Polycystic ovary syndrome (PCOS) is a common and heterogeneous endocrine abnormality affecting approximately 6–21% of reproductive-age females globally and is a major cause of infertility [1-3]. In the clinic, PCOS is mainly characterized by hyperandrogenism, irregular menstrual cycle, and polycystic ovaries morphology [4].
Hyperandrogenism is characterized clinically by hirsutism (~80% of PCOS patients), acne, amenorrhea, and alopecia, and is biochemically diagnosed by increasing androgens' serum levels, especially testosterone [5]. Besides, PCOS is associated with a wide range of metabolic disorders, such as insulin resistance, diabetes, obesity, hypertension, hepatic steatosis, and increased cardiovascular disorders [6-10]. Insulin resistance contributes to PCOS's reproductive and metabolic abnormalities and is found in 70-80% of women with PCOS [8, 11]. The chronic inflammatory state is one of the essential features of insulin resistance and has been suggested as one of the contributing agents in PCOS's pathogenesis. Recent evidence demonstrated that the levels of inflammatory markers, such as C-reactive protein (CRP), interleukin (IL)-18, IL-6, tumor necrosis factor-α (TNF-α), and monocyte chemotactic protein-1 (MCP-1) were increased in the patients with PCOS [12-14]. Due to the multifactorial nature of PCOS, there is no effective treatment for PCOS patients. In the last decade, many trials have shown that insulin sensitizers can inhibit inflammation and improve PCOS aspects [15, 16]. For a long time, dipeptidyl peptidase‐4 (DPP4) inhibitors have been used to treat insulin resistance, and in recent years, these inhibitors have been suggested to study against inflammation [17, 18]. One of the DPP4 inhibitors is sitagliptin, which is approved for utilization in treating type 2 diabetes (T2D) [19, 20]. DPP 4 selectively cleaves the N-terminal dipeptide of glucagon-like peptide-1 (GLP-1) and enhances the levels of active GLP-1. Since the GLP-1 is one of the primary regulators of post-prandial insulin secretion by β-cells in the pancreas, inhibition of DPP4 by the sitagliptin drug increases the bioavailability of GLP-1 and controls glucose homeostasis [21, 22]. In addition to the role of sitagliptin in regulating blood glucose, this drug's anti-inflammatory potential has also been reported [23]. A previous study reported that sitagliptin reduces inflammatory cytokines and improves pro-inflammatory (M1)/anti-inflammatory (M2)-like phenotypes of peripheral blood monocytes in diabetic patients [24, 25]. According to these findings, we hypothesized that sitagliptin might benefit PCOS patients. However, the present study was therefore designed to analyze PCOS's impact on inflammatory markers CRP, IL-6, TNF-α, IL-1β, and TGF-β in estradiol valerate induced PCOS rats.
Materials and Methods
Animals and ethics statement
Female Wistar rats (n=22) weighing 175–200 g were procured from the Laboratory Animal Facility of Shahid Sadoughi University of Medical Sciences, Yazd, Iran. The rats were housed under controlled laboratory conditions (22±2 °C and 12 hours' light/dark cycle) with free access to food and water. All treatments and animal care procedures were approved by the Animal Care and Ethics Committee of Shahid Sadoughi University of Medical Sciences, Yazd, Iran (1396.144.IR.SSU.MEDICINE.REC).
Vaginal smear test
Before inducing PCOS, the animal's regular estrous cycle must be ensured. A vaginal smears test was used for this purpose. To perform this test, 50 µl of normal saline 0.9% was injected into the vagina of each animal with a sampler, and then the same amount was sucked and placed on a slide. After drying, the samples were examined under a light microscope with magnification (10 ×), and different stages of the estrous cycle were identified in the samples based on classical cellular characteristics. This process was performed every morning for four consecutive days.
Experimental design and treatment
After confirmation of animals' regular estrous cycles, PCOS was induced. This study used estradiol valerate to induce PCOS, and
4 mg/kg of estradiol valerate was injected intraperitoneally for each animal. Three days after PCOS induction, animal treatments were started and were continued for 30 days. The animals were randomly distributed into four equal groups:
Group 1 (control group): The animals did not receive medication during the experimental period.
Group 2 (PCOS group): The animals in the PCOS group received the sitagliptin solvent (distilled water) daily during treatment.
Group 3 (treatment group): During treatment, PCOS rats received 25 mg/kg of sitagliptin daily.
Group 4 (treatment group): During treatment, PCOS rats received 50 mg/kg of sitagliptin daily.
Sitagliptin preparation procedure
Sitagliptin powder was prepared from Obeidi Pharmaceutical Company (Karaj-Iran). The drug was dissolved in water daily and administered to the animals by gavage at 25 and 50 mg/kg for one month.
Animal surgery
The animals were prepared for surgery at the end of 30 days of sitagliptin treatment. The animals were anesthetized entirely with ketamine and xylazine (a mixture of ketamine 90 mg/kg and xylazine 10 mg/kg was injected intraperitoneally for complete anesthesia). Next, the animals were laid on their backs, the abdomen's hairs were shaved, a transverse incision was made on the surface of the abdomen, and an upward longitudinal incision was made at each end. About 4 ml of blood was taken from the animal's heart, poured into a test tube, and allowed to stand for 40 minutes at room temperature, then centrifuged at 3000 rpm for 18 minutes to separate serum. The obtained serum was stored in a freezer at -70 °C for further evaluation. Simultaneously, ovarian specimens were removed and transferred to a -70 freezer for further evaluation.
CRP measurement
According to the kit instruction, serum concentrations of CRP were measured using an enzyme-linked immunosorbent assay (Rat C-Reactive Protein ELISA Kit, E0053Ra, China). Briefly, the sample was added to the pre-coated plate with Rat CRP antibody. Then, biotinylated Rat CRP Antibody was added and bound to CRP in the reaction solution. CRP antibody became visible by adding streptavidin-HRP, washing unbound Streptavidin-HRP and adding substarte solution. The reaction is terminated by adding an acidic stop solution, and absorbance is measured at 450 nm.
Real‐time quantitative polymerase chain reaction (PCR) analysis
The mRNA levels of IL-6, TNF-α, IL-1β, and TGF-β from the ovarian samples were analyzed with a Rotor-Gene Q (Qiagen, Germany) detection system. Briefly, total RNA from
all samples was extracted by RNA Extraction Kit (Cinnagen Company, Iran), and its concentration and purity were quantified with a Nanodrop 2000 spectrophotometer (at 260/280 nm). One µg of total RNA from each sample was used for single-stranded cDNA synthesis according to the instructions provided in the reverse transcriptase kit (Parstous, Iran) and finally amplified following the instructions provided in the PCR kit. A PCR reaction containing one μl cDNA, 10 μl SYBR green qPCR reaction mix (Amplicon, Denmark), one μl specific primers (10 pmol/μl each of forwarding and reverse primers) (Table 1), and eight μl sterile distilled water with a final volume of 20 μl. PCR was conducted based on the following thermal cycle for each given transcript: 15 min at 95 ˚C; 40 cycles of 95 ˚C for 30 s and 58-64 ˚C for 30 s, and one cycle of 70 ˚C for 30 s. After normalization with the Ribosomal protein L13 (RPL13 gene) as house keeping gene, each gene's expression level was calculated at the end of the reaction using the comparative Ct method (2−∆∆Ct).
Statistical analysis
One-way analysis of variance (ANOVA) and Tukey's posthoc test was used to find significant differences in mRNA expression ratio among experimental groups. The results were analyzed using GraphPad Prism software (Version 8), and the data obtained were expressed in Mean ± standard error of the mean. P <0.05 is considered a significant level in all tests.
Results
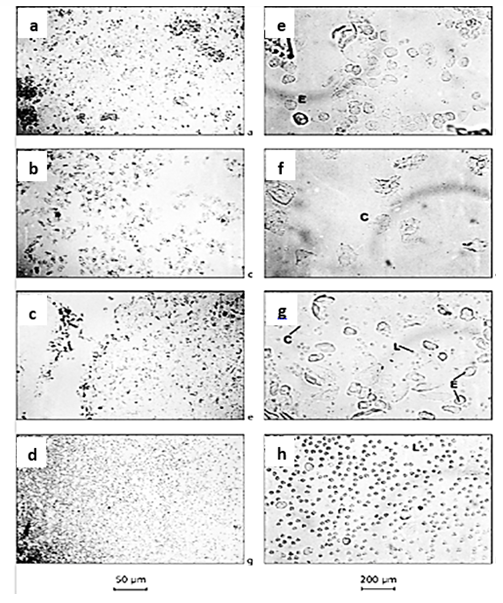
A vaginal smear test was performed every morning for four consecutive days, and the animals that showed 3 or 4 regular and consecutive estrous cycles were considered normal animals in terms of the sexual cycle and entered the intervention. The cytological appearance of cells obtained from the rat's vaginal smear test to determine four phases of the estrous cycle is shown in Figure 1 Vaginal smear obtained during proestrus and estrous phases consisted of multifaceted epithelial cells (Fig. 1a and 1b) and cornified cells (Fig. 1c and 1d). At the metestrus stage, an equal mixture of leukocytes, epithelial cells, and cornified cells was seen (Fig. 1e and 1f). Also, vaginal smear obtained during the diestrus stage consisted of leukocytes (Fig. 1g and 1h).
Table 1. The sequences of the primers of IL-6, TNF-α, IL-1β, TGF-β, and RPL13.
| Gene |
Forward primer |
Reverse primer |
| IL-6 |
5ʹ-GAC CAA GAC CAT CCA ACT CAT C-3ʹ |
5ʹ-TCC ACA AAC TGA TAT GCT TAG GC-3ʹ |
| TNF-α |
5ʹ- ACG CTC TTC TGT CTA CTG AAC TTC-3ʹ |
5ʹ-TGA TCT GAG TGT GAG GGT CTG G-3ʹ |
| IL-1β |
5ʹ-CCT TGT GCA AGT GTC TGA AG-3ʹ |
5ʹ-GGG CTT GGA AGC AAT CCT TA-3ʹ |
| TGF-β |
5ʹ-CAC CAT CCA TGA CAT GAA-3ʹ |
5ʹ-CAA CCC AGG TCC TTC CTA AA-3ʹ |
| RPL13 |
5ʹ-ATT GTG GCC AAG CAG GTA-3ʹ |
5ʹ-GTT GGT ATT CAT CCG CTT CC-3ʹ |
Fig. 1. The vaginal smears for the examination of estrous cycles. Proestrus phase (a and e), estrous phase (b and f), met-estrus phase (c and g), and di-estrus cycle (d and h) (magnification 10× for a-d and 40×c for e-h). C= Cornified cell; E= Epithelial cell; L= Lymphocytes.
Effect of different doses of sitagliptin on mRNA expression of inflammatory mediators
As shown in Figure 2, IL-1β mRNA expression in the PCOS group increased significantly compared to the control group (p ˂ 0.05). Also, sitagliptin reduced the mRNA expression of
IL-1β at doses of 25 and 50 mg/kg, but this decrease at 50 mg/kg was statistically significant between the sitagliptin and PCOS model groups (p ˂0.05). The mRNA level of IL-6 in the PCOS model group was markedly rose compared with the control group (p <0.01). The mRNA level of IL-6 was reduced in the sitagliptin treatment group compared with the PCOS group (p <0.01). TGF-β mRNA expression in the PCOS group showed a significant increase compared to the normal group (p ˂0.0001). TGF-β mRNA expression was significantly decreased at 50 mg/kg sitagliptin treatment groups compared with the PCOS group (p <0.01). According to Figure 2, TNF-α mRNA expression in the PCOS group significantly increased compared to the normal group (p ˂0.05). Treatment with sitagliptin resulted in a decrease in the expression of TNF-α inflammatory factor, and this decrease was significant in the treatment group with a dose of 50 mg/kg sitagliptin (p ˂0.01).
Effect of different doses of sitagliptin on CRP serum levels
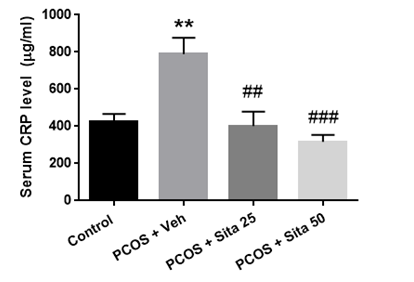
As shown in Figure 3, the serum's CRP concentration significantly increased in the PCOS group compared with the normal group (p <0.01). The CRP level in the groups treated with 25 and 50 mg/kg doses of sitagliptin was significantly reduced compared with the PCOS model group (p < 0.01).
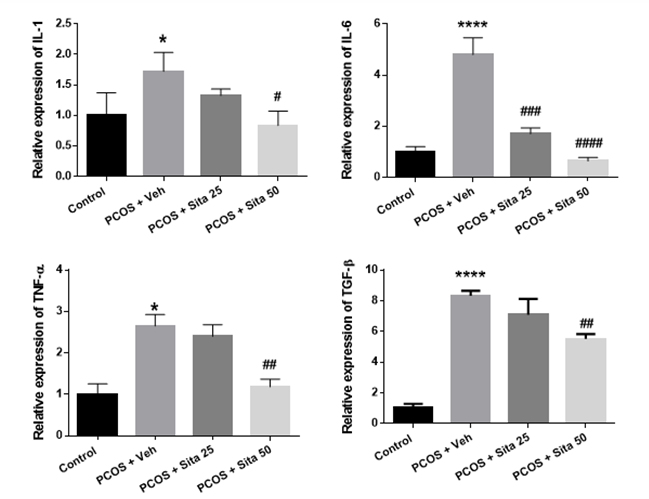
Fig. 2. The effect of sitagliptin on the mRNA expression of IL-1, IL-6, TGF-β, and TNF-α in PCOS rats' ovarian tissue. The results are expressed as means ± SEM. * Significantly different between PCOS and control groups. #, ##, ### and #### : p<0.05, p<0.01, and p<0.001 when compared to the PCOS group. IL-1= Interleukin 1; IL-6= Interleukin 6; TNF-α= Tumor necrosis factor-α; TGF-β= Transforming growth factor-β.
Fig. 3. The effect of sitagliptin on the serum level of CRP in rats. The results are expressed as means ± SEM. ** Significantly different between PCOS and control groups. ### Significantly different between sitagliptin treated groups and the PCOS group.
CRP= C-reactive protein
Discussion
The present study demonstrates that sitagliptin, a DPP-4 inhibitor, has anti-inflammatory effects in a PCOS rat model. We show that daily oral administration of sitagliptin for one month attenuated the levels of inflammatory markers in serum and ovaries of PCOS rats.
PCOS is one of the most common endocrine and metabolic disorders found in women, diagnosed through two out of three
features: polycystic ovaries morphology on ultrasound, hyperandrogenism, and menstrual irregularities [6]. Accumulating evidence demonstrates that insulin resistance is a common feature of PCOS affecting approximately 50–70% of females and is critically accompanied by this syndrome's reproductive and metabolic complications. In response to insulin resistance, hyper-insulinemia leads to increased androgen production and impaired ovarian function
[6, 26-28]. Additionally, there is a strong association between insulin resistance, hyperandrogenism, and inflammation in PCOS's pathogenesis. A previous study reported that androgens sensitize circulating mononuclear cells to promote glucose-induced inflammation in women with PCOS [29]. It has been showed that CRP was associated with insulin resistance, and its levels were significantly higher in the serum of PCOS patients [30]. In PCOS patients, elevated circulating levels of pro-inflammatory responses, such as TNF-α, IL-1β, and IL-6, are also observed [26, 31]. These cytokines control the reproductive processes, including folliculogenesis, ovulation, and fertilization [12, 26, 32]. Pro-inflammatory cytokines can stimulate the up-regulation of responsible enzymes for androgen production in ovarian theca cells and might impair ovulation control [14, 33-35].
Due to the central role of insulin resistance in PCOS's pathogenesis, it seems that improving insulin sensitivity by insulin-sensitizing agents may be effective in reducing inflammation and treating patients with PCOS. Previous study showed that Quercetin had a favorable therapeutic effect on the PCOS rats by improving insulin resistance and inflammation [36]. In 2017, Mohammadi et al. reported that the anti-inflammatory impacts of curcumin on the rat model of PCOS might be due to its inhibitory effect on TNF-α, IL-6, and CRP levels markers [12, 37]. Numerous studies have demonstrated that DPP-4 inhibitors induce insulin secretion, and there is increasing evidence that the DPP-4 inhibitors also have anti-inflammatory effects against inflammation states on various organs and tissues, including the kidney, pancreas, and liver [38-40]. Sitagliptin was the first DPP-4 inhibitor, which was approved by the Food and Drug Administration for the treatment of T2D [41]. Previous researches have reported that sitagliptin preserved renal tissue against diabetic nephropathy through decreased inflammatory factors in T2D animal models [32, 42]. In other study T2D patients has shown that sitagliptin significantly reduced serum levels of inflammatory markers, such as CRP, and TNF-α, and increased serum levels of anti-inflammatory markers such as IL-10 [24]. A study by Wang et al. suggested that sitagliptin had anti‐inflammatory and could mitigate hepatic fibrosis in cirrhosis patients. Researchers have shown that sitagliptin may inhibit the TGF‐β signal transduction pathway to inhibit collagen synthesis, thus influencing the fibrosis process [43]. Researchers have also shown that the TGF‐β level in the serum of PCOS patients is relatively higher than that in the serum of healthy subjects [44].
Due to the importance of the role of inflammation in PCOS's pathogenesis, in this study, we investigated the anti-inflammatory effect of sitagliptin in PCOS rats. In the present study, we show that the expression of a series of inflammation-related genes is dramatically increased in the ovaries and serum of a PCOS rat model. TNFα, TGF-β, IL-1β, and IL-6 gene expression are increased in PCOS rats, confirming that these inflammatory mediators may play vital roles in PCOS's pathogenesis. We also show that treatment with sitagliptin suppresses the increases in TNFα, TGF-β, IL-6, and IL-1β gene expression and CRP serum levels in the PCOS-like rat ovaries.
Conclusion
The data obtained in this manuscript show that sitagliptin treatment recovered the levels of inflammatory factors in the rat model of PCOS. Therefore, it may be concluded that sitagliptin may suggest a therapeutic potential in managing chronic inflammation in PCOS women. However, PCOS is a heterogeneous disease, and further studies are needed to confirm the role of sitagliptin in the improvement and management of patients with this syndrome.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgments
We would like to greatly acknowledge the department of physiology of Shahid Sadoughi University of Medical Sciences for their sincere support.
References
- Rencber SF, Ozbek SK, Eraldemır C, Sezer Z, Kum T, Ceylan S, et al. Effect of resveratrol and metformin on ovarian reserve and ultrastructure in PCOS: an experimental study. J Ovarian Res. 2018; 11(1): 55.
- Zhang Y, Hu M, Meng F, Sun X, Xu H, Zhang J, et al. Metformin ameliorates uterine defects in a rat model of polycystic ovary syndrome. EBioMedicine 2017; 18: 157-70.
- Fattah A, Asadi A, Shayesteh MRH, Hesari FH, Jamalzehi S, Abbasi M, et al. Fertility and infertility implications in rheumatoid arthritis; state of the art. Inflammation Research 2020; 69(8): 721-29.
- Mohammad MB, Seghinsara AM. Polycystic ovary syndrome (PCOS), diagnostic criteria, and AMH. APJCP. 2017; 18(1): 17.
- Dadachanji R, Shaikh N, Mukherjee S. Genetic variants associated with hyperandrogenemia in PCOS pathophysiology. Genetics Research Int. 2018; 1-13.
- Polak K, Czyzyk A, Simoncini T, Meczekalski B. New markers of insulin resistance in polycystic ovary syndrome. J Endocrinologic Invest. 2017; 40(1): 1-8.
- Zeng X, Xie Yj, Liu Yt, Long Sl, Mo Zc. Polycystic ovarian syndrome: correlation between hyperandrogenism, insulin resistance and obesity. Clinica Chimica Acta 2020; 502: 214-21.
- Crespo RP, Bachega TA, Mendonça BB, Gomes LG. An update of genetic basis of PCOS pathogenesis. Archiv Endocrinol Metabol. 2018; 62(3): 352-61.
- Abazari O, Divsalar A, Ghobadi R. Inhibitory effects of oxali-platin as a chemotherapeutic drug on the function and structure of bovine liver catalase. J Biomolecul Struct Dynam. 2020; 38(2): 609-15.
- Abbasi M, Asadi A, Musavi H. Association of liver aminotransferases with lipid profile in patients with type II diabetes mellitus. Medical Laboratory Journal. 2019; 13(6): 11-6.
- Alaei Sheini F, Tabnak M, Hasanzadeh Bezvan M, Mahdiannasser M, Musavi H, Choobineh H, et al. A systematic review of the evidence on the effects of cytomegalovirus on abortion. Int J Med Lab. 2018; 5(3): 173-81.
- Mohammadi S, Kayedpoor P, Karimzadeh-Bardei L, Nabiuni M. The effect of curcumin on TNF-α, IL-6 and CRP expression in a model of polycystic ovary syndrome as an inflammation state. J Reprod Infertil. 2017; 18(4): 352.
- Abazari O, Shafaei Z, Divsalar A, Eslami-Moghadam M, Ghalandari B, Saboury AA, et al. Interaction of the synthesized anticancer compound of the methyl-glycine 1, 10-phenanthroline platinum nitrate with human serum albumin and human hemoglobin proteins by spectroscopy methods and molecular docking. J Iran Chem Society 2020; 17(7): 1601-614.
- Fox CW, Zhang L, Sohni A, Doblado M, Wilkinson MF, Chang RJ, et al. Inflammatory stimuli trigger increased androgen production and shifts in gene expression in theca-interstitial cells. Endocrinology 2019; 160(12): 2946-958.
- Patil PD, Mahajan UB, Patil KR, Chaudhari S, Patil CR, Agrawal YO, et al. Past and current perspective on new therapeutic targets for type-II diabetes. Drug Design Develop Therap. 2017; 11: 1567.
- Shazly S, Laughlin-Tommaso SK. Menstrual disorders. Gynecology: Springer; 2020. 45-104.
- Aroor AR, Habibi J, Kandikattu HK, Garro-Kacher M, Barron B, Chen D, et al. Dipeptidyl peptidase-4 (DPP-4) inhibition with linagliptin reduces western diet-induced myocardial TRAF3IP2 expression, inflammation and fibrosis in female mice. Cardiovasc Diabetol. 2017; 16(1): 1-15.
- Gonçalves A, Almeida L, Silva AP, Fontes-Ribeiro C, Ambrósio AF, Cristóvão A, et al. The dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin ameliorates retinal endothelial cell dysfunction triggered by inflammation. Biomed Pharmacother. 2018; 102: 833-38.
- Goldenberg R, Gantz I, Andryuk PJ, O'Neill EA, Kaufman KD, Lai E, et al. Randomized clinical trial comparing the efficacy and safety of treatment with the once‐weekly dipeptidyl peptidase‐4 (DPP‐4) inhibitor omarigliptin or the once‐daily DPP‐4 inhibitor sitagliptin in patients with type 2 diabetes inadequately controlled on metformin monotherapy. Diabet Obesity Metabol. 2017; 19(3): 394-400.
- Asadi A, Nezhad DY, Javazm AR, Khanicheragh P, Mashouri L, Shakeri F, et al. In vitro effects of curcumin on transforming growth factor-β-mediated non-smad signaling pathway, oxidative stress, and pro‐inflammatory cytokines production with human vascular smooth muscle cells. Iran J Allergy, Asthma Immunol. 2020: 19(1): 84-93.
- Deacon CF. Physiology and pharmacology of DPP-4 in glucose homeostasis and the treatment of type 2 diabetes. Front Endocrinol. 2019; 10: 80.
- Mohamadi N, Kazemi SM, Mohammadian M, Toofani Milani A, Moradi Y, Yasemi M, et al. Toxicity of cisplatin-loaded poly butyl cyanoacrylate nanoparticles in a brain cancer cell line: Anionic polymerization results. Asian Pac J Cancer Prev. 2017; 18(3): 629-32.
- Carani AV, Dipti N. Sitagliptin recuperates oxidative stress and inflammatory cytokine expression in ovary of PCOS rats. J Drug Delivery Therapeut. 2019; 9(S4): 244-51.
- Satoh-Asahara N, Sasaki Y, Wada H, Tochiya M, Iguchi A, Nakagawachi R, et al. A dipeptidyl peptidase-4 inhibitor, sitagliptin, exerts anti-inflammatory effects in type 2 diabetic patients. Metabolism 2013; 62(3): 347-51.
- Shafaei Z, Abazari O, Divsalar A, Ghalandari B, Poursoleiman A, Saboury AA, et al. Effect of a synthesized amyl-glycine1, 10-phenanthroline platinum nitrate on structure and stability of human blood carrier protein, albumin: spectroscopic and modeling approaches. J fluorescence 2017; 27(5): 1829-838.
- González F. Inflammation in polycystic ovary syndrome: underpinning of insulin resistance and ovarian dysfunction. Steroids 2012;77(4): 300-305.
- Shorakae S, Ranasinha S, Abell S, Lambert G, Lambert E, de Courten B, et al. Inter‐related
effects of insulin resistance, hyperandrogenism, sympathetic dysfunction and chronic inflammation in PCOS. Clinic Endocrinol. 2018; 89(5): 628-33.
- Abbasi M. Effects of various contraceptive methods on clinical and metabolic parameters. Archiv Med Lab Sci. 2019; 4(2): 23-29.
- González F, Nair KS, Daniels JK, Basal E, Schimke JM. Hyperandrogenism sensitizes mononuclear cells to promote glucose-induced inflammation in lean reproductive-age women. American J Physiol-Endocrinol Metabol. 2012; 302(3): 297-306.
- Escobar-Morreale HF, Luque-Ramírez M, González F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and metaanalysis. Fertil Steril. 2011; 95(3): 1048-1058.
- Abbasi M, Namjoo AR, Khamesipour F. Ethanol effects on histobiochemical parameters of suckling pups borned from alcoholic rat mothers. Comparative Clinical Pathology 2016; 25(4): 833-39.
- Marques C, Mega C, Gonçalves A, Rodrigues-Santos P, Teixeira-Lemos E, Teixeira F, et al. Sitagliptin prevents inflammation and apoptotic cell death in the kidney of type 2 diabetic animals. Mediators of Inflammation 2014: 1-16.
- Samir MS, Glister C, Mattar D, Laird M, Knight PG. Follicular expression of pro-inflammatory cytokines tumour necrosis factor-α (TNFα), interleukin 6 (IL6) and their receptors in cattle: TNFα, IL6 and macrophages suppress thecal androgen production in vitro. Reproduction 2017; 154(1): 35-49.
- Duffy DM, Ko C, Jo M, Brannstrom M, Curry Jr TE. Ovulation: parallels with inflammatory processes. Endocrin Rev. 2019; 40(2): 369-416.
- Abbasi M, Namjoo A. Low dose effects of ethanol on suckling rats: Enzymes activity, histological alterations and growth parameters. Journal of Shahrekord Uuniversity of Medical Sciences 2013; 15(6): 64-54.
- Wang Z, Zhai D, Zhang D, Bai L, Yao R, Yu J, et al. Quercetin decreases insulin resistance in a polycystic ovary syndrome rat model by improving inflammatory microenvironment. Reprod Sci. 2017; 24(5): 682-90.
- Musavi H, Abazari O, Barartabar Z, Kalaki-Jouybari F, Hemmati-Dinarvand M, Esmaeili P, et al. The benefits of vitamin D in the COVID-19 pandemic: biochemical and immunological mechanisms. Archiv Physiol Biochem. 2020; 10(1): 1-9.
- Bae EJ. DPP-4 inhibitors in diabetic complications: role of DPP-4 beyond glucose control. Archiv Pharm Res. 2016; 39(8): 1114-128.
- Coppolino G, Leporini C, Rivoli L, Ursini F, di Paola ED, Cernaro V, et al. Exploring the effects of DPP-4 inhibitors on the kidney from the bench to clinical trials. Pharmacol Res. 2018; 129: 274-94.
- Musavi H, Tabnak M, Sheini FA, Bezvan MH, Amidi F, Abbasi M. Effect of garlic (Allium sativum) on male fertility: a systematic review. J Herbmed Pharmacol. 2018; 7(4): 306-12.
- Drucker D, Easley C, Kirkpatrick P. Sitagliptin. Nature Reviews Drug Discovery 2007; 6(2): 109-11.
- Abazari O, Shafaei Z, Divsalar A, Eslami-Moghadam M, Ghalandari B, Saboury AA. Probing the biological evaluations of a new designed Pt (II) complex using spectroscopic and theoretical approaches: Human hemoglobin as a target. J Biomol Struct Dynamic. 2016; 34(5): 1123-131.
- Wang F, Zhang ZF, He YR, Wu HY, Wei SS. Effects of dipeptidyl peptidase‐4 inhibitors on transforming growth factor‐β1 signal transduction pathways in the ovarian fibrosis of polycystic ovary syndrome rats. J Obstetric Gynaecol Res. 2019; 45(3): 600-608.
- Raja-Khan N, Urbanek M, Rodgers RJ, Legro RS. The role of TGF-β in polycystic ovary syndrome. Reprod Sci. 2014; 21(1): 20-31.










































 , Mahin Izadi
, Mahin Izadi 

 , Fatemeh Zare Mehrjerdi
, Fatemeh Zare Mehrjerdi 

 , Maryam Yadegari
, Maryam Yadegari 

 , Mohammad Ebrahim Rezvani *
, Mohammad Ebrahim Rezvani *