Chronic myeloid leukemia (CML) is a stem cell neoplastic disorder associated with BCR-ABL fusion oncoprotein and the presence of the Philadelphia chromosome [1, 2]. Translocation t(9;22) (q34.1;q11.21) occurs in about 95% of all CML patients and creates the chimeric BCR-ABL gene [3]. The incidence rates vary from 0.6 to 2.0 cases per 100 000 habitants, develop with age, and are higher in males than females [4]. The annual prevalence of newly diagnosed CML in the United States has been reported at nearly 1.0 to 1.3 per 100,000 or nearly 4800 to 5200 new cases [5].
Curcumin (diferuloylmethane, Cur) is a polyphenol compound derived from the rhizome of turmeric (Curcuma longa L) and the main component of the different curry recipes [6]. Pharmacological effects of Curcumin include anti-tumor, anti-inflammatory, and anti-oxidant properties [7]. In addition, Curcumin is well known to be a potent inhibitor of the proliferation of several tumor cells [8]. Curcumin has such effects as the suppressor of the tumor initiation, promotion, and metastasis [9] in Xammatory, anti-bacterial, fungal, anti-oxidant, anticarcinogen/antimutagen, and anticancer properties [10]. Curcumin has been described as a potent inhibitor of protein kinase C, tyrosine kinase receiver epidermal growth factor and kinase IkappaB [11]. Curcumin prevents NFkappaB activation and expression of c-jun, c-fos, c-myc, and inducible nitric oxide synthase [12], increases cell cytotoxicity, and down-regulates cyclin D1 [13]. It also has been demonstrated to have more or less the same anti-proliferative effect on K562 p210 BCR/ABL-positive cells [12].
Imatinib mesylate is an inhibitor of tyrosine kinase, which suppresses the activity of the BCR-ABL fusion protein [14], ABL-related gene protein [15], platelet-derived growth factor (PDGF) receptor, and Kit [16, 17]. Imatinib suppresses the proliferation and induces apoptosis of the leukemic cells [18]; approved for use in the treatment of CML [19].
K562 cells are based on chronic myelocytic leukemia blastic crisis [20]. They reported that Curcumin inhibited the proliferation of K562 cells associated with the downregulation of the P210 BCR/ABL protein [21] and inhibited the proliferation of K562 cells. The inhibition effect was associated with the down-regulation of p210 CR/ABL/Ras/Raf/MEK-1/ERK/Elk-1 and p210 CR/ABL/Ras/MEKK/SEK/JNK/c-Jun signal transduction pathway [21]. This study used the P210 CR/ABL-positive K562 cell line as a CML cell model for drug detection. Therefore, we aimed to examine Curcumin and Imatinib's effects on p210 BCR/ABL expression and proliferation of k562 cells.
Materials and Methods
Chemicals
Nanomicelle-Curcumin was obtained from Exir Nano Sina Company (Tehran, Iran). Each nano-Curcumin soft gel contains 60 mg of Curcumin (60 mg/ml).
Imatinib was produced by the Sobhan company (Tehran, Iran). Each Imatinib capsule contains 100 mg of Imatinib (100 mg/ml).
Cell lines and cell culture
Cell lines based on a patient with CML in blast crisis (K562; Cat No: C122) were bought from Cell Bank, Institute Pasteur, Iran (Teheran, Iran). All the reagents and mediums made in this study were supplied immediately before use. The American Type Culture Collection (ATCC) cell line is called Cell-bank: CCL-243.
Cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 (Gibco; Germany) fortified with 10% fetal bovine serum (Gibco; Germany) and 1% pen strep at 37°C in 5% CO2 until confluent.
3-[4,5-dimethylthiazole-2-yl]-2,5-diphenylte trazolium bromide (MTT) assay
The effects of different concentrations of Nano-Curcumin and Imatinib on the K562 cell line were investigated in this study. This effect was dose-dependent. Initially, in each 96-well culture plate, 105 non-stick cells (cell/ml) showed rapid growth, containing 100 µl aliquots of growth medium and incubated for 48 hours with concentrations (Table 1) of nano-Curcumin and Imatinib in triplicate. After 48 hours of incubation, 5 mg/ml of MTT solution was added to all plates. Finally, the plates were incubated for 4 hours at 37°C. The Formosan precipitate was dissolved by adding 100 µl of Dimethyl Sulfoxide solution. The absorbance was read using the enzyme-linked immunosorbent assay plate reader (Anthos, Australia) at 570 and 630 nm. The middle-maximal inhibitory concentration (IC50) of every group was calculated. The cells' viability was determined by measuring the absorbance of the samples against negative controls.
Cell line preparation before RNA extraction
To ready the cells line, a million cells were primarily transported on a six-well plaque. A dose of 46×10-3 mmol/ml Curcumin nanomicelle (IC50) was added to the first well. Also, a dose of 36 ×10-3 mmol/ml Imatinib (IC50) was added to the latter. The treated 48hr with the IC50 dose of nanomicelle and Imatinib; afterward, 106 untreated k562 cells were included in the three wells as a control sample. The 6-well plate was incubated for 48 h at 37°C at 5% CO2.
Molecular assessment
The RNAs of K562 cells treated with Nano-Curcumin and Imatinib with the mentioned concentrations were extracted using the RNA extraction kit (Jena Bioscience; Cat No: pp-210s). All stages of testing were performed in an environment free of RNAs. The extracted RNAs were quantitatively evaluated using Nano Drop (Thermo Scientific, Wilmington, USA) apparatus and qualitatively investigated with gel electrophoresis.
To make the cDNA using a cDNA kit (parstous Co, Cat#A101231 Tehran, Iran), 1 microliter of RNA was used. Factory instructions were used to perform the process. Primers for quantitative real-time polymerase chain reaction (PCR) are presented in Table 2. Quantitative real time-PCR (qRT-PCR) of BCR/ABL was performed with the SYBR Green (Batch: 18A0802) technique in a LightCycler® 96 device sequence detection system. (2 microtubes with synthesized cDNA after treatment and one microtube as a control sample with synthesized cDNA from the untreated cell line).
The gene used as housekeeping was glycerol aldehyde 3 phosphates (GAPDH). The results were expressed in the form of relative fold changes in gene expression and then normalized to the corresponding levels of the reference gene (GAPDH) (primers in Table 2).
Based on the data obtained for target genes (BCR / ABL) and reference (GAPDH), the value of (ΔCT) was calculated, the results of each drug were compared with each other, and (ΔΔCT) was expressed as 2-∆∆CT.
Ethical approval
Mashhad University of Medical Sciences Research Committee approved the research protocol (IRMUMSMEDICAL. REC. 1397. 056). All data were kept confidential.
Statistical analysis
The pairwise comparison between the groups was performed using the Tukey post hoc test, and statistical differences were analyzed using ANOVA. Analysis of CT values obtained from the real-time PCR device using formula 2(-∆∆ct) indicates a decrease in BCR-ABL gene expression compared to the reference gene expression.
Result
MTT assay
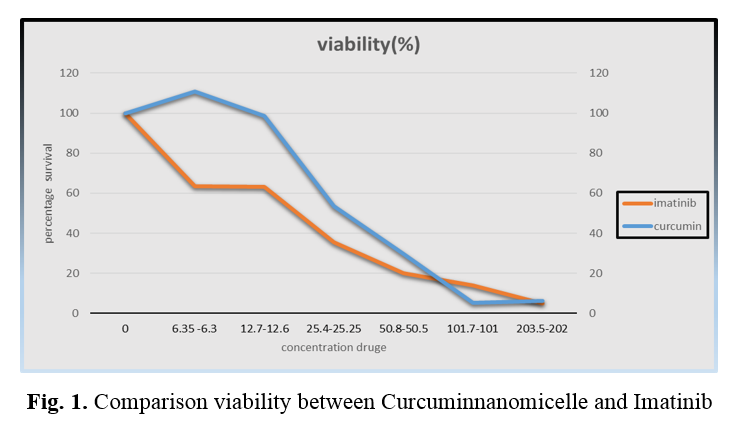
We evaluated the growth inhibition effects of nanomicelle of Curcumin and Imatinib on k562 cells by MTT assay. The results indicated that Curcumin significantly inhibited the k562 cells in a time- and dose-dependent manner. Since the samples were studied in three replicates, the percentage of viability of the control sample was computed at 100%. Using PRISM software (version 8), we calculated IC50 for Curcumin nanomicelle and Imatinib. MTT assay concentrations were obtained, 59×10-3μmol/ml (after 48 h) and 36×10 -3μmol/ml (after 48 h) as 50% killing dose (IC50), respectively, for Curcumin nanomicelle and Imatinib (Figure 1).
Table 1. The concentration of Curcumin nanomicelle and Imatinib in culture media for MTT assay
| concentration |
| Curcumin (umol/l) |
203.5 |
101.7 |
50.8 |
25.4 |
12.7 |
6.35 |
3.175 |
|
|
| Imatinib (umol/l) |
202 |
101 |
50.5 |
25.25 |
12.6 |
6.3 |
3.15 |
Table 2. Primers used in this study for the target gene and housekeeping gene in Real-time PCR
| Primers |
Sequence 5'à 3' |
| GAPDH |
Forward |
5´-GGT GGT CTC CTC TGA CTT CAA CA-3´ |
| Reverse |
5´-GTT GCT GTA GCC AAA TTC GTT GT-3' |
| BCR/ABL |
Forward |
5´-CTGGGTTCTGGGTGATTCAGTGG-3´ |
| Reverse |
5´-CTTCACAGCCCACCATTCTGATAGCCC-3´ |
|
|
|
|
RNA extraction and cDNA synthesis
The RNA extracted from the cells was treated with the mentioned doses at the next step. Then, RNA concentration and purity were determined by optical absorbance. Ratio 260/280 nm in NanoDrop estimated at 1.98, 1.96 and 2.03 for cells treated with Curcumin nanomicelle, Imatinib, and untreated, respectively. Like the above-order mean concentration of extracted RNA, it was 131 ng/µl, 374ng/µl, and 532.5ng/µl, respectively.
In the next step, cDNA was manufactured from the extracted RNA with the mentioned doses.
Real-time PCR results
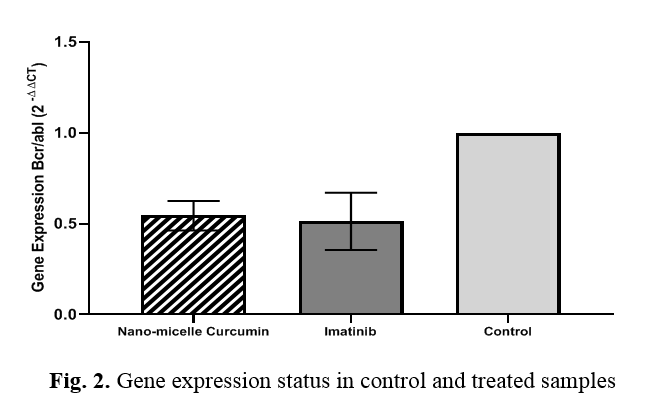
In the fourth step, CT figures out for each sample; then, ΔΔCT was estimated and earned using the gene ratio law, the gene expression ratio. The gene expression ratio yielded the amount of 0.497 while treating with Imatinib; this amount was 0.540 while treating with Curcumin nanomicelle. The pairwise comparison between the groups was performed using Tukey's post hoc test. The BCR/ABL gene expression of the samples treated with Curcumin nanomicelle was not significantly different from the Imatinib (Fig. 2).
Discussion
CML results from a mutation in a pluripotent stem cell with progressive granulocytosis, hypercellularity of the bone marrow, and splenomegaly [22]. CML is the first cancer treated with a tyrosine kinase inhibitor. The Philadelphia chromosome produces CML. Imatinib was identified as the first-line treatment method Imatinib is an "Abelson Cluster Point Fracture Inhibitor" (BCR-ABL) inhibitor [23]. Although Imatinib, the first molecular medicine developed for CML, has been remarkably successful, the appearance of resistance to this agent attenuates the prospect of a cure for this leukemia [24]. Research on the controlled administration of Curcumin in target tissues and limbs has been a significant topic over the past decade. Although the benefit of Curcumin has been investigated in many studies, supplementary research is needed for its utilization [25].
Due to insufficient bioavailability in the free formulation, various strategies have been tried to modify the bioavailability of Curcumin in the form of nanomicelles and nanoparticles [26]. In this study, the BCR/ABL gene expression was 0.497 and 0.540 for Imatinib, and Curcumin nanomicelle, respectively, which was not statistically significant. Our finding suggested that treating K562 cell lines with Curcumin Nanomicelle IC50 dose might decrease the BCR/ABL gene expression compared to the untreated ones. Although this reduction in expression was not statistically significant, increased medication concentrations might yield better results. One of the most important genetic changes that cause leukemia is the bilateral translocation between the long arms of chromosomes 9 and 22 and the formation of the Philadelphia chromosome, which leads to the formation of the BCR/ABL hybrid gene. The molecular result of the Philadelphia chromosome is the production of oncogenes BCR-ABL which code for oncoprotein BCR-ABL chimeric, with creator kinase activity that increases the growth overtaking of the leukemic cells [27]. This fusion is crucial in activating the enzyme tyrosine kinase and the abnormal tyrosine kinase activity and is vital for many hematological disorders and leukemia initiation [28]. The has not adjusted the tyrosine kinase activity of BCR-ABL has been essential enough to preserve CML's leukemia phenotype [24]. It indicates a severe issue in an attempt to project molecular therapies. Imatinib is an antineoplastic substance. The drug is an oral chemotherapy medication used to treat cancer [29]. Imatinib stops the BCR-ABL chimeric protein tyrosine kinase, a non-typical enzyme constructed by chronic myeloid leukemia cells that possess the Philadelphia chromosome [30]. Moreover, the drug prevents the receptor tyrosine kinases for a platelet-derived growth factor and stem cell factor (SCF)/c-kit [31]. This drug stops the increase and causes apoptosis in cells overexpressing such oncoproteins [32, 33]. Notably, it is utilized for chronic and acute lymphocytic leukemia that possess Philadelphia chromosome-positive, some kinds of gastrointestinal stromal tumors, and several other malignancies [34].
Some studies have shown that Curcumin nanomicelle of plant origin has fewer toxic properties than chemical drugs. Animal and human studies have noted the lack of Curcumin's side effects. It is also economical, and its raw material is available for production. In a study conducted in 2019 by Hosseini et al., the anti-tumor activity of Curcumin capsules was investigated, and this study showed that Curcumin inhibited the growth and migration of breast cancer cells [35]. This study shows that Curcumin nanomicelles have a lethal effect on the K 562 method by the MTT method, and the study of gene expression shows a decrease in expression compared to the control groups. It cannot be evaluated simultaneously. Imatinib and nano Curcumin muscles can show better results. Also, In 2019, Curcumin nanoparticles' anti-oxidant and anti-inflammatory effects on drug-induced acute myocardial infarction in diabetic rats were investigated, and their effect was confirmed [36].
Conclusion
The present study revealed that treating cellular category k562 with Curcumin nanomicelle and Imatinib reduces the BCR-ABL gene expression. Also, data obtained from the MTT assay showed that the Curcumin nanomicelle and Imatinib induced the apoptotic process.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
The Mashhad University of Medical Science Research Council supported this research (MUMS/961809). This study is part of an MSc thesis in Biology at Islamic Azad University, Mashhad, Iran.
References
- Sampaio MM, Santos MLC, Marques HS, de Souza Gonçalves VL, Araújo GRL, Lopes LW, et al. Chronic myeloid leukemia-from the Philadelphia chromosome to specific target drugs: A literature review. World J Clinic Oncol. 2021; 12(2): 69.
- Kim JH, Kang KW, Lee BH, Park Y, Kim BS. Generation of induced pluripotent stem cell line KUMi002-A with normal karyotype from a patient with Philadelphia chromosome-positive chronic myeloid leukemia. Stem Cell Res. 2021; 55(8): 102465.
- Chim CS, Wan TS, Wong KY, Fung TK, Drexler HG, Wong KF. Methylation of miR-34a, miR-34b/c, miR-124-1 and miR-203 in Ph-negative myeloproliferative neoplasms. J Transl Med. 2011; 9(1): 197.
- Zhang MY, Fung TK, Chen FY, Chim CS. Methylation profiling of SOCS1, SOCS2, SOCS3, CISH and SHP1 in Philadelphia‐negative myeloproliferative neoplasm. J Cel Mol Med. 2013; 17(10): 1282-290.
- Huang X, Cortes J, Kantarjian H. Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer 2012; 118(12): 3123-137.
- Hofmann WK, Tsukasaki K, Takeuchi N, Takeuchi S, Koeffler HP. Methylation analysis of cell cycle control genes in adult T-cell leukemia/lymphoma. Leukemia Lymphoma 2001; 42(5): 1107-109.
- Kusy S, Larsen CJ, Roche J. p14ARF, p15INK4b and p16INK4a methylation status in chronic myelogenous leukemia. Leukemia Lymphoma 2004; 45(10): 1989-994.
- Almatroodi SA, Alrumaihi F, Alsahli MA, Alhommrani MF, Khan A, Rahmani AH. Curcumin, an active constituent of turmeric spice: implication in the prevention of lung injury induced by benzo (a) pyrene (BaP) in rats. Molecules 2020; 25(3): 724.
- Almatroodi SA, Syed MA, Rahmani AH. Potential therapeutic targets of Curcumin, most abundant active compound of turmeric spice: Role in the management of various types of cancer. Recent Patents on Anti-Cancer Drug Discovery 2021; 16(1): 29-33.
- Hesari A, Ghasemi F, Salarinia R, Biglari H, Tabar Molla Hassan A, Abdoli V, et al. Effects of Curcumin on NF‐κB, AP‐1, and Wnt/β‐catenin signaling pathway in hepatitis B virus infection. J Cel Biochem. 2018; 119(10): 7898-904.
- Chim C, Wan T, Fung T, Wong K. Methylation of TET2, CBL and CEBPA in Ph-negative myeloproliferative neoplasms. J Clinic Pathol. 2010; 63(10): 942-46.
- Rasmussen KD, Jia G, Johansen JV, Pedersen MT, Rapin N, Bagger FO, et al. Loss of TET2 in hematopoietic cells leads to DNA hypermethylation of active enhancers and induction of leukemogenesis. Gen Develop. 2015; 29(9): 910-22.
- Hosseini S, Chamani J, Rahimi H, Azmoodeh N, Ghasemi F, Hassan Abadi P. An in vitro study on Curcumin delivery by nano-micelles for esophageal squamous cell carcinoma (KYSE-30). Rep Biochem Mol Biol. 2018; 6(2): 137-43.
- Huang ZL, Gao M, Ji MS, Tao K, Xiao Q, Zhong L, et al. TAT-CC fusion protein depresses the oncogenicity of BCR-ABL in vitro and in vivo through interrupting its oligomerization. Amino Acids 2013; 44(2): 461-72.
- Okuda K, Weisberg E, Gilliland DG, Griffin JD. ARG tyrosine kinase activity is inhibited by STI571. Blood 2001; 97(8): 2440-448.
- Buchdunger E, Cioffi CL, Law N, Stover D, Ohno-Jones S, Druker BJ, et al. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exper Therapeut. 2000; 295(1): 139-45.
- Heinrich MC, Griffith DJ, Druker BJ, Wait CL, Ott KA, Zigler AJ. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood 2000; 96(3): 925-32.
- Zhou X, Yuan P, Liu Q, Liu Z. LncRNA MEG3 regulates imatinib resistance in chronic myeloid leukemia via suppressing microRNA-21. Biomol Therapeut. 2017; 25(5): 490.
- Phillips LN, Hijiya N. Tyrosine kinase inhibitors and beyond for chronic myeloid leukemia in children. Pediatric Drugs 2021; 23(2): 241-51.
- Han Y, Wang Y, Xu Z, Li J, Yang J, Li Y, et al. effect of bone marrow mesenchymal stem cells from blastic phase chronic myelogenous leukemia on the growth and apoptosis of leukemia cells. Oncol Rep. 2013; 30(2): 1007-1013.
- Aggerholm A, Guldberg P, Hokland M, Hokland P. Extensive intra-and interindividual heterogeneity of p15INK4B methylation in acute myeloid leukemia. Cancer Res. 1999; 59(2): 436-41.
- Buzaid AN, Al-Amri AM. Sudden visual loss as an initial manifestation of chronic myeloid leukemia. Saudi J Med Sci. 2017; 5(3): 278.
- Al-Hadiya BM, Bakheit AH, Abd-Elgalil AA. Imatinib mesylate. Profiles Drug Subst Excip Relat Methodol. 2014; 39(1): 265-97.
- Jiang L, Wen C, He Q, Sun Y, Wang J, Lan X, et al. Pseudolaric acid B induces mitotic arrest and apoptosis in both imatinib-sensitive and-resistant chronic myeloid leukaemia cells. Euro J Pharmacol. 2020; 876(6): 173064.
- Cao H, Wang Y, He X, Zhang Z, Yin Q, Chen Y, et al. Codelivery of sorafenib and Curcumin by directed self-assembled nanoparticles enhances therapeutic effect on hepatocellular carcinoma. Mol Pharmaceut. 2015; 12(3): 922-31.
- Ahangari N, Kargozar S, Ghayour‐Mobarhan M, Baino F, Pasdar A, Sahebkar A, et al. Curcumin in tissue engineering: A traditional remedy for modern medicine. Biofactors 2019; 45(2): 135-51.
- Rahimi HR, Jaafari MR, Mohammadpour AH, Abnous K, Ghayour Mobarhan M, Ramezanzadeh E, et al. Curcumin: reintroduced therapeutic agent from traditional medicine for alcoholic liver disease. Asia Pacific J Med Toxicol. 2015; 4(1): 25-30.
- Melo JV, Barnes DJ. Chronic myeloid leukaemia as a model of disease evolution in human cancer. Nat Rev Cancer 2007; 7(6): 441-53.
- Heisterkamp N, Jenster G, Ten Hoeve J, Zovich D, Pattengale PK, Groffen J. Acute leukaemia in bcr/abl transgenic mice. Nature 1990; 344(6263): 251-53.
- Wei L, Yang Y, Gupta P, Wang A, Zhao M, Zhao Y, et al. A small molecule inhibitor, OGP46, is effective against imatinib-resistant BCR-ABL mutations via the BCR-ABL/JAK-STAT pathway. Molecular Therapy-Oncolytics 2020; 18(1): 137-48.
- Croom KF, Perry CM. Imatinib mesylate. Drugs 2003; 63(5): 513-22.
- Joensuu H, Dimitrijevic S. Tyrosine kinase inhibitor imatinib (STIS71) as an anticancer agent for solid tumours. Ann Med. 2001; 33(7): 451-55.
- Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nature Med. 1996; 2(5): 561-66.
- El Jurdi N, Bankoff M, Klein A. Perforation of the colon during imatinib mesylate (Gleevec) treatment in a patient with chronic myeloid leukemia (CML). Cureus 2016; 8(6): 660.
- Hosseini SA, Zand H, Cheraghpour M. The influence of Curcumin on the downregulation of MYC, insulin and IGF-1 receptors: a possible mechanism underlying the anti-growth and anti-migration in chemoresistant colorectal cancer cells. Medicina 2019; 55(4): 90.
- Boarescu PM, Boarescu I, Bocșan IC, Gheban D, Bulboacă AE, Nicula C, et al. Antioxidant and anti-inflammatory effects of Curcumin nanoparticles on drug-induced acute myocardial infarction in diabetic rats. Antioxidants 2019; 8(10): 504.









































 , Parisa Bagheri
, Parisa Bagheri 
 , Saeedeh Hajebi Khaniki
, Saeedeh Hajebi Khaniki 
 , Zahra Chehreghani
, Zahra Chehreghani 
 , Hamid Reza Rahimi *
, Hamid Reza Rahimi * 



