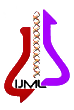BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijml.ssu.ac.ir/article-1-396-en.html
Introduction
Leishmaniasis is a worldwide, wide-range disorder without any available definitive cure, vaccine, or pesticide. It has no sterile immunity, and efforts in this field have not been successful placing it in the sixth rank of most important infectious diseases of the world and tropical regions by the World Health Organization (WHO). Cutaneous leishmaniasis (CL) is one of the zoonotic parasite diseases that is endemic in 88 countries of the four continents of the world (22 countries in Europe and America, and 66 countries in Asia and Africa). It is regarded as the most important disease of tropical and subtropical diseases after Malaria [1, 2]. With an average incidence of more than 150 per 100 000 population, it has the highest rates of infectious diseases. The number of reported CL cases increased from 13729 in 2002 to more than 24000 in 2006. Thereafter, the disease prevalence increased, and new CL foci emerged in Iran [3-5]. Although the diagnosis of CL in its typical form in sporadic areas is not a difficult task, atypical forms of the disease resemble various skin disorders such as eczema, lupus erythematosus, furuncle, atypical mycobacterium infections, deep mycosis, sarcoidosis, leprosy, syphilis, foreign body granuloma, and even sometimes malignant skin tumors which justify initiating the treatment only when the lesion is parasitologically confirmed. Routine diagnosis of CL is based on the presence of Leishmania amastigote in a direct smear prepared by scratching the periphery of a suspected lesion. Usually, research facilities in endemic areas perform culture on Novy–Nicolle–McNeal (NNN) medium in addition to direct smear [6]. Surveys of molecular epidemiology suggest that sensitivity and specificity identification of the type of CL in the area has increased the scope of the problem and the type of treatment. This study, explored for the first time the status and the proportions of CL induced by Leishmania major (L. major) and Leishmania Tropica (L. Tropica) among CL suspected patients referred to the Health Centers of Abarkouh, Ardakan, Bafgh, and Khatam cities, Yazd Province, Iran.
Materials and Methods
Sample collection
Sample collection was based on national guidelines and was equivalent to the diagnosis and treatment method of cutaneous leishmaniasis ordered by the WHO. Each patient was interviewed, and the characteristics of his or her largest wound were selected. The wound and surrounding skin were cleansed and sterilized using 70-degree ethyl alcohol. Samples were collected by shaving the skin from the margin of the lesion, and three samples were used for direct diagnosis.
Direct smear
The sample was smeared on a glass slide and stained using Giemsa (10%) stain, and then the slide was checked under the microscope in search for Leishmania Amastigote; sometimes, sampling was done more than once, twice or thrice when the first attempt failed to see the parasite, when the slide was not taken appropriately or when the sample showed superinfection.
DNA extraction
All positive and negative slides were considered to extract DNA using the Gen All Exgene Cell SV (#106-101, Gen All, Korea) according to the manufacturer's instructions. The extracted DNA was analyzed using a spectrophotometer and electrophoresis.
Detection and identification
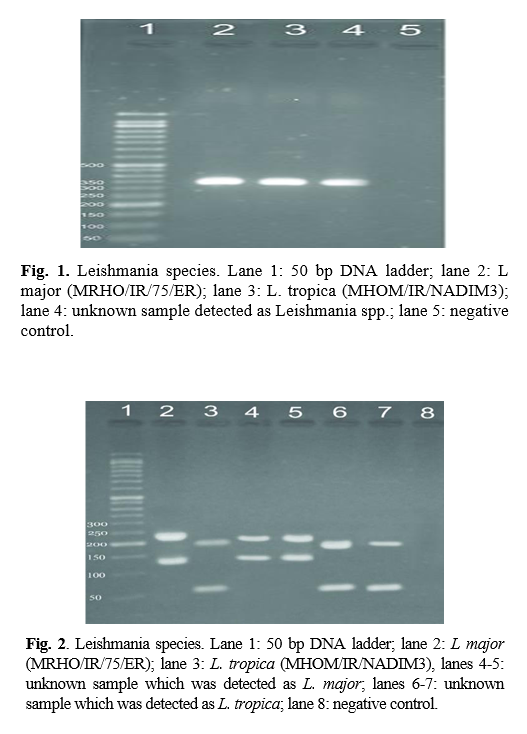
Genus detection was done using ITS1-polymerase chain reaction (PCR) by the specific primers of LITSR (5′-CTG GAT CAT TTT CCG ATG- 3′) and L58S (5′-TGA TAC CAC TTA TCG CAC TT-3′). The reaction PCR included the final concentration of 1x PCR buffer, 0.5 nm each primer, 0.2 mm dNTP, 1.5 mm MgCl2, and 5 mm DNA. The condition of amplification was done by the first denaturation of 94°C and followed by 40 cycles of denaturation at 94°C, annealing at 50°C, and extension at 72°C, each in 45 s. The final extension was done at 72°C for 5 min. The PCR product was assessed using 1% agarose gel electrophoresis alongside with 50 bp DNA ladder. The species identification was performed using restriction fragment length polymorphism) RFLP( analysis using Hae III restriction enzyme with the concentration of 10 U in each reaction of 20 µl volume. The digestive assessment was done using 3% agarose gel electrophoresis alongside with 50 bp DNA ladder. This research was approved by the Ethics Committee of Shahid Sadoughi University of Medical Sciences, Yazd, Iran (IR.SSU.REC.1394.13).
Statistical analysis
Statistical analysis was performed using SPSS16 and the related statistical tests for each type of analysis (p<0.05).
Results
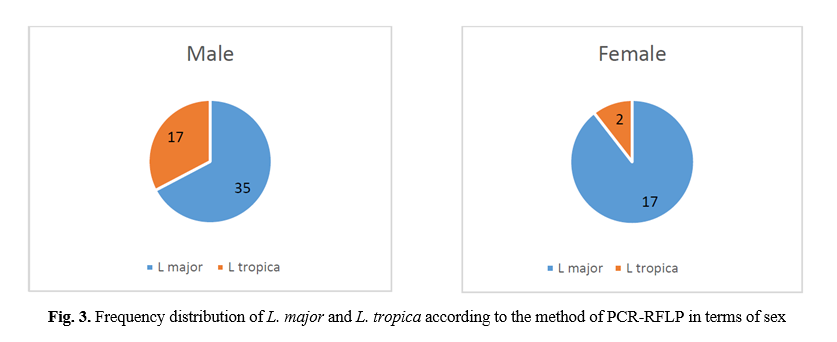
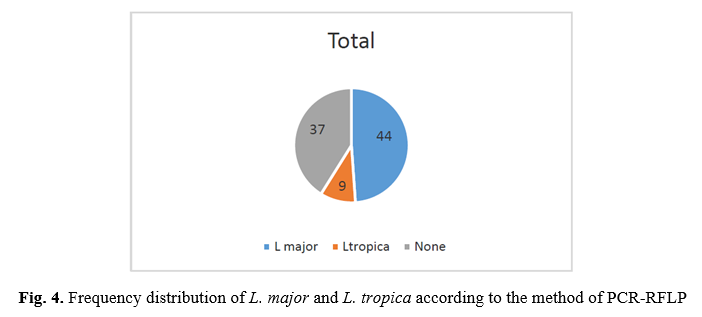
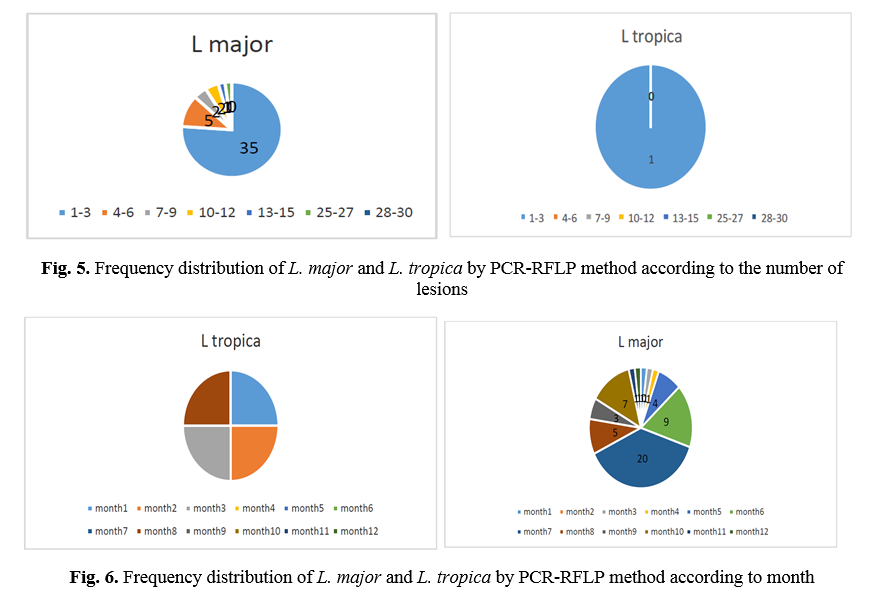
RFLP results: after species identification, all PCR products of L major and L. tropica from the skin smears of CL patients were digested by Taq1 restriction enzyme to detect potential genetic polymorphism. Digestion products showed that all isolates displayed two DNA bands of approximately 300 and 350 bp (Fig. 1 & 2). If bands 220 and 140 bp were observed after digestion of PCR products with enzyme HaeIII, it was related to L. major, and if bands 200 and 60 bp were observed, it was related to L. tropica. A total of 90 samples that were suspected patients were referred to the health centers of Abarkouh, Ardakan, Bafgh, and Khatam, Yazd Province, Iran (during 2015-2016). Of these, 64 (71.1%) samples were male, and 26 (27.9%) samples were female (Fig. 3). Thirty (33.3%) samples were from Ardakan, 29 samples from Bafgh, 21 from Abarkouh, and 10 from Khatam. The results showed that, in macroscopic examination, 90 of the patients had 59 lesions: patients with lesion(s) induced by L. major =52 (M=35, 67.3%, F=17, 32.7%), lesion(s) induced by L. tropica =4 (M=2, 50.0%, F=2, 50.0%) and lesion(s) induced by false-positive =33(36.4%). Moreover, in microscopic method, 90 of the patients showed 59 lesions: patients with lesion(s) induced by L. major =44 (M=29, 65.9%, F=15, 34.1%), lesion(s) induced by L. tropica =9 (M =8, 88.8%, F=1, 11.2%) and lesion(s) induced by false-positive =37 (M=28, 73.6%, F=9, 24.4%) (Figure 5).
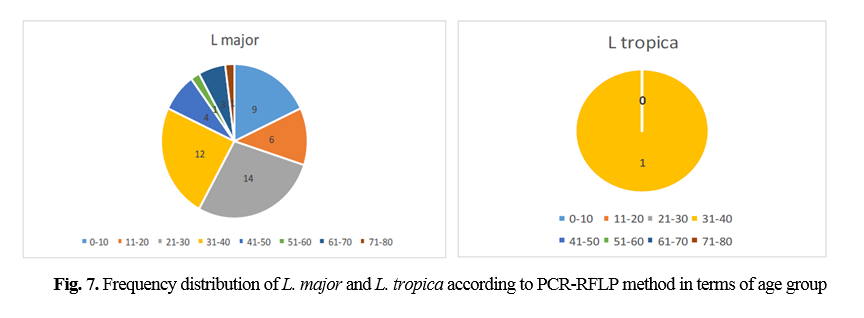
The comparison of mean L. major and L. tropica species between microscopic and molecular methods was conducted by chi-square test, which was significant (P=0.000). The history of travel to the endemic area was not always correlated with isolated Leishmania species. A total of 90 samples consisting of suspected patients who were referred to the health centers of Abarkouh, Ardakan, Bafgh, and Khatam, Yazd Province, Iran (during 2014-2015) were selected according to month and age group (Figs. 6 and 7).
Discussion
Leishmaniasis is an important disease that has been investigated by many researchers in various aspects [7-10]. The maintenance of this disease has been reported from various parts of the world due to immigration, population growth, presence of infected Phlebotomus spp., and suitable environment [11-13]. Analysis of clinical data obtained from referrals to health centers in Yazd showed that out of 897 people with leishmaniasis, 457 were men and 439 were women, and this rate is high for Yazd. Although various methods have been used for diagnosis, PCR is a sensitive and specific method used to identify leishmaniasis in Iran and this study [14-19]. The main purpose of this study was to identify different species, including L. major and L. tropica molecularly. In this study, out of 372 samples, 159 samples were positive by PCR, including 87 cases (54.7%) of L. major species and 73 cases (45.3%) of L. tropica species [20-21]. The prevalence of the two main cutaneous leishmaniases, i.e., urban and rural leishmaniasis, was almost identical in Yazd. Although L. major was higher in rural areas, L. tropica was higher in urban areas. Our data and results are consistent with most results in Iran [22-30]. CL in Yazd Province follows climatic and ecological features. In the suburbs and villages, more proximity to desert areas and the availability of rodents as a reservoir, and the lack of drugs, pesticides, and ineffective parasite control programs cause the spread of rural leishmaniasis. Urban species of CL in developed urban areas due to continuous traffic from other provinces and neighboring countries (Afghanistan, Pakistan, Iraq, and others) cause a steady outbreak of leishmaniasis. Additionally, indigenous and immigrated populations, whether domestic or foreign, lack of appropriate drugs to treat patients, lack of pesticides to control vector mosquitoes, and lack of coherent planning for further control and prevention, insecticides, and ineffective parasite control programs cause the spread of rural leishmaniasis.
Conclusion
Confirmation of the parasite's diagnosis is the only reliable evidence for starting treatment, even though a history of travel to or from a known native area is an important factor in suspecting a lesion known as cutaneous leishmaniasis. Cutaneous leishmaniasis is a combination of urban and rural leishmaniasis in Yazd Province. However, the distribution of Indus is different. In rural and less developed areas, the predominant rural species is dominant whereas, in urban and developed areas, urban-type is prevalent. Nevertheless, in total, 50% of leishmaniasis in Yazd Province is rural and 50% is urban. Planning to control and reduce the incidence and prevalence of cutaneous leishmaniasis and combating reservoirs in Yazd province can be done by specifying the extent and type of the problem.
Conflicts of Interest
There was no conflict of interest.
Acknowledgments
The authors would like to thank the authorities of the school of medicine for their financial support and those who have assisted in the experimental procedure, especially to Mrs. Marziyeh Modares Sanavi and Mrs.Tayebe Karimi of the medical parasitology and mycology department, School of Medicine.
References
[1]. Ardehali S, Moattari A, Hatam G, Hosseini S, Sharifi I. Characterization of Leishmania isolated in Iran: Serotyping with species specific monoclonal antibodies. Acta tropica 2000; 75(3): 301-307.
[2]. Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet 2005; 366 (9496): 1561-577.
[3]. Como N, Pipero P, Meta E, Kraja D. Clinical aspects of visceral leishmaniasis in albanian adults. Anglisticum Journal 2016; 3(5): 132-40.
[4]. No JH. Visceral leishmaniasis: Revisiting current treatments and approaches for future discoveries. Acta tropica 2016; 155(3): 113-23.
[5]. Hoare CA. Early discoveries regarding the parasite of oriental sore. Transactions of the Royal Society of Tropical Medicine and Hygiene 1938; 32(1): 12-92.
[6]. Wright JH. Protozoa in a case of tropical ulcer (“Delhi sore”). J Med Res. 1903; 10(3): 472.
[7]. Hassanshahi Gh, Alavi SE, Khorramdelazad H, Ahmadi A, Fattahi Bafghi A, Abdollahi, et al. Serum levels of CC chemokine ligands in cutaneous Leishmaniasis patients. J Parasit Dis. 2020; 44(3): 1-6.
[8]. Eslami G, Fattahi Bafghi A, Lotfi MH, Mirzaei F, Ahmadi S, Tajfiroozeh AA, et al. Isolation and molecular identification of leishmania spp. agents in patients with cutaneous Leishmaniasis in Yazd Province, endemic region of central Iran. 2020; Iran J Public Hlth. 2020; 49(5): 975-80.
[9]. Fattahi Bafghi A, Vahidi AR, Anvari MH, Barzegar K, Ghafourzadeh M. In vivo Antileishmanial activity of alcoholic extract from Nigella sativa seeds. African J Microbiol Res. 2011; 5(12): 1504-510.
[10]. Fattahi Bafghi A, Noorbala M, Noorbala MT, Aghabagheri M. Anti Leishmanial effect of zinc sulphate on the viability of Leishmania tropica and L. major Promastigotes, Jundishapur J Microbiol. 2014; 7(9): 11192.
[11]. Fata A, Salehi Sangani G, Rafatpanah H, Mousavi Bazzaz M, Mohaghegh MA, Movahedi A. Identification of Leishmania species by kinetoplast DNA-polymerase chain reaction for the first time in Khaf district, Khorasan-e-Razavi province, Iran. Trop Parasitol. 2015; 5(1): 50-4.
[12]. Fattahi Bafghi A, Dehghani Ashkezari M, Vakili M, Pournasir S. Polyurethane sheet impregnated with Arabinogalactan can lead to increase of attachment of promastigotes and Amastigote of Leishmania major (MRHO/IR/75/ER) by GP63 and HSP70 genes. Material Sci Engineer. 2018; 91(2): 292-96.
[13]. Ayatollahi J, Fattahi Bafghi A, Shahcheraghi SH. Rare variants of cutaneous leishmaniasis presenting as eczematous lesions. Med J Islam Repub Iran 2014; 28(7): 71.
[14]. Ayatollahi J, Fattahi Bafghi A, Shahcheraghi SH. Chronic zoster-form: a rare variant of cutaneous leishmaniasis, 2015; Reviews Med Microbiol. 2015; 26(3): 114-15.
[15]. Russell R, Iribar MP, Lambson B, Brewster S, Blackwell JM, Dye C, et al. Intra and inter-specific microsatellite variation in the Leishmania subgenus Viannia. Mol Biochem Parasitol. 1999; 103(1): 71-7.
[16]. Fattahi Bafghi A, Bagheri SM, Hejazian SH. Antileishmanial activity of Ferula asafetida oleo gum resin (asafetida) against Leishmania major: An in vitro study, J Ayurveda Integr Med. 2014; 5(4): 223-26.
[17]. Lesmes ACZ, Castro EC, Bello F. Characterization of cell cultures derived from Lutzomyia spinicrassa (Diptera: Psychodidae) and their susceptibility to infection with Leishmania (Viannia) braziliensis. Med Sci Monitor 2005; 11(12): 457-64.
[18]. Alimoradi S, Hajjaran H, Mohebali M, Mansouri F. Molecular identification of Leishmania species isolated from human cutaneous leishmaniasis by RAPD-PCR. Iran J Public Hlth. 2009; 38(2): 44-50.
[19]. Razmjou S, Hejazy H, Motazedian MH, Baghaei M, Emamy M, Kalantary M. A new focus of zoonotic cutaneous leishmaniasis in Shiraz, Iran. Transactions of the Royal Society of Tropical Medicine and Hygiene 2009; 103(7): 727-30.
[20]. Wortmann G, Hochberg LP, Arana BA, Rizzo NR, Arana F, Ryan JR. Diagnosis of cutaneous leishmaniasis in Guatemala using a real-time polymerase chain reaction assay and the SmartCycler®. Am J Tropic Med Hygiene 2007; 76(5): 906-908.
[21]. Isabel M, Gabriele S, Safar F, et al. Leishmania major: genetic heterogeneity of Iranian isolates by single-strand conformation polymorphism and sequence analysis of ribosomal DNA internal transcribed spacer. Acta tropica. 2006; 98(1): 52-8.
[22]. Tashakori M, Ajdary S, Kariminia A, Mahboudi F, Alimohammadian MH. Characterization of Leishmania species and L. major strains in different endemic areas of cutaneous leishmaniasis in Iran. Iran Biomed J. 2003; 7(2): 43-50.
[23]. Genetu A, Gadisa E, Aseffa A, Barr S, Lakew M, Jirata D, et al. Leishmania aethiopica: strain identification and characterization of superoxide dismutase-B genes. Experiment parasitol. 2006; 113(4): 221-26.
[24]. Spotin A, Rouhani S, Ghaemmaghami P, Haghighi A, Zolfaghari MR, Amirkhani A, et al. Different morphologies of Leishmania major amastigotes with no molecular diversity in a neglected endemic area of zoonotic cutaneous leishmaniasis in Iran. Iran Biomed J. 2015; 19(3): 149.
[25]. Mahmoudvand H, Ezzatkhah F, Sharififar F, Sharifi I, Dezaki. Antileishmanial and cytotoxic effects of essential oil and methanolic extract of myrtus communis L. Korean J Parasitol. 2015; 53(1): 21-7.
[26]. Behravan M, Moin-Vaziri V, Haghighi A, Rahbarian N, Taghipour N, Abadi A, et al. Molecular identification of leishmania species in a re-emerged focus of cutaneous Leishmaniasis in varamin district, Iran. J Arthropod-Borne Dis. 2017; 11(1): 124-31.
[27]. Feiz-Haddad MH, Kassiri H, Kasiri N, Panahandeh A, Lotfi M. Prevalence and epidemiologic profile of acute cutaneous leishmaniasis in an endemic focus, Southwestern Iran. J Acute Dis. 2015; 4 (4): 292-97.
[28]. Canberk S, Longatto‐Filho A, Schmitt F. Molecular diagnosis of infectious diseases using cytological specimens. Diagnost Cytopathol. 2016; 44(2): 156-64.
[29]. Pirstani M, Sadraei J, Dalimi A, Vaeznia H. Determination of Leishmania species causing cutaneous leishmaniasis in Mashhad by PCR-RFLP method. Archiv Razi Ins. 2016; 64(1): 39-44.
[30]. Mirzaei1 F, Fattahi Bafghi A, Mohaghegh MA, Zarei Jaliani H, Faridnia R, Kalani H. In vitro anti-Leishmanial activity of Satureja hortensis and Artemisia dracunculus extracts on Leishmania major promastigotes. J Parasit Dis. 2016; 40(4): 1571-574.
Received: 2021/01/1 | Accepted: 2021/08/28 | Published: 2021/09/25
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |