Thu, Jan 29, 2026
[Archive]
Volume 9, Issue 4 (November 2022)
IJML 2022, 9(4): 295-303 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Naseri M R, Kalantar S M, Nikoonahad N, Shafienia H, Montazeri F. Differential Expression of Circulating miRNA-103 in Women with and without Polycystic Ovary Syndrome. IJML 2022; 9 (4) :295-303
URL: http://ijml.ssu.ac.ir/article-1-448-en.html
URL: http://ijml.ssu.ac.ir/article-1-448-en.html
Differential Expression of Circulating miRNA-103 in Women with and without Polycystic Ovary Syndrome
Mohammad Reza Naseri 

 , Seyed Mehdi Kalantar
, Seyed Mehdi Kalantar 

 , Narges Nikoonahad
, Narges Nikoonahad 

 , Hanieh Shafienia
, Hanieh Shafienia 

 , Fateme Montazeri *
, Fateme Montazeri * 




 , Seyed Mehdi Kalantar
, Seyed Mehdi Kalantar 

 , Narges Nikoonahad
, Narges Nikoonahad 

 , Hanieh Shafienia
, Hanieh Shafienia 

 , Fateme Montazeri *
, Fateme Montazeri * 


Abortion Research Center, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. Department of Biology, Faculty of Science, Science and Art University, Yazd, Iran
Full-Text [PDF 303 kb]
(454 Downloads)
| Abstract (HTML) (1563 Views)
References
Full-Text: (789 Views)
Introduction
Stein and Leventhal first described polycystic ovary syndrome (PCOS) in women without menstrual periods and with polycystic ovaries in 1935. PCOS is a common and heterogeneous endocrine disorder in women of reproductive age [1, 2]. Across the world, 116 million women are involved with this syndrome, which is estimated to affect approximately 4-8% of women in the community [3]. In Pakistan, about half of women of reproductive age were affected by PCOS in 2009. According to various diagnostic criteria, 4-14% of Iranian women have been reported with this syndrome [3, 4]. The main features of this chronic ovulation syndrome are menstrual irregularity, increased serum androgen levels, changes in the ratio of LH/FSH, and polycystic ovaries. Other symptoms include hirsutism, glucose intolerance, diabetes mellitus, and cardio-vascular disorders, representing endocrine and metabolic disorders [5, 6]. Being overweight in women with PCOS is more than normal; about 30-80% of PCOS women are obese [7-9].
Given that the etiology of PCOS is unclear, there is evidence of the role of environmental, chemical, and genetic factors [9]. Besides, insulin resistance and obesity have been suggested to be associated with this syndrome [10]. In addition, having a family history of PCOS is considered a decisive risk factor. The main evidence of this claim is the high prevalence of this syndrome among first-degree relatives [2, 11].
MicroRNAs (miRNAs) are small noncoding endogenous RNA sequences. These single-stranded sequences are around 22 nucleotides long and negatively regulate the expression of their target genes. Furthermore, the type of expression of the microRNAs in each tissue is controlled. They can have an induction or inhibitory effect on other miRNAs or play a role in a signaling pathway. It is estimated that miRNAs can regulate the expression of more than 30% of genes and may play a vital regulatory role in most biological processes [12-14].
Recent findings showed that miRNAs could be detected in specific tissue and circulation [15]. These small noncoding RNAs, especially extracellular miRNAs (circulating miRNAs), have gained more attention as biomarker candidates. Therefore, investigating miRNAs in serum or plasma might point to the overall circumstance of the whole body in different conditions. The strengths of circulating miRNAs as a biomarker include high stability, selectivity, noninvasive, cheap and fast accessibility, and specificity for a particular tissue or a specific pathology/injury. These characteristics make circulating miRNAs predictive and reflective biomarkers of disease development or clinical diagnosis [16, 17]. Unusual expression of circulating miRNAs has been reported in numerous metabolic disorders, counting obesity, diabetes, and newly, PCOS. It is known that circulating miRNAs have a significant role in maintaining metabolic homeostasis [18, 19]. Therefore, miRNAs show potential for regulating the signaling pathways involved in PCOS. The role of miRNAs in PCOS pathogenesis and development is not well defined and has only been investigated in a few studies [18, 20, 21].
MiR-103 is a paralog of miR-107, and both genes are completely protected in all vertebrates [22, 23]. The highest expression of this miRNA has been observed in the brain. However, its expression in different tissues, as well as human serum, has been reported in several studies [22, 24].
Literature review and bioinformatics studies predict that miR-103/7 will likely regulate the body's metabolism [22]. It seems to play its role by influencing lipids' metabolism and acetyl CoA [22, 25-27]. Also, this miRNA indirectly affects peroxisome proliferators-activated receptor [22, 28], leading to insulin resistance in PCOS women. Considering the potential role of this miRNA in lipid metabolism and its association with overweight and insulin resistance, we will compare the expression level of circulating miR-103 in PCOS and healthy women. To the best of our knowledge, there has been no study investigating whether miR-103 expresses differentially in PCOS women, and that could be a potential noninvasive biomarker for early diagnosis of PCOS.
Materials and Methods
A total of 50 serum samples were collected from the peripheral blood of the Iranian women's population referred to Yazd Reproductive Sciences Institute. Twenty-five serum samples from PCOS patients and 25 healthy women without any evidence of androgen excess or ovulatory dysfunction were included in the study. PCOS diagnosing was according to Rotterdam Criteria [9]. The mean age of the participants in the PCOS group was 28 ± 4.4 years, and in the healthy group was 28 ± 5.3 years, and the mean body mass index (BMI) was 27.37 ± 3.38 kg/m2 and 25.02 ± 3.87 kg/m2 in the patients and healthy group, respectively.
In this study, 10 mL of peripheral blood was received from each patient. The whole blood was centrifuged twice, once at 4000 rpm for 10 min, then for 15 minutes at 1200 rpm to remove cell particles completely, and finally, serum was stored at −80 ˚C for further analysis. Total RNA was isolated from serum samples using the High Pure miRNA Isolation Kit from Roche Company (made in Germany). The concentration and purity of the extracted RNA were measured by a Nanodrop spectrophotometer (ND-1000). MiR-103 Stem-loop cDNAs were specifically synthesized by ABI kit (Applied Biosystems, Carlsbad, CA, USA) according to the manufacturer's instructions, by specific stem-primer with following sequence:
5'- GTCGTATGCAGAGCAGGGTCCGAGGTA-3'
5'-TTCGCACTGCATACGACTCATAG-3'.
Quantitative reverse transcription-polymerase chain reaction (Q-PCR) using SYBR premix Ex Taq II kit (Amplicon) was performed for evaluating miR-103 and Snord as a reference. Aliquots of the reaction mixture with a total volume of 25 µl were used for quantitative PCR by the following conditions: initial denaturation at 95 ˚C for 10 min followed by 40 cycles of 95 ˚C for 15 s, 60 ˚C for 1 min and 72 ˚C for 45 s. All PCR experiments were performed in duplicate. This experimental study was conducted following the Declaration of Helsinki. Also, this study was approved by the Ethical Committee of Yazd Reproductive Sciences Institute (Code no.: IR.SSU.RSI.REC.1396.8), and all the participants signed informed consent before their inclusion.
Statistical analysis
All statistical analyses were performed using SPSS software version 23.0 (SPSS, Inc., Chicago, USA). The case and control samples were compared using the Mann-Whitney U test. The frequency distributions between the two groups were analyzed using Fisher's exact test and Chi-square analysis. All hypotheses were two-sided, and the significant level was considered p < 0.05.
Results
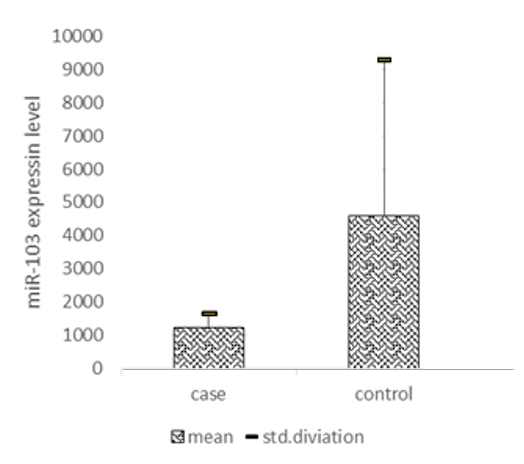
In this study, we evaluated the expression of circulating miR-103 in the plasma of PCOS women. The characteristics of all participants in this study are summarized in Table 1, including 25 PCOS and 25 Non-PCOS women. They were well-matched in age, and we found no differences in serum estradiol levels. However, the BMI and serum anti-mullerian hormone (AMH) levels were significantly higher in PCOS women (p < 0.05). Q-PCR was applied to evaluate the expression level of miRNA-103 relative to the expression of reference miRNA, SNORD, which has consistent expression in the serum of both groups. A significant decrease in the expression of miRNA-103 was observed in serum derived from patients with PCOS compared with non-PCOS women (p = 0.02) (Fig. 1).
Despite a significant difference between groups regarding the expression level of miRNA-103 and AMH level, the correlation between AMH and estradiol with the expression level of miRNA-103 in PCOS women was statically non-significant at the 0.05 level. Also, any significant correlation between age and BMI of patients with the expression level of miRNA-103 in PCOS women was observed.
Given that the etiology of PCOS is unclear, there is evidence of the role of environmental, chemical, and genetic factors [9]. Besides, insulin resistance and obesity have been suggested to be associated with this syndrome [10]. In addition, having a family history of PCOS is considered a decisive risk factor. The main evidence of this claim is the high prevalence of this syndrome among first-degree relatives [2, 11].
MicroRNAs (miRNAs) are small noncoding endogenous RNA sequences. These single-stranded sequences are around 22 nucleotides long and negatively regulate the expression of their target genes. Furthermore, the type of expression of the microRNAs in each tissue is controlled. They can have an induction or inhibitory effect on other miRNAs or play a role in a signaling pathway. It is estimated that miRNAs can regulate the expression of more than 30% of genes and may play a vital regulatory role in most biological processes [12-14].
Recent findings showed that miRNAs could be detected in specific tissue and circulation [15]. These small noncoding RNAs, especially extracellular miRNAs (circulating miRNAs), have gained more attention as biomarker candidates. Therefore, investigating miRNAs in serum or plasma might point to the overall circumstance of the whole body in different conditions. The strengths of circulating miRNAs as a biomarker include high stability, selectivity, noninvasive, cheap and fast accessibility, and specificity for a particular tissue or a specific pathology/injury. These characteristics make circulating miRNAs predictive and reflective biomarkers of disease development or clinical diagnosis [16, 17]. Unusual expression of circulating miRNAs has been reported in numerous metabolic disorders, counting obesity, diabetes, and newly, PCOS. It is known that circulating miRNAs have a significant role in maintaining metabolic homeostasis [18, 19]. Therefore, miRNAs show potential for regulating the signaling pathways involved in PCOS. The role of miRNAs in PCOS pathogenesis and development is not well defined and has only been investigated in a few studies [18, 20, 21].
MiR-103 is a paralog of miR-107, and both genes are completely protected in all vertebrates [22, 23]. The highest expression of this miRNA has been observed in the brain. However, its expression in different tissues, as well as human serum, has been reported in several studies [22, 24].
Literature review and bioinformatics studies predict that miR-103/7 will likely regulate the body's metabolism [22]. It seems to play its role by influencing lipids' metabolism and acetyl CoA [22, 25-27]. Also, this miRNA indirectly affects peroxisome proliferators-activated receptor [22, 28], leading to insulin resistance in PCOS women. Considering the potential role of this miRNA in lipid metabolism and its association with overweight and insulin resistance, we will compare the expression level of circulating miR-103 in PCOS and healthy women. To the best of our knowledge, there has been no study investigating whether miR-103 expresses differentially in PCOS women, and that could be a potential noninvasive biomarker for early diagnosis of PCOS.
Materials and Methods
A total of 50 serum samples were collected from the peripheral blood of the Iranian women's population referred to Yazd Reproductive Sciences Institute. Twenty-five serum samples from PCOS patients and 25 healthy women without any evidence of androgen excess or ovulatory dysfunction were included in the study. PCOS diagnosing was according to Rotterdam Criteria [9]. The mean age of the participants in the PCOS group was 28 ± 4.4 years, and in the healthy group was 28 ± 5.3 years, and the mean body mass index (BMI) was 27.37 ± 3.38 kg/m2 and 25.02 ± 3.87 kg/m2 in the patients and healthy group, respectively.
In this study, 10 mL of peripheral blood was received from each patient. The whole blood was centrifuged twice, once at 4000 rpm for 10 min, then for 15 minutes at 1200 rpm to remove cell particles completely, and finally, serum was stored at −80 ˚C for further analysis. Total RNA was isolated from serum samples using the High Pure miRNA Isolation Kit from Roche Company (made in Germany). The concentration and purity of the extracted RNA were measured by a Nanodrop spectrophotometer (ND-1000). MiR-103 Stem-loop cDNAs were specifically synthesized by ABI kit (Applied Biosystems, Carlsbad, CA, USA) according to the manufacturer's instructions, by specific stem-primer with following sequence:
5'- GTCGTATGCAGAGCAGGGTCCGAGGTA-3'
5'-TTCGCACTGCATACGACTCATAG-3'.
Quantitative reverse transcription-polymerase chain reaction (Q-PCR) using SYBR premix Ex Taq II kit (Amplicon) was performed for evaluating miR-103 and Snord as a reference. Aliquots of the reaction mixture with a total volume of 25 µl were used for quantitative PCR by the following conditions: initial denaturation at 95 ˚C for 10 min followed by 40 cycles of 95 ˚C for 15 s, 60 ˚C for 1 min and 72 ˚C for 45 s. All PCR experiments were performed in duplicate. This experimental study was conducted following the Declaration of Helsinki. Also, this study was approved by the Ethical Committee of Yazd Reproductive Sciences Institute (Code no.: IR.SSU.RSI.REC.1396.8), and all the participants signed informed consent before their inclusion.
Statistical analysis
All statistical analyses were performed using SPSS software version 23.0 (SPSS, Inc., Chicago, USA). The case and control samples were compared using the Mann-Whitney U test. The frequency distributions between the two groups were analyzed using Fisher's exact test and Chi-square analysis. All hypotheses were two-sided, and the significant level was considered p < 0.05.
Results
In this study, we evaluated the expression of circulating miR-103 in the plasma of PCOS women. The characteristics of all participants in this study are summarized in Table 1, including 25 PCOS and 25 Non-PCOS women. They were well-matched in age, and we found no differences in serum estradiol levels. However, the BMI and serum anti-mullerian hormone (AMH) levels were significantly higher in PCOS women (p < 0.05). Q-PCR was applied to evaluate the expression level of miRNA-103 relative to the expression of reference miRNA, SNORD, which has consistent expression in the serum of both groups. A significant decrease in the expression of miRNA-103 was observed in serum derived from patients with PCOS compared with non-PCOS women (p = 0.02) (Fig. 1).
Despite a significant difference between groups regarding the expression level of miRNA-103 and AMH level, the correlation between AMH and estradiol with the expression level of miRNA-103 in PCOS women was statically non-significant at the 0.05 level. Also, any significant correlation between age and BMI of patients with the expression level of miRNA-103 in PCOS women was observed.
Table 1. The characteristics and clinical variables of participants in this study
| Characteristics | Groups | N | Mean | Std. Deviation | p-value |
| Age | PCOS | 25 | 28.08 | 4.40 | 0.29 |
| Control | 25 | 28.72 | 5.38 | ||
| Body mass index | PCOS | 25 | 27.37 | 3.38 | 0.026 |
| Control | 25 | 25.02 | 3.87 | ||
| Estradiol level | PCOS | 25 | 2846.36 | 1595.3 | 0.195 |
| Control | 25 | 2140.91 | 1324.54 | ||
| Anti-mullerian hormone level | PCOS | 25 | 8.8356 | 4.26 | 0.010 |
| Control | 25 | 4.4524 | 2.20 | ||
| miR-103 expression | PCOS | 25 | 1229.54 | 431.05 | 0.02 |
| Control | 25 | 4604.13 | 4711.09 |
PCOS= Polycystic ovary syndrome
Table 2. Biological Functions of the Predicted Targets for the miRNA-103, which can be related to the etiology and progression of PCOS
| Target gene | Full name | Function of genes | Ref | |
| Hormone | ||||
| Steroid hormone receptor activity | HSD3B7 | hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 7 | Encodes an enzyme in liver cells that stimulate the bile process, including absorbing fats and fat-soluble vitamins. Bile acids are generated from cholesterol in a multi-step process. | [29] |
| Metabolism | ||||
| Lipoprotein | LRP1 | low-density lipoprotein receptor-related protein 1 | Encodes a lipoprotein receptor that mediates some cellular procedures, containing intracellular signaling, lipid metabolism, and removal of apoptotic bodies. | [30] |
| LRP2 | low-density lipoprotein receptor-related protein 2 | This gene constructs a receptor protein named megalin. After binding, ligands activate signaling pathways involved in cellular development. The role of this gene varies depending on the target tissue and the ligand attached. They are also involved in many functions containing vitamin absorption, immune activities, stress response, and fat metabolism. | [31, 32] | |
| LRP1B | low-density lipoprotein receptor-related protein 1B | This gene encodes a receptor that functions variably, depending on the ligand attached in many cellular processes in normal cells and some cancerous conditions. | [33] | |
| Insulin signaling pathway | ACACA | acetyl-CoA carboxylase alpha | Encodes a biotin-containing enzyme that involves in fatty acid biosynthesis. | [34, 35] |
| CRKL | CRK like proto-oncogene | Encodes a protein kinase with SH2 and SH3 domains and activates many kinase signaling pathways, including insulin metabolism. | [36] | |
| PHKA1 | phosphorylase kinase regulatory subunit alpha 1 | This kinase is responsible for regulating cellular energy by activating glycogen phosphorylase b which catalyzes glycogen. | [37] | |
| PRKAB2 | protein kinase AMP-activated non-catalytic subunit beta 2 | This gene control cellular energy status by the effect on AMPK. Non-catalytic subunit of this kinase is involved in lipid and carbohydrate biosynthesis. Expression of AMPK was observed in cellular stresses, growth, and proliferation. |
[38] | |
| RPS6KB1 | ribosomal protein S6 kinase B1 | This gene involves the serine/threonine kinases system by effecting the ribosomal S6 kinase. Includes mTOR signaling to stimulate protein synthesis, cellular growth, and proliferation. | [39] | |
| Response to insulin stimulus | Cav1 | caveolin-1 | Overexpression in Caveolin-1 is related to miR-103/107 inactivation in adipocytes and involved in insulin signaling. miR-103 was identified as a key factor for insulin sensitivity and a novel target for treating type 2 diabetes and obesity. | [40] |
| PI3K | Phosphoinositide 3-kinase | Overexpression of PI3K in oocytes related to premature ovarian failure (POF) is one of the symptoms of PCOS | [41] | |
| Sexual reproduction | ||||
| Progesterone-mediated oocyte maturation | PGRMC2 | progesterone receptor membrane component 2 | Localized to female reproductive tracts and expressed in the oocyte. Essential for the maintenance of a normal female reproductive lifespan. | [42] |
| CDC25A | cell division cycle 25A | It is necessary to transfer from G1 to the S phase of the cell cycle and to be involved in regulating phosphatase activity, protein levels, and protein-protein interactions, especially degraded in response to DNA damage. |
[43] |

Fig. 1. Comparison of miR-103 expression levels between PCOs individuals and healthy women (p = 0.02)
Discussion
MicroRNAs have been known as novel regulatory factors that control many biological functions through different signaling pathways, including metabolic pathways. New studies have revealed that miRNAs could be detected in different human body fluids, such as follicular fluid and serum. Our finding confirms these results [12, 15]. Our knowledge about circulating miRNAs, specifically their role concerning PCOS is currently at a very early stage. Since PCOS is a metabolic disorder, the same pathways and miRNAs are likely involved.
Further studies could help to understand the molecular mechanisms behind this heterogenic syndrome. Considering the multiple roles of this miRNA in regulating involved processes in PCOS pathogenesis, its serum expression level was measured to understand its molecular mechanism. Recently, it has been reported that the expression of miR-103 increased in obese women with PCOS while it decreased in obese women without PCOS, which recommended that obesity and androgen concentrations control the expression of this miRNA [18, 44, 45]. An original study compared the expression of four miRNAs: miR-21, miR-27b, miR-103, and miR-155 in PCOS women with healthy females and male controls. Their findings revealed that obesity significantly decreases understudied miRNAs but leans towards displaying an increase in expression in PCOS women [22]. However, our result was incompatible with the study of Murri and colleagues; this was carried out on a larger number of PCOS patients with BMI relatively similar to the control group [18, 19]. According to the bioinformatics studies and original research, miRNA-103 has a role in many cellular processes, including the immune and inflammatory processes, hormone and intermediate metabolic pathways, adipogenesis, insulin signaling, and reproduction. Furthermore, these processes interact; almost all are involved in PCOS pathogenesis [3, 12, 13, 46]. Considering a single miRNA could regulate numerous 'target' mRNAs or, in other words, a complete metabolic pathway, we could suggest some miRNAs like miR-103 and miR-107 regulate metabolic pathways in PCOS women [22, 47-49]. Compared with healthy women, serum expression of miR-21 is increased in PCOS patients. By targeting LATS1, miR-21 could prompt PCOS progression and act as a novel noninvasive biomarker for diagnosing PCOS [50].
Conclusion
These findings significantly extend previous results and show that serum levels of miRNA-103 were PCOS-specific and could be used to distinguish between PCOS patients and healthy women [51]. Therefore, it is valuable to suggest that investigating PCOS-specific miRNAs could raise the sensitivity and specificity of a PCOS diagnosis. In addition, these results can provide advantageous information about the etiological causes of this multifactorial syndrome to researchers and scientists.
Conflict of Interest
The authors declare that they have no competing interests.
Acknowledgments
We sincerely appreciate our colleagues in the ART section of Yazd Reproductive Sciences Institute and Yazd Genome laboratory for their kind collaboration. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Further studies could help to understand the molecular mechanisms behind this heterogenic syndrome. Considering the multiple roles of this miRNA in regulating involved processes in PCOS pathogenesis, its serum expression level was measured to understand its molecular mechanism. Recently, it has been reported that the expression of miR-103 increased in obese women with PCOS while it decreased in obese women without PCOS, which recommended that obesity and androgen concentrations control the expression of this miRNA [18, 44, 45]. An original study compared the expression of four miRNAs: miR-21, miR-27b, miR-103, and miR-155 in PCOS women with healthy females and male controls. Their findings revealed that obesity significantly decreases understudied miRNAs but leans towards displaying an increase in expression in PCOS women [22]. However, our result was incompatible with the study of Murri and colleagues; this was carried out on a larger number of PCOS patients with BMI relatively similar to the control group [18, 19]. According to the bioinformatics studies and original research, miRNA-103 has a role in many cellular processes, including the immune and inflammatory processes, hormone and intermediate metabolic pathways, adipogenesis, insulin signaling, and reproduction. Furthermore, these processes interact; almost all are involved in PCOS pathogenesis [3, 12, 13, 46]. Considering a single miRNA could regulate numerous 'target' mRNAs or, in other words, a complete metabolic pathway, we could suggest some miRNAs like miR-103 and miR-107 regulate metabolic pathways in PCOS women [22, 47-49]. Compared with healthy women, serum expression of miR-21 is increased in PCOS patients. By targeting LATS1, miR-21 could prompt PCOS progression and act as a novel noninvasive biomarker for diagnosing PCOS [50].
Conclusion
These findings significantly extend previous results and show that serum levels of miRNA-103 were PCOS-specific and could be used to distinguish between PCOS patients and healthy women [51]. Therefore, it is valuable to suggest that investigating PCOS-specific miRNAs could raise the sensitivity and specificity of a PCOS diagnosis. In addition, these results can provide advantageous information about the etiological causes of this multifactorial syndrome to researchers and scientists.
Conflict of Interest
The authors declare that they have no competing interests.
Acknowledgments
We sincerely appreciate our colleagues in the ART section of Yazd Reproductive Sciences Institute and Yazd Genome laboratory for their kind collaboration. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Dewailly D, Lujan ME, Carmina E, Cedars MI, Laven J, Norman RJ, et al. Definition and significance of polycystic ovarian morphology: a task force report from the Androgen Excess and Polycystic Ovary Syndrome Society. Human Reproduction Update 2014; 20(3): 334-52.
- Chatterjee T. Polycystic Ovary syndrome–A review. Res Rev Biosci. 2016; 11(4): 104.
- Mobeen H, Afzal N, Kashif M. Polycystic ovary syndrome may be an autoimmune disorder. Scientifica 2016; 4071735: 1-7.
- Rashidi H, Ramezani Tehrani F, Bahri Khomami M, Rostami Dovom M, Noroozzadeh M, Azizi F. The Prevalence of various phenotypes of polycystic ovary syndrome: a community-based study in Southwest of Iran. Iranian Journal of Endocrinology and Metabolism 2014; 16(2): 119-26.
- Casarini L, Simoni M, Brigante G. Is polycystic ovary syndrome a sexual conflict? A review. Reproductive Biomedicine Online 2016; 32(4): 350-61.
- McCartney CR, Marshall JC. Polycystic ovary syndrome. New England Journal of Medicine 2016; 375(1): 54-64.
- Vrbikova J, Hainer V. Obesity and polycystic ovary syndrome. Obesity Facts 2009; 2(1): 26-35.
- De Leo V, Musacchio M, Cappelli V, Massaro M, Morgante G, Petraglia F. Genetic, hormonal and metabolic aspects of PCOS: an update. Reproductive Biology and Endocrinology 2016; 14(1): 38.
- Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocrine Reviews 2015; 36(5): 487-525.
- Polak K, Czyzyk A, Simoncini T, Meczekalski B. New markers of insulin resistance in polycystic ovary syndrome. Journal of Endocrinological Investigation 2017; 40(1): 1-8.
- Kahsar-Miller M, Azziz R. The development of the polycystic ovary syndrome: family history as a risk factor. Trends in Endocrinology & Metabolism 1998; 9(2): 55-8.
- Maalouf S, Liu W, Pate JL. MicroRNA in ovarian function. Cell and Tissue Research 2016; 363(1): 7-18.
- Iorio MV, Visone R, Di Leva G, Donati V, Petrocca F, Casalini P, et al. MicroRNA signatures in human ovarian cancer. Cancer Research 2007; 67(18): 8699-707.
- Jesintha Mary M, Deecaraman M, Vijayalakshmi M, Umashankar V. A systemic review on differential regulation of genes in polycystic ovarian syndrome disease. International Journal of Pharma and Biosciences 2015; 6(2): 893-900.
- Sørensen AE, Wissing ML, Salö S, Englund ALM, Dalgaard LT. MicroRNAs related to polycystic ovary syndrome (PCOS). Genes 2014; 5(3): 684-708.
- Viereck J, Thum T. Circulating noncoding RNAs as biomarkers of cardiovascular disease and injury. Circulation Research 2017; 120(2): 381-99.
- Cortez MA, Bueso-Ramos C, Ferdin J, Lopez-Berestein G, Sood AK, Calin GA. MicroRNAs in body fluids-the mix of hormones and biomarkers. Nature Reviews Clinical Oncology. 2011; 8(8): 467.
- Murri M, Insenser M, Fernández-Durán E, San-Millán JL, Escobar-Morreale HF. Effects of polycystic ovary syndrome (PCOS), sex hormones, and obesity on circulating miRNA-21, miRNA-27b, miRNA-103, and miRNA-155 expression. The Journal of Clinical Endocrinology & Metabolism 2013; 98(11): 1835-44.
- Fernández-Hernando C, Ramírez CM, Goedeke L, Suárez Y. MicroRNAs in metabolic disease. Arteriosclerosis, Thrombosis, and Vascular Biology 2013; 33(2): 178-85.
- Hossain MM, Cao M, Wang Q, Kim JY, Schellander K, Tesfaye D, et al. Altered expression of miRNAs in a dihydrotestosterone-induced rat PCOS model. Journal of Ovarian Research 2013; 6(1): 36.
- Imbar T, Eisenberg I. Regulatory role of microRNAs in ovarian function. Fertility and Sterility 2014; 101(6): 1524-530.
- Wilfred BR, Wang W-X, Nelson PT. Energizing miRNA research: a review of the role of miRNAs in lipid metabolism, with a prediction that miR-103/107 regulates human metabolic pathways. Molecular Genetics and Metabolism 2007; 91(3): 209-17.
- Kumar M, Nath S, Prasad HK, Sharma G, Li Y. MicroRNAs: a new ray of hope for diabetes mellitus. Protein & Cell 2012; 3(10): 726-38.
- Babak T, Zhang W, Morris Q, Blencowe BJ, Hughes TR. Probing microRNAs with microarrays: tissue specificity and functional inference. RNA 2004; 10(11): 1813-819.
- Dean C, Jackman R. M. VitaMindTM practitioner information. Strauss: Herb Company; 2004.
- Jackowski S, Rock C. Regulation of coenzyme A biosynthesis. Journal of Bacteriology 1981; 148(3): 926-32.
- Rock CO, Calder RB, Karim MA, Jackowski S. Pantothenate kinase regulation of the intracellular concentration of coenzyme A. Journal of Biological Chemistry 2000; 275(2): 1377-383.
- Pan S, Yang X, Jia Y, Li R, Zhao R. Microvesicle‐shuttled mir‐130b reduces fat deposition in recipient primary cultured porcine adipocytes by inhibiting PPAR‐γ expression. Journal of Cellular Physiology 2014; 229(5): 631-39.
- Subramaniam P, Clayton PT, Portmann BC, Mieli-Vergani G, Hadžic N. Variable clinical spectrum of the most common inborn error of bile acid metabolism-3β-hydroxy-Δ5-C27-steroid dehydrogenase deficiency. Journal of Pediatric Gastroenterology and Nutrition 2010; 50(1): 61-6.
- Klar J, Schuster J, Khan TN, Jameel M, Mäbert K, Forsberg L, et al. Whole exome sequencing identifiesLRP1as a pathogenic gene in autosomal recessive keratosis pilaris atrophicans. Journal of Medical Genetics 2015; 52(9): 599-606.
- Christensen EI, Birn H. Megalin and cubilin: multifunctional endocytic receptors. Nature Reviews Molecular Cell Biology 2002; 3(4): 258-67.
- Christensen EI, Nielsen R. Role of megalin and cubilin in renal physiology and pathophysiology. Reviews of Physiology, Biochemistry and Pharmacology: Springer Berlin Heidelberg; 2006: 1-22.
- Xing P, Liao Z, Ren Z, Zhao J, Song F, Wang G, et al. Roles of low-density lipoprotein receptor-related protein 1 in tumors. Chinese Journal of Cancer 2016; 35(1): 6.
- Hunkeler M, Hagmann A, Stuttfeld E, Chami M, Guri Y, Stahlberg H, et al. Structural basis for regulation of human acetyl-CoA carboxylase. Nature 2018; 558(7710): 470-74.
- Colbert CL, Kim CW, Moon YA, Henry L, Palnitkar M, McKean WB, et al. Crystal structure of Spot 14, a modulator of fatty acid synthesis. Proc Natl Acad Sci USA. 2010; 107(44): 18820-825.
- Song Q, Yi F, Zhang Y, Jun Li DK, Wei Y, Yu H, et al. CRKL regulates alternative splicing of cancer-related genes in cervical cancer samples and HeLa cell. BMC Cancer 2019; 19(1): 499.
- Brushia RJ, Walsh DA. Phosphorylase kinase: the complexity of its regulation is reflected in the complexity of its structure. Front Biosci. 1999; 4(5): 618-41.
- Kazgan N, Williams T, Forsberg LJ, Brenman JE. Identification of a nuclear export signal in the catalytic subunit of AMP-activated protein kinase. Molecular Biology of the Cell 2010; 21(19): 3433-442.
- Dufner A, Thomas G. Ribosomal S6 kinase signaling and the control of translation. Experimental Cell Research 1999; 253(1): 100-109.
- Trajkovski M, Hausser J, Soutschek J, Bhat B, Akin A, Zavolan M, et al. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature 2011; 474(7353): 649-53.
- Adhikari D, Risal S, Liu K, Shen Y. Pharmacological inhibition of mTORC1 prevents over-activation of the primordial follicle pool in response to elevated PI3K signaling. PLoS One 2013; 8(1): 53810.
- Parker CG, Galmozzi A, Wang Y, Correia BE, Sasaki K, Joslyn CM, et al. Ligand and target discovery by fragment-based screening in human cells. Cell 2017; 168(3): 527-41.
- Hoffmann I, Draetta G, Karsenti E. Activation of the phosphatase activity of human cdc25A by a cdk2‐cyclin E dependent phosphorylation at the G1/S transition. The EMBO J 1994; 13(18): 4302-10.
- Nteeba J, Ross JW, Perfield Ii JW, Keating AF. High fat diet induced obesity alters ovarian phosphatidylinositol-3 kinase signaling gene expression. Reproductive Toxicology 2013; 42(1): 68-77.
- Rad HM, Mowla SJ, Ramazanali F, Valojerdi MR. Characterization of altered microRNAs related to different phenotypes of polycystic ovarian syndrome (PCOS) in serum, follicular fluid, and cumulus cells. Taiwanese Journal of Obstetrics and Gynecology 2022; 61(5): 768-79.
- Bahmyari S, Jamali Z, Khatami SH, Vakili O, Roozitalab M, Savardashtaki A, et al. microRNAs in female infertility: An overview. Cell Biochemistry and Function 2021; 39(8): 955-69.
- Sørensen A, Wissing M, Salö S, Englund A, Dalgaard L. MicroRNAs related to polycystic ovary syndrome (PCOS). Genes 2014; 5(3): 684-708.
- Kurylowicz A. microRNAs in human adipose tissue physiology and dysfunction. Cells 2021; 10(12): 3342.
- El-Ashmawy NE, Gawaly AM, Batanony HAE, Khedr NF. Regulation of MicroRNA 103 and 107 in Obese T2DM Patients Maintained on Metformin. Research Square 2021: 1-16.
- Jiang L, Li W, Wu M, Cao S. Ciculating miRNA-21 as a biomarker predicts polycystic ovary syndrome (PCOS) in patients. Clin Lab. 2015; 61: 1009-1015.
- Sørensen AE, Wissing ML, Englund ALM, Dalgaard LT. MicroRNA species in follicular fluid associating with polycystic ovary syndrome and related intermediary phenotypes. The Journal of Clinical Endocrinology & Metabolism 2016; 101(4): 1579-589.
Type of Study: Research |
Subject:
Genetics/ Biotechnology
Received: 2022/05/17 | Accepted: 2022/12/3 | Published: 2022/12/31
Received: 2022/05/17 | Accepted: 2022/12/3 | Published: 2022/12/31
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |