutary or detrimental: the two faces of Janus. Am J Physiol-Heart Circul Physiol. 2003; 284(4): 1043-1047.
[3]. Verdecchia P, Schillaci G, Guerrieri M, Gatteschi C, Benemio G, Boldrini F, et al. Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation 1990; 81(2): 528-36.
[4]. El-Hosseiny L, Alqurashy N, Sheweita S. Oxidative stress alleviation by sage essential oil in co-amoxiclav induced hepatotoxicity in rats. Int J Biomed Sci. 2016; 12(2): 71-9.
[5]. Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Therapeutics 2010; 128(1): 191-227.
[6]. McLenachan JM, Dargie HJ. A review of rhythm disorders in cardiac hypertrophy. Am J Cardiol. 1990; 65(14): 42-4.
[7] . Nguyen TD, Shingu Y, Amorim PA, Schwarzer M, Doenst T. Triheptanoin alleviates ventricular hypertrophy and improves myocardial glucose oxidation in rats with pressure overload. J Cardi Fail. 2015; 21(11): 906-915.
[8]. Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell 1995; 80(2): 285-91.
[9]. Magyar J, Szentandrássy N, Bányász T, Fülöp L, Varró A, Nánási PP. Effects of terpenoid phenol derivatives on calcium current in canine and human ventricular cardiomyocytes. Euro J Pharmacol. 2004; 487(1-3): 29-36.
[10]. Szentandrássy N, Szigeti G, Szegedi C, Sárközi S, Magyar J, Bányász T, et al. Effect of thymol on calcium handling in mammalian ventricular myocardium. Life Sci. 2004; 74(7): 909-21.
[11]. Peixoto Neves D, Silva Alves K, Gomes M, Lima F, Lahlou S, Magalhães P, et al. Vasorelaxant effects of the monoterpenic phenol isomers, carvacrol and thymol, on rat isolated aorta. Fundament Clin Pharmacol. 2010; 24(3): 341-50.
[12]. Yu W, Liu Q, Zhu S. Carvacrol protects against acute myocardial infarction of rats via anti-oxidative and anti-apoptotic pathways. Biological and Pharmaceutical Bulletin. 2013; 36(4): 579-84.
[13]. Teiger E, Than V, Richard L, Wisnewsky C, Tea B-S, Gaboury L, et al. Apoptosis in pressure overload-induced heart hypertrophy in the rat. J Clin Invest. 1996; 97(12): 2891-897.