Wed, Feb 4, 2026
[Archive]
Volume 10, Issue 3 (August 2023)
IJML 2023, 10(3): 254-263 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Fallah Raoufi M, Mokhlesabadi Farahani T, Sadat Mousavi Sadr Jadidi E, Moradi Gardeshi T. Protective Effects of Ginger Extract on Oxidative Stress and Steroidogenesis-related Genes in The Ovary of Streptozotocin-induced Diabetic Rats. IJML 2023; 10 (3) :254-263
URL: http://ijml.ssu.ac.ir/article-1-452-en.html
URL: http://ijml.ssu.ac.ir/article-1-452-en.html
Mehri Fallah Raoufi 
 , Tahereh Mokhlesabadi Farahani
, Tahereh Mokhlesabadi Farahani 

 , Elnaz Sadat Mousavi Sadr Jadidi
, Elnaz Sadat Mousavi Sadr Jadidi 

 , Tohid Moradi Gardeshi *
, Tohid Moradi Gardeshi * 



 , Tahereh Mokhlesabadi Farahani
, Tahereh Mokhlesabadi Farahani 

 , Elnaz Sadat Mousavi Sadr Jadidi
, Elnaz Sadat Mousavi Sadr Jadidi 

 , Tohid Moradi Gardeshi *
, Tohid Moradi Gardeshi * 


Department of Veterinary Sciences, Garmsar Branch, Islamic Azad University, Garmsar, Iran
Full-Text [PDF 452 kb]
(839 Downloads)
| Abstract (HTML) (994 Views)


Discussion
References
Full-Text: (650 Views)
Introduction
Diabetes mellitus is a serious public health challenge characterized by the disturbed metabolism of carbohydrates, proteins, and lipids due to inappropriate insulin secretion, insulin resistance, or both [1]. The prevalence and burden of diabetes mellitus has dramatically increased worldwide and will affect around 700 million people by 2045 [2]. Under diabetes conditions, hyperglycemia may affect female reproductive functions at several levels due to the impairment of the endocrine regulation of folliculogenesis, steroidogenesis, and oocyte maturation. Ovarian steroidogenesis is a series of enzymatic reactions through which theca and granulosa cells synthesize steroid hormones 17β-estradiol (E2), progesterone, and androgens [3]. However, decreases in the transcript level of steroidogenic genes such as steroidogenic acute regulatory protein (StAR) and 3β-hydroxysteroid dehydrogenase (3βHSD) have also been reported in a hyperglycemic state. There is growing interest in understanding the controlling of these enzymatic reactions under diabetic conditions [4, 5].
Extensive evidence has elucidated that hyperglycemia-induced oxidative stress is a potent contributor to ovarian disorders [6, 7]. Excessive production of reactive oxygen species (ROS) can trigger multiple cellular signaling pathways that serve as critical mediators in the pathogenesis of diabetes-caused reproductive disorders [8-10]. Much evidence has highlighted that excessive ROS levels can involve the apoptosis induction of granulosa cells which impairs folliculogenesis and steroidogenesis. Therefore, counteracting excessive ROS levels might prevent diabetes-induced ovarian damage [11, 12]. Using herbal remedies effectively treats diseases, including diabetes [13, 14]. Ginger, Zingiber Officinale Roscoe, has been used for spice and medicine for thousands of years. Gingerols and shogaols are ginger's main phenolic compounds, accounting for various biological activities, including antioxidant, anti-inflammatory, and anti-cancer activities [15, 16]. Many studies have reported that ginger can manage multiple diseases, such as cardiovascular diseases, obesity, neurodegenerative diseases, and diabetes mellitus. The anti-diabetic effects of ginger have been proven through its anti-oxidant and anti-inflammatory properties [17, 18].
Given that the anti-diabetic activity of ginger has been confirmed, limited investigations have been carried out on the protective impact of ginger against the damage caused by hyperglycemia on the female reproductive system so far. Accordingly, the current work was designed to explore the impacts of ginger extract on reproductive hormones (progesterone, E2, testosterone) in the serum and some oxidant/anti-oxidant markers [(malondialdehyde (MDA) and glutathione peroxidase (GPx)] and steroidogenic genes (3βHSD and StAR) in the ovarian homogenate of STZ- stimulated diabetic rats.
Materials and Methods
Plant preparation and extraction
Dried rhizomes of ginger have concurred from Gol Darou Company (Isfahan, Iran). For extraction, 200 g ginger rhizomes were firstly ground to powder in an electric blender. Then, the rhizome powder was soaked in 70% methanol solution (v/v) at 25 °C for three days. The obtained extract was filtered, evaporated, freeze-dried, and then stored at -20 °C until later experiments [19].
Animals
The current research was an interventional study, and all procedures were confirmed by the animal care committee of the Garmsar Branch, Islamic Azad University (IR.IAU. SHAHROOD.REC.1400.069). In brief, a total of 96 female Wistar rats (220–250 g) were prepared from Garmsar Branch, Islamic Azad University., All animals were maintained at 25 ± 2 °C and a 12 h light/dark cycle for seven days before experimentations.
Experimental design
Diabetes was induced in overnight fasted rats by intraperitoneal injection of 60 mg/kg streptozotocin (STZ; Sigma) dissolved in 0.1 M citrate buffer (pH 4.5) [20]. After 72 hours, the fasting blood glucose levels were monitored, and the rats. Diabetes was confirmed according to blood glucose levels above 220 mg/ dl. All rats were randomly distributed into four groups (24 animals per each group), as follows:
1- Control: Healthy control animals were gavaged with distilled water daily for eight weeks.
2- Diabetes: Diabetic animals were gavaged with distilled water daily for eight weeks.
3- Diabetes +200 mg/kg ginger extract: Diabetic animals were gavaged 200 mg/kg ginger extract daily for eight weeks.
4- Diabetes+400 mg/kg ginger extract: Diabetic animals were gavaged 400 mg/kg ginger extract daily for eight weeks.
The ginger extract doses were selected based on prior studies [21, 22].
Samples collection and preparation
After eight weeks of treatment, the whole blood samples were directly taken by cardiac puncture after anesthetizing animals. The whole blood samples were immediately centrifuged (3500 rpm for 15 min), and the serum was kept at −80 °C for biochemical assays. Subsequently, all rats were then sacrificed, and the ovaries were removed. Ovarian homogenates were kept at −80 °C for later assays.
Biochemical assays
Determination of serum glucose and insulin
The serum glucose level was determined using a colorimetric assay kit from Pars Azmoon Company (Tehran, Iran) following instructions and an automatic biochemical analyzer. Following the manufacturer's instructions, blood insulin level was assayed using a commercial rat enzyme-linked immunosorbent assay (ELISA) kit (Monobind, USA).
Determination of MDA and GPx
The amount of MDA and activity of GPx were investigated in the ovarian homogenate using suitable kits obtained by Navand Health Company (Iran) following the manufacturer's instructions.
Determination of hormones
Progesterone, 17β-estradiol, and testosterone levels in the serum of all groups were assayed
by ELISA kits purchased from the Monobind Company (USA), following the manufacturer's recommendations.
Gene expression
RNA isolation was performed in the ovarian homogenate for gene expression analyses using the commercially available RNA isolation kit (Yekta Tajhiz, Iran). The quantity and integrity of total RNA were assessed using the nanodrop spectrophotometer (Thermo Scientific NanoDrop™ 1000) and 1.5% agarose gel electrophoresis. Trace to moderate amounts of genomic DNA contamination during the total RNA isolation is a frequent cause of false-positive signals in RT-qPCR-based analysis. Therefore, we performed an additional genomic DNA removal by DNase during total RNA extraction. Subsequently, about one µg of total RNA was subjected to cDNA synthesis using the reverse transcriptase kit (Yekta Tajhiz, Iran). Quantitative real-time PCR was done on a quantitative PCR system (ABI7500, USA) using SYBR® Green Master Mix (Yekta Tajhiz, Iran). The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was applied as a reference gene. Relative gene expression was analyzed using the 2-∆∆CT formula. The sequences of desired primers are provided in Table 1.
Statistical analysis
GraphPad Prism 8 software (San Diego, California, USA) analyzed data and drew graphs. All data are displayed as mean ± standard deviation (SD), and the difference among experimental groups was analyzed using one-way ANOVA and Tukey's posthoc. p < 0.05 were set as significant levels.
Results
The ginger extract improves glycemic indices
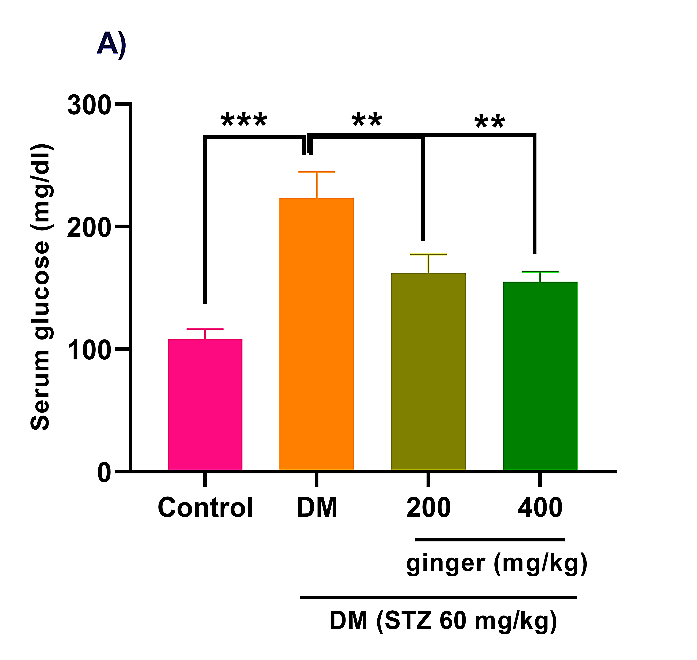
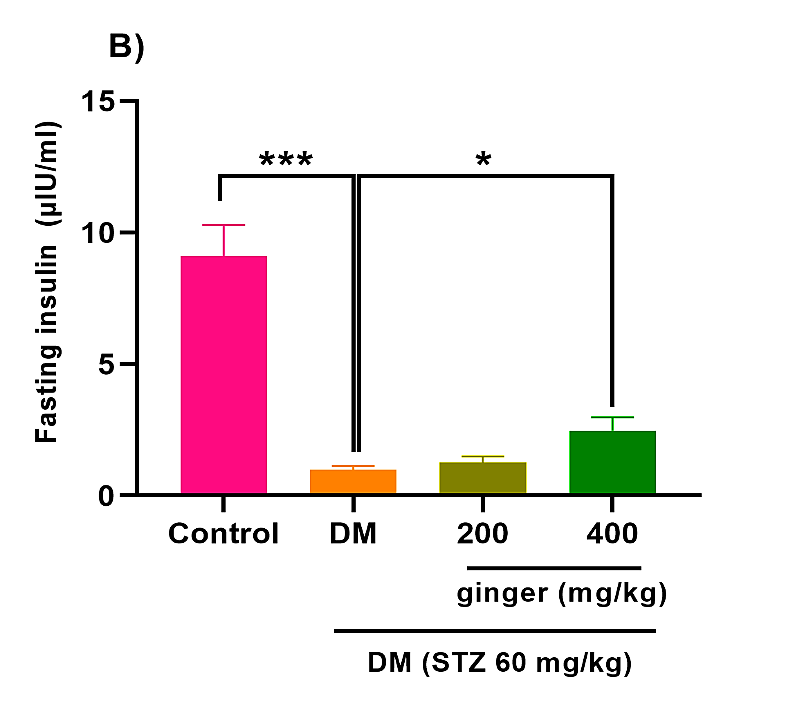
As depicted in Figure 1A, STZ induction in rats greatly increased fasting blood glucose concentration compared to the healthy group (p<0.001). However, the administration of diabetic rats with ginger extract (200 and 400 mg/kg) remarkably attenuated fasting blood glucose levels (p<0.01). As expected, the serum level of insulin was markedly reduced in the diabetic animals compared to the control animals (p<0.001). However, diabetic rats administrated with 400 mg/kg ginger extract exhibited an obvious improvement in the insulin level (Fig. 1B; p < 0.05).
Extensive evidence has elucidated that hyperglycemia-induced oxidative stress is a potent contributor to ovarian disorders [6, 7]. Excessive production of reactive oxygen species (ROS) can trigger multiple cellular signaling pathways that serve as critical mediators in the pathogenesis of diabetes-caused reproductive disorders [8-10]. Much evidence has highlighted that excessive ROS levels can involve the apoptosis induction of granulosa cells which impairs folliculogenesis and steroidogenesis. Therefore, counteracting excessive ROS levels might prevent diabetes-induced ovarian damage [11, 12]. Using herbal remedies effectively treats diseases, including diabetes [13, 14]. Ginger, Zingiber Officinale Roscoe, has been used for spice and medicine for thousands of years. Gingerols and shogaols are ginger's main phenolic compounds, accounting for various biological activities, including antioxidant, anti-inflammatory, and anti-cancer activities [15, 16]. Many studies have reported that ginger can manage multiple diseases, such as cardiovascular diseases, obesity, neurodegenerative diseases, and diabetes mellitus. The anti-diabetic effects of ginger have been proven through its anti-oxidant and anti-inflammatory properties [17, 18].
Given that the anti-diabetic activity of ginger has been confirmed, limited investigations have been carried out on the protective impact of ginger against the damage caused by hyperglycemia on the female reproductive system so far. Accordingly, the current work was designed to explore the impacts of ginger extract on reproductive hormones (progesterone, E2, testosterone) in the serum and some oxidant/anti-oxidant markers [(malondialdehyde (MDA) and glutathione peroxidase (GPx)] and steroidogenic genes (3βHSD and StAR) in the ovarian homogenate of STZ- stimulated diabetic rats.
Materials and Methods
Plant preparation and extraction
Dried rhizomes of ginger have concurred from Gol Darou Company (Isfahan, Iran). For extraction, 200 g ginger rhizomes were firstly ground to powder in an electric blender. Then, the rhizome powder was soaked in 70% methanol solution (v/v) at 25 °C for three days. The obtained extract was filtered, evaporated, freeze-dried, and then stored at -20 °C until later experiments [19].
Animals
The current research was an interventional study, and all procedures were confirmed by the animal care committee of the Garmsar Branch, Islamic Azad University (IR.IAU. SHAHROOD.REC.1400.069). In brief, a total of 96 female Wistar rats (220–250 g) were prepared from Garmsar Branch, Islamic Azad University., All animals were maintained at 25 ± 2 °C and a 12 h light/dark cycle for seven days before experimentations.
Experimental design
Diabetes was induced in overnight fasted rats by intraperitoneal injection of 60 mg/kg streptozotocin (STZ; Sigma) dissolved in 0.1 M citrate buffer (pH 4.5) [20]. After 72 hours, the fasting blood glucose levels were monitored, and the rats. Diabetes was confirmed according to blood glucose levels above 220 mg/ dl. All rats were randomly distributed into four groups (24 animals per each group), as follows:
1- Control: Healthy control animals were gavaged with distilled water daily for eight weeks.
2- Diabetes: Diabetic animals were gavaged with distilled water daily for eight weeks.
3- Diabetes +200 mg/kg ginger extract: Diabetic animals were gavaged 200 mg/kg ginger extract daily for eight weeks.
4- Diabetes+400 mg/kg ginger extract: Diabetic animals were gavaged 400 mg/kg ginger extract daily for eight weeks.
The ginger extract doses were selected based on prior studies [21, 22].
Samples collection and preparation
After eight weeks of treatment, the whole blood samples were directly taken by cardiac puncture after anesthetizing animals. The whole blood samples were immediately centrifuged (3500 rpm for 15 min), and the serum was kept at −80 °C for biochemical assays. Subsequently, all rats were then sacrificed, and the ovaries were removed. Ovarian homogenates were kept at −80 °C for later assays.
Biochemical assays
Determination of serum glucose and insulin
The serum glucose level was determined using a colorimetric assay kit from Pars Azmoon Company (Tehran, Iran) following instructions and an automatic biochemical analyzer. Following the manufacturer's instructions, blood insulin level was assayed using a commercial rat enzyme-linked immunosorbent assay (ELISA) kit (Monobind, USA).
Determination of MDA and GPx
The amount of MDA and activity of GPx were investigated in the ovarian homogenate using suitable kits obtained by Navand Health Company (Iran) following the manufacturer's instructions.
Determination of hormones
Progesterone, 17β-estradiol, and testosterone levels in the serum of all groups were assayed
by ELISA kits purchased from the Monobind Company (USA), following the manufacturer's recommendations.
Gene expression
RNA isolation was performed in the ovarian homogenate for gene expression analyses using the commercially available RNA isolation kit (Yekta Tajhiz, Iran). The quantity and integrity of total RNA were assessed using the nanodrop spectrophotometer (Thermo Scientific NanoDrop™ 1000) and 1.5% agarose gel electrophoresis. Trace to moderate amounts of genomic DNA contamination during the total RNA isolation is a frequent cause of false-positive signals in RT-qPCR-based analysis. Therefore, we performed an additional genomic DNA removal by DNase during total RNA extraction. Subsequently, about one µg of total RNA was subjected to cDNA synthesis using the reverse transcriptase kit (Yekta Tajhiz, Iran). Quantitative real-time PCR was done on a quantitative PCR system (ABI7500, USA) using SYBR® Green Master Mix (Yekta Tajhiz, Iran). The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was applied as a reference gene. Relative gene expression was analyzed using the 2-∆∆CT formula. The sequences of desired primers are provided in Table 1.
Statistical analysis
GraphPad Prism 8 software (San Diego, California, USA) analyzed data and drew graphs. All data are displayed as mean ± standard deviation (SD), and the difference among experimental groups was analyzed using one-way ANOVA and Tukey's posthoc. p < 0.05 were set as significant levels.
Results
The ginger extract improves glycemic indices
As depicted in Figure 1A, STZ induction in rats greatly increased fasting blood glucose concentration compared to the healthy group (p<0.001). However, the administration of diabetic rats with ginger extract (200 and 400 mg/kg) remarkably attenuated fasting blood glucose levels (p<0.01). As expected, the serum level of insulin was markedly reduced in the diabetic animals compared to the control animals (p<0.001). However, diabetic rats administrated with 400 mg/kg ginger extract exhibited an obvious improvement in the insulin level (Fig. 1B; p < 0.05).
Table 1. The primer sequences
| Genes | Primer sequences (5'- 3') | Product length | Tm | Ref. |
| 3βHSD | Forward: CCCTGCTCTACTGGCTTGC Reverse: TCTGCTTGGCTTCCTCCC |
189 | 60.45 58.92 |
[23] |
| StAR | Forward: CCCAAATGTCAAGGAAATCA Reverse: AGGCATCTCCCCAAAGTG |
187 | 53.73 56.49 | [24] |
| GAPDH | Forward: TGCCAAGTATGATGACATCAAGAAG Reverse: AGCCCAGGATGCCCTTTAGT |
71 | 59.41 60.92 |
[25] |


Fig. 1. Effect of ginger extract on (A) glucose level and (B) insulin level in the serum of studied groups
All data are expressed as mean ± standard deviation. Twenty-four animals in each group were employed for this experiment. *p˂0.05, **p ˂ 0.01, and ***p ˂ 0.001. DM= Diabetes mellitus; STZ= Streptozotocin.
All data are expressed as mean ± standard deviation. Twenty-four animals in each group were employed for this experiment. *p˂0.05, **p ˂ 0.01, and ***p ˂ 0.001. DM= Diabetes mellitus; STZ= Streptozotocin.
Ginger extract ameliorates oxidative stress
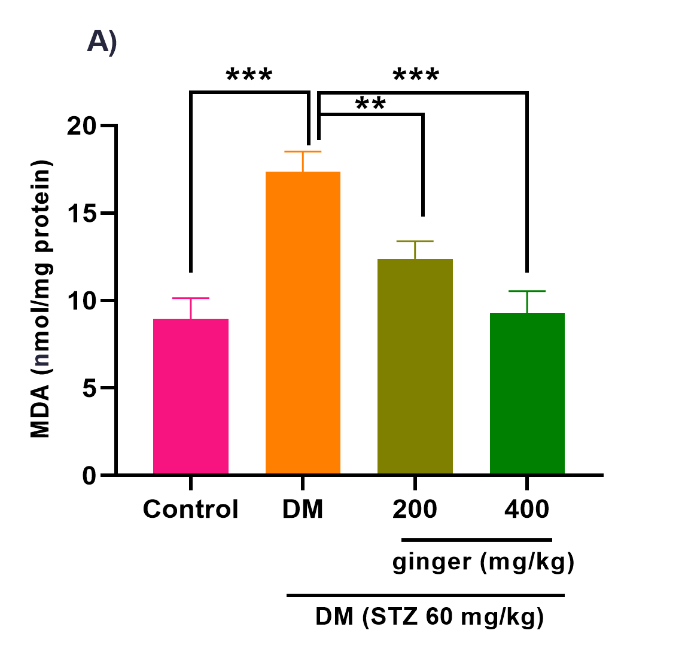
The diabetic animals indicated a considerable increase in ovarian MDA compared to the healthy animals (p < 0.001). However, MDA levels in the ovary of diabetic rats were markedly mitigated after the treatment of diabetic rats with ginger extract (p < 0.01; p < 0.001; Fig. 2A).
As depicted in Figure 2B, the activity of GPx was diminished in the ovarian homogenate of the diabetic group compared to the healthy group (p < 0.01). Diabetic group recipients of 200 mg/kg ginger exhibited a remarkable improvement in ovarian GPx activity (Fig. 2B; p<0.05). However, the higher ginger concentration greatly enhanced GPx activity (p < 0.01) in the ovarian homogenate of diabetic animals.
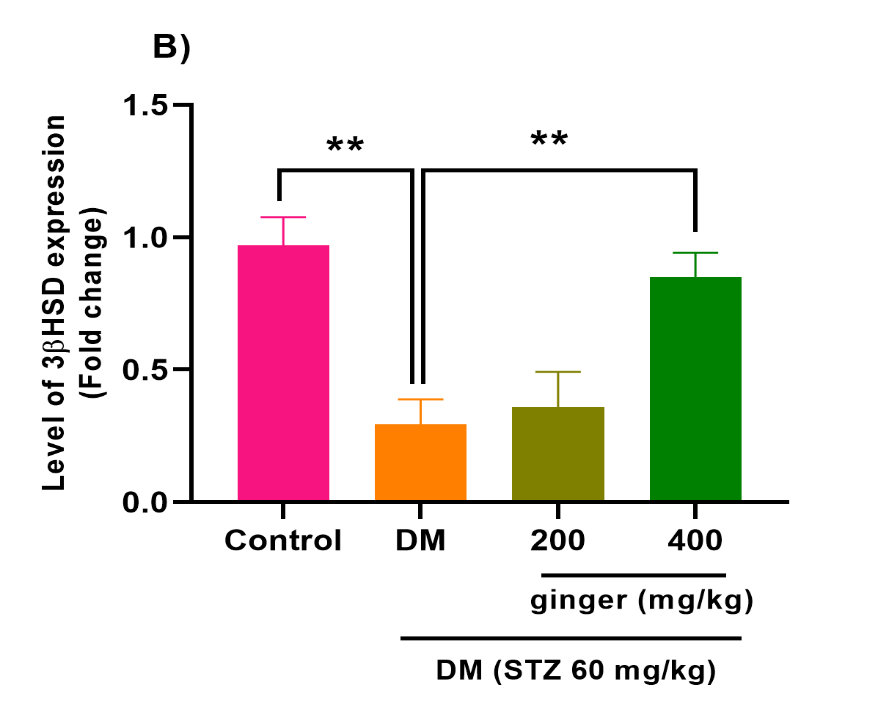
Ginger extract ameliorates 3βHSD and StAR mRNA expression
The q-RT-PCR analysis indicated that the StAR mRNA expression was markedly attenuated in the ovary of the diabetic group relative to the control group (p < 0.01). At the same time, a remarkable up-regulation was seen in the StAR mRNA expression in diabetic animals recipients of ginger extract compared to the untreated diabetic animals (p < 0.05; p < 0.01; Fig. 3A). Similarly, a significant down-regulation was seen in the 3βHSD mRNA levels in the diabetic group relative to the control group (p<0.01). Diabetic rats' recipients of 400 mg/kg (p < 0.01) of ginger extract for eight weeks exhibited a significant increment in the 3βHSD mRNA levels. However, no statistical change in the 3βHSD mRNA levels was observed among the 200 mg/kg ginger-treated diabetic group and the untreated diabetic group (Fig. 3B).
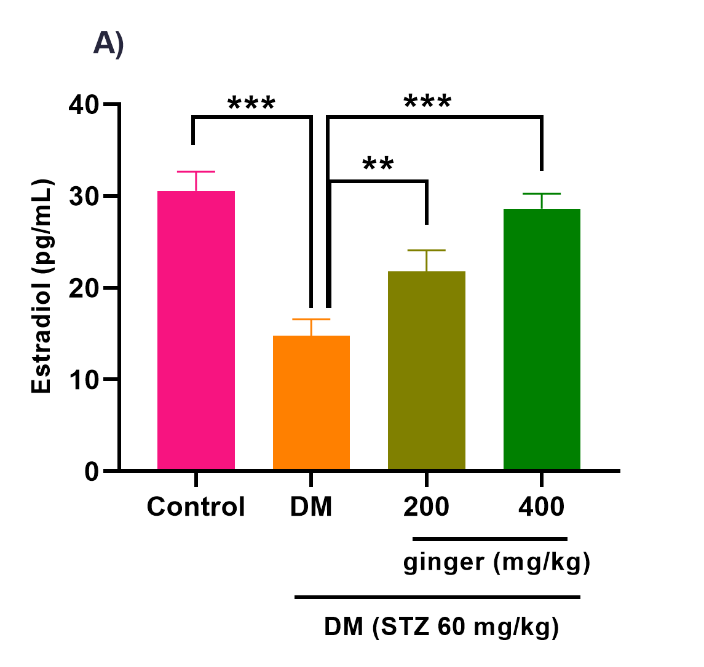
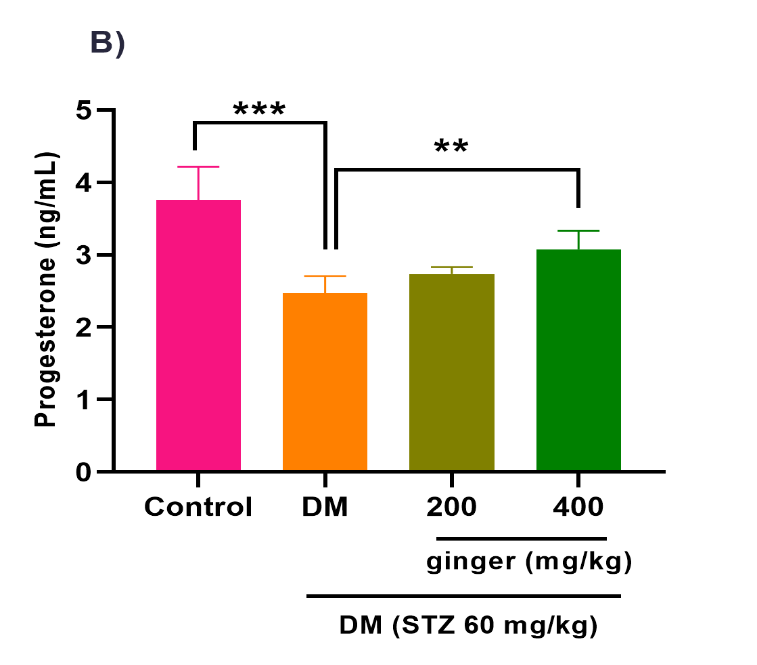
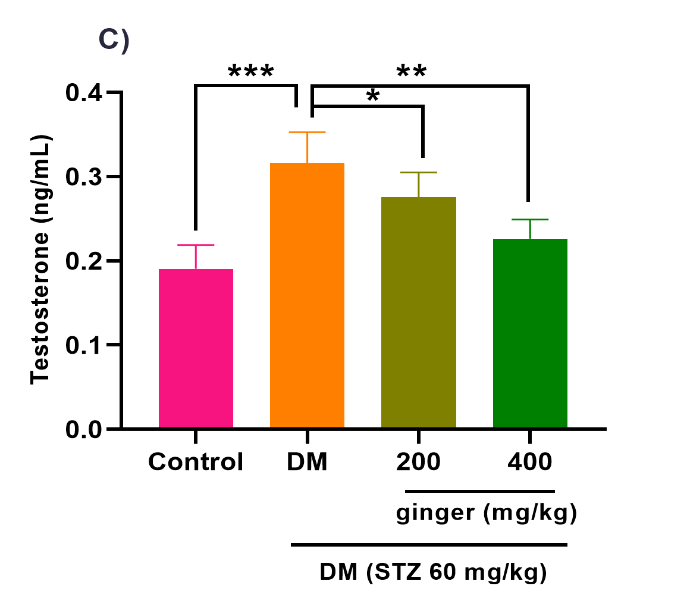
Ginger extract ameliorates steroid hormones production
As illustrated in Figures 4A-C, the level of testosterone hormone (p<0.001) was increased, whereas the levels of E2 (p<0.001) and progesterone (p<0.001) hormones were diminished in the serum of the diabetic group compared to the healthy group. After treatment of diabetic rats with 200 mg/kg of ginger extract for eight weeks, the serum testosterone levels (p < 0.05) were significantly attenuated, whereas the serum E2 greatly increased (p<0.01). No statistical difference was detected in the progesterone level between the 200 mg/kg ginger-treated diabetic group and the untreated diabetic group (Fig. 4B). However, the administration of diabetic rats with 400 mg/kg of ginger extract exhibited a substantial decrement in testosterone (p< 0.01) and a remarkable increment in the E2 (p< 0.001) and progesterone (p<0.01) levels relative to the untreated diabetic rats.
The diabetic animals indicated a considerable increase in ovarian MDA compared to the healthy animals (p < 0.001). However, MDA levels in the ovary of diabetic rats were markedly mitigated after the treatment of diabetic rats with ginger extract (p < 0.01; p < 0.001; Fig. 2A).
As depicted in Figure 2B, the activity of GPx was diminished in the ovarian homogenate of the diabetic group compared to the healthy group (p < 0.01). Diabetic group recipients of 200 mg/kg ginger exhibited a remarkable improvement in ovarian GPx activity (Fig. 2B; p<0.05). However, the higher ginger concentration greatly enhanced GPx activity (p < 0.01) in the ovarian homogenate of diabetic animals.
Ginger extract ameliorates 3βHSD and StAR mRNA expression
The q-RT-PCR analysis indicated that the StAR mRNA expression was markedly attenuated in the ovary of the diabetic group relative to the control group (p < 0.01). At the same time, a remarkable up-regulation was seen in the StAR mRNA expression in diabetic animals recipients of ginger extract compared to the untreated diabetic animals (p < 0.05; p < 0.01; Fig. 3A). Similarly, a significant down-regulation was seen in the 3βHSD mRNA levels in the diabetic group relative to the control group (p<0.01). Diabetic rats' recipients of 400 mg/kg (p < 0.01) of ginger extract for eight weeks exhibited a significant increment in the 3βHSD mRNA levels. However, no statistical change in the 3βHSD mRNA levels was observed among the 200 mg/kg ginger-treated diabetic group and the untreated diabetic group (Fig. 3B).
Ginger extract ameliorates steroid hormones production
As illustrated in Figures 4A-C, the level of testosterone hormone (p<0.001) was increased, whereas the levels of E2 (p<0.001) and progesterone (p<0.001) hormones were diminished in the serum of the diabetic group compared to the healthy group. After treatment of diabetic rats with 200 mg/kg of ginger extract for eight weeks, the serum testosterone levels (p < 0.05) were significantly attenuated, whereas the serum E2 greatly increased (p<0.01). No statistical difference was detected in the progesterone level between the 200 mg/kg ginger-treated diabetic group and the untreated diabetic group (Fig. 4B). However, the administration of diabetic rats with 400 mg/kg of ginger extract exhibited a substantial decrement in testosterone (p< 0.01) and a remarkable increment in the E2 (p< 0.001) and progesterone (p<0.01) levels relative to the untreated diabetic rats.


Fig. 2. Effect of ginger extract on (A) malondialdehyde (MDA) level and (B) glutathione peroxidase (GPX) level in the ovarian of studied groups
All results are demonstrated as mean ± standard deviation. Twenty-four animals in each group were employed for this experiment. *p ˂ 0.05, **p ˂ 0.01, and ***p ˂ 0.001. DM= Diabetes mellitus; STZ= Streptozotocin.
All results are demonstrated as mean ± standard deviation. Twenty-four animals in each group were employed for this experiment. *p ˂ 0.05, **p ˂ 0.01, and ***p ˂ 0.001. DM= Diabetes mellitus; STZ= Streptozotocin.


Fig. 3. Effect of ginger extract on (A) steroidogenic acute regulatory (StAR) transcript level and (B) 3β-hydroxysteroid dehydrogenase (3βHSD), transcript level in the ovarian of studied groups. All results are indicated as mean ± standard deviation. Twenty-four animals in each group were employed for this experiment. *P˂0.05 and **P˂0.01. DM= Diabetes mellitus; STZ= Streptozotocin.



Fig. 4. Effect of ginger extract on (A) estradiol, (B) progesterone, and (C) testosterone level in the serum of studied groups
All results are indicated as mean ± standard deviation. Twenty-four animals in each group were employed for this experiment. *p ˂ 0.05, **p ˂ 0.01, and ***p ˂ 0.001. DM= Diabetes mellitus; STZ= Streptozotocin.
All results are indicated as mean ± standard deviation. Twenty-four animals in each group were employed for this experiment. *p ˂ 0.05, **p ˂ 0.01, and ***p ˂ 0.001. DM= Diabetes mellitus; STZ= Streptozotocin.
Discussion
Previous evidence has elucidated that hyperglycemia-provoked oxidative stress can promote the downstream events implicated in ovarian disorders [11, 12, 26]. There is increasing evidence of ginger extract's favorable effect in managing hyperglycemia [27]. Herein, we studied the beneficial impacts of ginger extract against oxidative stress and its related complications in diabetic rats.
STZ is an alkylating agent involved in DNA alkylation and the β cell death, leading to hyperglycemia [28]. This work observed a significant increment in the glucose levels of STZ-treated rats following prior reports [29, 30]. Hyperglycemia is associated with chronic injury to various organs, including ovaries [31, 32]. Therefore, achieving an optimal glycemic state is necessary to control or delay hyperglycemia complications. Previously published articles described the favorable impacts of medicinal plants on hyperglycemia in STZ-induced diabetic rats. Karimi et al. demonstrated that silymarin successfully alleviated retinal microvascular damage in STZ-provoked diabetic rats [14]. Al Hroob et al. reported that ginger protects rats against diabetic nephropathy by alleviating hyperglycemia [27]. Yi et al. evaluated the effects of 6-shogaol, a polyphenolic compound of ginger, on glucose and insulin levels in the serum of STZ-treated mice. The mice treated with 6-Shogaol exhibited a considerable improvement in glucose and insulin [33]. In the present work, diabetic rats treated with ginger extract exhibited a remarkable amelioration in serum glucose and a great elevation in insulin levels. Ginger was found to provoke liver glycolytic enzymes' cellular activity, which contained pyruvate kinase, phosphofructokinase, and glucokinase [27]. Growing evidence elucidated that hyperglycemia-induced oxidative stress primarily contributes to diabetes complications [8]. For instance, Almatroodi et al. reported that STZ-induced diabetes caused the generation of oxidative stress and renal damage. Their results exhibited that 6-gingerol decreased oxidative stress in diabetic rats, thereby reducing renal injury [34]. Oxidative stress in different organs arose due to excessive cellular ROS production and reduced anti-oxidant agents' activity [35]. Excessive ROS levels can cause extensive damage to cellular biomolecules, including lipids, proteins, and nucleic acids [36]. Lipid peroxidation is the most common indicator of cell membrane destruction. GPx is one of the major members of the anti-oxidant defense system, and the increased activity of this enzyme is an important therapeutic strategy in oxidative stress conditions [37]. Herein, diabetic induction caused a substantial enhancement in the MDA amount and declined GPx activity in the ovary of rats. The oral administration of ginger extract ameliorated MDA levels and boosted GPx activity in the ovary of diabetic rats. Diabetes in women is related to amenorrhea and disturbance in ovarian hormone secretion [38]. Previous studies have revealed that hyperglycemia-provoked oxidative stress was associated with altered folliculogenesis and steroidogenesis in diabetic female rats [12, 39]. Chabrolle et al. highlighted that the serum concentrations of progesterone and E2 hormones were lowered in STZ-treated rats than in controls, indicating that ovarian function was changed. They also assayed the impact of hyperglycemia on the levels of steroid hormones and the enzymes involved in the steroidogenesis pathway in rat granulosa cells. Their findings indicated that in the hyperglycemic conditions, the transcript levels of StAR, p450scc, p450 aromatase, 3βHSD, and the production of E2 and progesterone were reduced [40]. Therefore, an imbalance in the oxidant and anti-oxidant activities due to diabetes negatively affects steroidogenic function and ovarian hormone levels. Diabetes-related ovarian hormonal disturbance can lead to various health consequences and reduce folliculogenesis [38, 41, 42]. In 2017, Atashpour et al. evaluated the impacts of ginger extract on the concentrations of sex hormones in the ovaries of rats with polycystic ovary syndrome. Their data demonstrated that ginger extract (350 mg/ kg) has beneficial impacts on improving polycystic ovary syndrome [43]. In the current project, our findings revealed a decrease in the mRNA expression of 3βHSD and StAR steroidogenic genes and the levels of E2 and progesterone and an increase in the testosterone level in diabetic rats. However, our results indicated that the mRNA levels of 3βHSD and StAR steroidogenic genes increased in the ovary of the diabetic rats compared to the healthy rats. The ginger extract also improved E2 and progesterone levels and caused a significant reduction in testosterone levels. One of the limitations of this study is the lack of assessment of StAR and 3βHSD markers at the protein levels. Also, other steroidogenic genes were not investigated in ovarian tissue samples of diabetic rats.
Conclusion
Our findings indicated that ginger extract exerts a protective impact in STZ-treated rats. Ginger attenuated diabetes-provoked oxidative stress and improved the steroidogenic function in the ovary of diabetic rats. Further research is recommended to elucidate the underlying molecular mechanisms associated with improving ovarian dysfunctions by ginger under diabetic conditions.
Conflict of Interest
The authors declare that there is no conflict of interest associated with this work.
Acknowledgments
We would like to express our gratitude to Dr. Mojtaba Abbasi, Dr. Ruzbeh Berijani, and Dr. Oveys Pourmahdi for their constructive comments and help with preparing the manuscript and formatting the whole article.
STZ is an alkylating agent involved in DNA alkylation and the β cell death, leading to hyperglycemia [28]. This work observed a significant increment in the glucose levels of STZ-treated rats following prior reports [29, 30]. Hyperglycemia is associated with chronic injury to various organs, including ovaries [31, 32]. Therefore, achieving an optimal glycemic state is necessary to control or delay hyperglycemia complications. Previously published articles described the favorable impacts of medicinal plants on hyperglycemia in STZ-induced diabetic rats. Karimi et al. demonstrated that silymarin successfully alleviated retinal microvascular damage in STZ-provoked diabetic rats [14]. Al Hroob et al. reported that ginger protects rats against diabetic nephropathy by alleviating hyperglycemia [27]. Yi et al. evaluated the effects of 6-shogaol, a polyphenolic compound of ginger, on glucose and insulin levels in the serum of STZ-treated mice. The mice treated with 6-Shogaol exhibited a considerable improvement in glucose and insulin [33]. In the present work, diabetic rats treated with ginger extract exhibited a remarkable amelioration in serum glucose and a great elevation in insulin levels. Ginger was found to provoke liver glycolytic enzymes' cellular activity, which contained pyruvate kinase, phosphofructokinase, and glucokinase [27]. Growing evidence elucidated that hyperglycemia-induced oxidative stress primarily contributes to diabetes complications [8]. For instance, Almatroodi et al. reported that STZ-induced diabetes caused the generation of oxidative stress and renal damage. Their results exhibited that 6-gingerol decreased oxidative stress in diabetic rats, thereby reducing renal injury [34]. Oxidative stress in different organs arose due to excessive cellular ROS production and reduced anti-oxidant agents' activity [35]. Excessive ROS levels can cause extensive damage to cellular biomolecules, including lipids, proteins, and nucleic acids [36]. Lipid peroxidation is the most common indicator of cell membrane destruction. GPx is one of the major members of the anti-oxidant defense system, and the increased activity of this enzyme is an important therapeutic strategy in oxidative stress conditions [37]. Herein, diabetic induction caused a substantial enhancement in the MDA amount and declined GPx activity in the ovary of rats. The oral administration of ginger extract ameliorated MDA levels and boosted GPx activity in the ovary of diabetic rats. Diabetes in women is related to amenorrhea and disturbance in ovarian hormone secretion [38]. Previous studies have revealed that hyperglycemia-provoked oxidative stress was associated with altered folliculogenesis and steroidogenesis in diabetic female rats [12, 39]. Chabrolle et al. highlighted that the serum concentrations of progesterone and E2 hormones were lowered in STZ-treated rats than in controls, indicating that ovarian function was changed. They also assayed the impact of hyperglycemia on the levels of steroid hormones and the enzymes involved in the steroidogenesis pathway in rat granulosa cells. Their findings indicated that in the hyperglycemic conditions, the transcript levels of StAR, p450scc, p450 aromatase, 3βHSD, and the production of E2 and progesterone were reduced [40]. Therefore, an imbalance in the oxidant and anti-oxidant activities due to diabetes negatively affects steroidogenic function and ovarian hormone levels. Diabetes-related ovarian hormonal disturbance can lead to various health consequences and reduce folliculogenesis [38, 41, 42]. In 2017, Atashpour et al. evaluated the impacts of ginger extract on the concentrations of sex hormones in the ovaries of rats with polycystic ovary syndrome. Their data demonstrated that ginger extract (350 mg/ kg) has beneficial impacts on improving polycystic ovary syndrome [43]. In the current project, our findings revealed a decrease in the mRNA expression of 3βHSD and StAR steroidogenic genes and the levels of E2 and progesterone and an increase in the testosterone level in diabetic rats. However, our results indicated that the mRNA levels of 3βHSD and StAR steroidogenic genes increased in the ovary of the diabetic rats compared to the healthy rats. The ginger extract also improved E2 and progesterone levels and caused a significant reduction in testosterone levels. One of the limitations of this study is the lack of assessment of StAR and 3βHSD markers at the protein levels. Also, other steroidogenic genes were not investigated in ovarian tissue samples of diabetic rats.
Conclusion
Our findings indicated that ginger extract exerts a protective impact in STZ-treated rats. Ginger attenuated diabetes-provoked oxidative stress and improved the steroidogenic function in the ovary of diabetic rats. Further research is recommended to elucidate the underlying molecular mechanisms associated with improving ovarian dysfunctions by ginger under diabetic conditions.
Conflict of Interest
The authors declare that there is no conflict of interest associated with this work.
Acknowledgments
We would like to express our gratitude to Dr. Mojtaba Abbasi, Dr. Ruzbeh Berijani, and Dr. Oveys Pourmahdi for their constructive comments and help with preparing the manuscript and formatting the whole article.
References
- Association AD. Introduction: Standards of Medical Care in Diabetes_2022. Am Diabetes Assoc. 2021; p. S1-S2.
- Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Research and Clinical Practice 2019; 157: 107843.
- Yazawa T, Imamichi Y, Sekiguchi T, Miyamoto K, Uwada J, Khan M, et al. Transcriptional regulation of ovarian steroidogenic genes: recent findings obtained from stem cell-derived steroidogenic cells. BioMed Research International 2019: 8973076.
- Samie KA, Tabandeh MR, Afrough M. Betaine ameliorates impaired steroidogenesis and apoptosis in mice granulosa cells induced by high glucose concentration. Systems Biology in Reproductive Medicine 2020; 66(6): 400-409.
- Zare Z, Dizaj TN, Lohrasbi A, Sheikhalishahi ZS, Asadi A, Zakeri M, et al. Silibinin inhibits TGF-β-induced MMP-2 and MMP-9 through Smad Signaling pathway in colorectal cancer HT-29 cells. Basic and Clinical Cancer Research 2020; 12(2): 81-90.
- Nowotny K, Jung T, Höhn A, Weber D, Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015; 5(1): 194-222.
- Papalou O, Victor VM, Diamanti-Kandarakis E. Oxidative stress in polycystic ovary syndrome. Current Pharmaceutical Design. 2016; 22(18): 2709-722.
- Yaribeygi H, Sathyapalan T, Atkin SL, Sahebkar A. Molecular mechanisms linking oxidative stress and diabetes mellitus. Oxidative Medicine and Cellular Longevity 2020: 8609213.
- Zhang P, Li T, Wu X, Nice EC, Huang C, Zhang Y. Oxidative stress and diabetes: antioxidative strategies. Frontiers of Medicine 2020: 1-18.
- Panji M, Behmard V, Zare Z, Malekpour M, Nejadbiglari H, Yavari S, et al. Suppressing effects of Green tea extract and Epigallocatechin-3-gallate (EGCG) on TGF-β-induced Epithelial-to-mesenchymal transition via ROS/Smad signaling in human cervical cancer cells. Gene 2021: 145774.
- Masjedi F, Keshtgar S, Zal F, Talaei-Khozani T, Sameti S, Fallahi S, et al. Effects of vitamin D on steroidogenesis, reactive oxygen species production, and enzymatic antioxidant defense in human granulosa cells of normal and polycystic ovaries. The Journal of Steroid Biochemistry and Molecular Biology 2020; 197: 105521.
- Yu H, Kuang M, Wang Y, Rodeni S, Wei Q, Wang W, et al. Sodium arsenite injection induces ovarian oxidative stress and affects steroidogenesis in rats. Biological Trace Element Research 2019; 189(1): 186-93.
- Pang GM, Li FX, Yan Y, Zhang Y, Kong LL, Zhu P, et al. Herbal medicine in the treatment of patients with type 2 diabetes mellitus. Chinese Medical Journal 2019; 132(1): 78-88.
- Karimi R, Bakhshi A, Dayati P, Abazari O, Shahidi M, Savaee M, et al. Silymarin reduces retinal microvascular damage in streptozotocin-induced diabetic rats. Scientific Reports 2022; 12(1): 15872.
- Mao QQ, Xu XY, Cao SY, Gan RY, Corke H, Beta T, et al. Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods 2019; 8(6): 185.
- Kiyama R. Nutritional implications of ginger: Chemistry, biological activities and signaling pathways. The Journal of Nutritional Biochemistry 2020; 86: 108486.
- Shahrajabian MH, Sun W, Cheng Q. Clinical aspects and health benefits of ginger (Zingiber officinale) in both traditional Chinese medicine and modern industry. Acta Agriculturae Scandinavica, Soil & Plant Science 2019; 69(6): 546-56.
- Shahidi M, Moradi A, Dayati P. Zingerone attenuates zearalenone-induced steroidogenesis impairment and apoptosis in TM3 Leydig cell line. Toxicon 2022; 211(5): 50-60.
- Bordbar H, Esmaeilpour T, Dehghani F, Panjehshahin MR. Stereological study of the effect of ginger's alcoholic extract on the testis in busulfan-induced infertility in rats. Iranian Journal of Reproductive Medicine. 2013; 11(6): 467.
- Satheesh MA, Pari L. Effect of pterostilbene on lipids and lipid profiles in streptozotocin-nicotinamide induced type 2 diabetes mellitus. Journal of applied biomedicine. 2008; 6(1): 31-37.
- Lamuchi-Deli N, Aberomand M, Babaahmadi-Rezaei H, Mohammadzadeh G. Effects of the hydroalcoholic extract of Zingiber officinale on arginase i activity and expression in the retina of streptozotocin-induced diabetic rats. International Journal of Endocrinology and Metabolism 2017; 15(2): 42161.
- Abdi T, Mahmoudabady M, Marzouni HZ, Niazmand S, Khazaei M. Ginger (Zingiber Officinale Roscoe) extract protects the heart against inflammation and fibrosis in diabetic rats. Canadian Journal of Diabetes 2021; 45(3): 220-27.
- Guo JJ, Ma X, Wang CQ, Ge YF, Lian QQ, Hardy DO, et al. Effects of luteinizing hormone and androgen on the development of rat progenitor Leydig cells in vitro and in vivo. Asian journal of andrology 2013;15(5): 685-91.
- Wu K, Li Y, Pan P, Li Z, Yu Y, Ma F, et al. Gestational vinclozolin exposure suppresses fetal Leydig cell development in rats. Ecotoxicology and Environmental Safety 2020; 203(10): 111053.
- Li G, Liu X, Du J, Chen J, She F, Wu C, et al. Positive shift of Nav1. 8 current inactivation curve in injured neurons causes neuropathic pain following chronic constriction injury. Molecular Medicine Reports 2015; 12(3): 3583-590.
- Panji M, Behmard V, Zare Z, Malekpour M, Nejadbiglari H, Yavari S, et al. Synergistic effects of green tea extract and paclitaxel in the induction of mitochondrial apoptosis in ovarian cancer cell lines. Gene 2021; 787: 145638.
- Al Hroob AM, Abukhalil MH, Alghonmeen RD, Mahmoud AM. Ginger alleviates hyperglycemia-induced oxidative stress, inflammation and apoptosis and protects rats against diabetic nephropathy. Biomedicine & Pharmacotherapy 2018;106: 381-89.
- Wang-Fischer Y, Garyantes T. Improving the reliability and utility of streptozotocin-induced rat diabetic model. Journal of Diabetes Research 2018; 2018: 8054073.
- Molehin OR, Oloyede OI, Adefegha SA. Streptozotocin-induced diabetes in rats: effects of white butterfly (Clerodendrum volubile) leaves on blood glucose levels, lipid profile and antioxidant status. Toxicology Mechanisms and Methods 2018; 28(8): 573-86.
- Eser N, Buyuknacar HS, Cimentepe OO, Gocmen C, Ucar Y, Erdogan S, et al. The effect of Ferula elaeochytris root extract on erectile dysfunction in streptozotocin-induced diabetic rat. International Journal of Impotence Research 2020; 32(2): 186-94.
- Olawale F, Aninye I, Ajaja U, Nwozo SO. Long-term hyperglycemia impairs hormonal balance and induces oxidative damage in ovaries of streptozotocin-induced diabetic wistar rat. Nigerian Journal of Physiological Sciences 2020; 35(1): 46-51.
- Zare Z, Dizaj TN, Lohrasbi A, Sheikhalishahi ZS, Panji M, Hosseinabadi F, et al. The effect of piperine on MMP-9, VEGF, and e-cadherin expression in breast cancer MCF-7 cell line. Basic & Clinical Cancer Research 2020; 12(3): 112-19.
- Yi JK, Ryoo ZY, Ha JJ, Oh DY, Kim MO, Kim SH. Beneficial effects of 6-shogaol on hyperglycemia, islet morphology and apoptosis in some tissues of streptozotocin-induced diabetic mice. Diabetology & Metabolic Syndrome 2019; 11(1): 1-13.
- Almatroodi SA, Alnuqaydan AM, Babiker AY, Almogbel MA, Khan AA, Husain Rahmani A. 6-Gingerol, a bioactive compound of ginger attenuates renal damage in streptozotocin-induced diabetic rats by regulating the oxidative stress and inflammation. Pharmaceutics 2021; 13(3): 317.
- Sies H. Oxidative stress: Concept and some practical aspects. Antioxidants 2020; 9(9): 852.
- Costantini D. Understanding diversity in oxidative status and oxidative stress: the opportunities and challenges ahead. Journal of Experimental Biology 2019; 222(13): 194688.
- Brigelius-Flohé R, Flohé L. Regulatory phenomena in the glutathione peroxidase superfamily. Antioxidants & Redox Signaling. 2020; 33(7): 498-516.
- Thong EP, Codner E, Laven JS, Teede H. Diabetes: a metabolic and reproductive disorder in women. The Lancet Diabetes & Endocrinology 2020; 8(2): 134-49.
- Hong Y, Yin Y, Tan Y, Hong K, Zhou H. The flavanone, naringenin, modifies antioxidant and steroidogenic enzyme activity in a rat model of letrozole-induced polycystic ovary syndrome. Medical science monitor: International Medical Journal of Experimental and Clinical Research 2019; 25: 395.
- Chabrolle C, JeanPierre E, Tosca L, Ramé C, Dupont J. Effects of high levels of glucose on the steroidogenesis and the expression of adiponectin receptors in rat ovarian cells. Reproductive Biology and Endocrinology 2008; 6(1): 1-14.
- Wellons MF, Matthews JJ, Kim C. Ovarian aging in women with diabetes: An overview. Maturitas 2017; 2(96): 109-13.
- Fattah A, Morovati A, Niknam Z, Mashouri L, Asadi A, Rizi ST, et al. The synergistic combination of cisplatin and piperine induces apoptosis in MCF-7 cell line. Iranian Journal of Public Health 2021; 50(5): 1037-1047.
- Atashpour S, Jahromi HK, Jahromi ZK, Maleknasab M. Comparison of the effects of Ginger extract with clomiphene citrate on sex hormones in rats with polycystic ovarian syndrome. International Journal of Reproductive BioMedicine 2017; 15(9): 561-68.
Type of Study: Research |
Subject:
Biochemistry
Received: 2022/06/29 | Accepted: 2022/08/14 | Published: 2023/10/2
Received: 2022/06/29 | Accepted: 2022/08/14 | Published: 2023/10/2
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |