Toxocariasis is a zoonotic parasitic disease with worldwide distribution caused by the larval stage of ascaridoid nematodes containing human and animal species, such as Toxocara canis (T. canis), T. cati, T. vitulorum and, T. leonina [1, 2]. T. canis is one of the most widespread public health and economically zoonotic parasitic infections humans share with dogs, cats, and wild canids [3, 4]. Epidemiological surveys have reported that the prevalence of T. canis in dogs ranged from 6.3-29% in different parts of Iran [5-7]. The prevalence of human toxocariasis in various parts of Iran ranges from 1.39 to 34.48% [8-13]. Significantly, humans become infected by accidentally ingesting viable eggs from contaminated soil and food. After hatching, emerging larvae are incapable of completing their life cycle and develop into mature adult worms in the human host and cause a range of clinical syndromes such as ocular larva migrans, visceral larva migrans, eosinophilic meningoencephalitis, neurotoxocariasis and covert toxocariasis [1, 2, 14, 15]. Traditionally, Toxocara species are known to be consistent with their morphological characteristics and predilection in a specific host species [16, 17]. Since the morphological identification of some ascaridoid nematodes especially at the larval and egg stages, is challenging [18], thus the analysis of genetic variation is necessary for studying genetic structures and population biology of parasites [19]. Currently, molecular methods such as polymerase chain reaction (PCR) of a selected target restriction fragment length polymorphism (RFLP) using ribosomal and mitochondrial markers have been developed and used widely for the diagnosis and genetic differentiation of Toxocara species [20-22]. Genetic variation plays a significant role in the adaptability and survival of a parasite once its environment changes. Accurate analysis of this variation is appropriate for studies on pathogenesis, taxonomy, population biology, and epidemiology of parasites. Various studies have been reported that certain genetic regions, such as the nuclear ribosomal DNA (rDNA) and mitochondrial could certify reliable markers for determining the phylogenetic relationships and genetic variation within and among the Toxocara species [21, 23-25]. The mitochondrial and ribosomal gene sequences have been used to analyze genetic variations of Toxocara in various parts of the world [26-28]. Previous studies
are done on the molecular characterization Toxascara species. For the first time, Jacobs
et al. (1997) reported the differentiation of nematodes of T. canis, T. cati and T. leonina based on second internal transcribed spacer (ITS2) sequences [20]. In respect of the high prevalence of T. canis in dogs in the world and shedding Toxocara eggs in the environment, which are transferable to humans, the identification of T. canis is substantial for the planning of prevention and control programs in human and animal communities [29]. Despite the high prevalence of T. canis in Zabol dogs (27.5%) [13] and their medical importance, there is no information from genetic analysis of those parasites from Zabol city. Therefore, this study was designed for the characterization and analysis of genetic variation within isolates of T. canis in stray dogs in Zabol by sequencing mitochondrial cytochrome c oxidase subunit 1 COX1 and second internal transcribed spacer ITS-2 of nuclear rDNA.
Materials and Methods
Collection of T. canis eggs
This cross-sectional study was meted out from June to December 2018 in Zabol, located in Southeast Iran, with 394,029 populations having a hot and dry climate. The average annual precipitation and temperature are 60 mm and 22°C, respectively. The sample volume needed to determine the contamination with T. canis was estimated according to the study of Emampour et al. in 2013 [5]. A complete of 200 stool samples were collected randomly from dogs in public parks and streets from different regions (urban and rural) of Zabol; samples were placed in labeled containers and transferred to the parasitology laboratory of Zabol University of Medical Sciences. This research project was reviewed and approved by the Ethics Committee of Zabol University of Medical Sciences, Iran. Project No. IR.ZBMU.REC.1397.056.
Microscopy method
30 samples containing Eggs were isolated from the feces using formalin ether 10% and centrifugal flotation [30], and eggs were preserved in 70% (v/v) ethanol at -20 °C till extraction of genomic DNA.
DNA extraction
Samples were entirely washed in distilled water to make ethanol. Total genomic DNA was extracted using QIAamp DNA Mini Kit (Qiagen, Hilden, Germany), with the subsequent modifications: Once the addition of 50 μL lysis buffer, the samples were subjected to three manual sonication cycles (1/5 min each) followed by three freeze-thaw cycles (liquid nitrogen/water bath at 56 °C) and proteinase K digestion was performed overnight. The DNAs were kept at -20 °C till PCR amplification.
PCR amplification
The complete nuclear ITS2 sequence and sequences of the mitochondrial COX1 were amplified by PCR using the following primers: ITS2 (forward: 5′-AGTATGATGGGCGCGCCAAT-3′, reverse: 5′-TAGTTTCTTTTCCTCCGCT-3′); COX1 (forward: 5′-TTTTTTGGGCATCCTG AGGTTTAT-3′,
reverse: 5′-TAAAGAAAGAACATAATGAAAATG-3′) [31, 32]. The PCR amplification was performed in 50 μl reaction volumes containing 25 μl 2× Taq PCR MasterMix (Cinnagen, Iran), 10 μl DNA template, 13 μl deionized distilled water, and 1 μl of each primer (25 pmol/μl; Cinnagen, Iran). The temperature specification was one cycle of 94 °C for 5 min (Initial denaturation), followed by 35 cycles of 94 °C for 30 s for denaturation, 55 °C for 30 s for annealing, and 72 °C for the 30s for extension, and final extension of 72 °C for 5 min. A sample including distilled water instead of template DNA was contained in each run as a negative control. PCR products were separated by electrophoresis on a 1.5% agarose gel in TAE (Tris 0.09 M, Acetic acid 0.09 M, EDTA 0.5 M) at 70V for 1h. Gels were stained with 5.3 μl ethidium bromide 10% (Roche, Germany), and the bands were visualized using a UV transilluminator and digitally photographed. ITS2 and COX1 PCR products (5 μl) were digested with 0.5 μl restriction endonuclease RsaI (New England Biolabs (NEB), R0167S) for 3h at 37 °C. Restriction fragments of amplicons were electrophoresed using a 3 % (w/v) agarose gel at 70 V for 1 hour, visualized on a UV transilluminator, and digitally photographed [33].
Sequencing and phylogenetic analysis
PCR sequencing was performed to stabilize the results of the PCR-RFLP method. The consensus sequences were compared with one another and GenBank reference sequences using the BioEdit software (http://www.mbio. ncsu.edu/bioedit/bioedit.html) and BLAST program (http://www.ncbi.nlm.nih.gov/) and sequence alignments by MEGA 7.0 software.
Results
For all samples, amplicons of about 388 and 422 base pairs (bp) were produced by PCR for ITS2 and COX1, respectively (Figs. 1, 2). After purification of the PCR product, all samples were sequenced by Takapozist company, and also the sequencing sources for ITS2 and COX1 were carefully examined using Chromas software. In this study, results of multiple alignments to compare sequences, identification of variable regions, and the number of transitions and transformations in the studied sequences, multiple alignments were performed exploitation MULTALIN software (http://multalin.toulouse.inra.fr/ multalin/multalin.h) software.
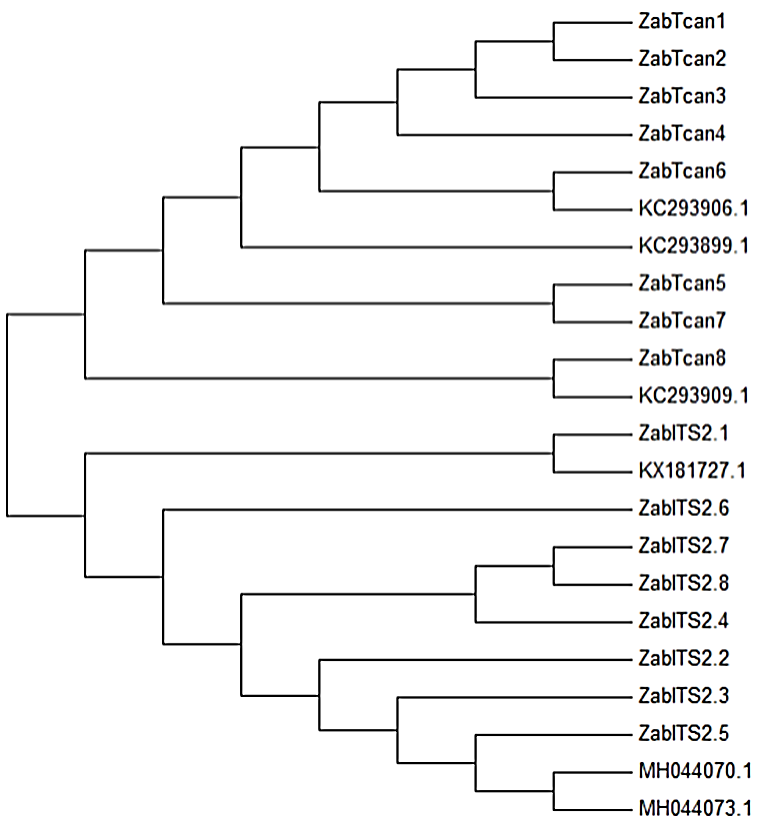
T. canis isolates were sequenced and compared with each other and with other parts of the world and subjected to multiple alignments. The phylogenetic relationship of ITS2 and COX1 sequences of isolates of T. canis from Zabol city using MEGA7.0 software showed that the identified genotypes are the same and there are no different from each other and other parts of the world (Fig. 3). In the present study, exploiting the BLAST program, all 30 isolates from dogs were identified as T. canis.
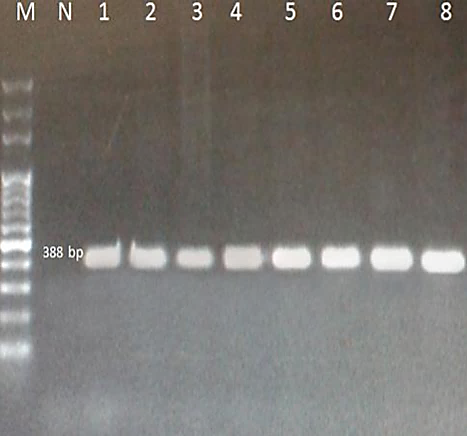
Fig. 1. PCR amplification of ITS-2 (~388 bp) of T. canis from the feces of stray dogs in Zabol on 1.5% agarose gel. M – 100bp DNA ladder, N: negative control
Fig. 2. PCR amplification of COX1 (422bp) of T. canis from the feces of stray dogs in Zabol on 1.5% agarose gel. M – 100bp DNA ladder, N: negative control
Fig. 3. Phylogenetic relationship of COX1 and ITS2 sequences of isolates of T. canis from Zabol using MEGA7.0 software
Discussion
The genetic analysis of parasites is an important factor in determining epidemiology and the management of parasitic diseases in humans and animals [34]. T. canis is a common parasitic nematode of dogs with public health considerations in dogs and humans [35, 36]. The prevalence of T. canis has been reported to be nearly high in Zabol dogs [13]. However, Toxocara's genetic and phylogenetic situation in this region has never been investigated. So as that understand the genetic structure and particular phylogenetic position of T. canis obtained from dogs in Zabol, genomic DNA was extracted, and COX1 and ITS2 genes were amplified. Extending from previous studies, specific PCR techniques are sensitive and specific and provide molecular tools for designation and molecular epidemiological studies of Toxocara infections in humans and animals [20, 37-39]. The PCR-RFLP is a suitable and reliable method for identifying Toxocara species that is based on the digestion of the PCR products by restriction enzymes or endonucleases and an important technique for epidemiological studies, particularly of environmental samples as a result of it permits the differentiation of Toxocara eggs isolated from the soil [20, 33, 40, 41]. Ribosomal genes such as first ITS-1 and the second ITS-2 are almost preserved and are typically used as genetic markers within the specific identification of nematodes. Previous studies confirm that ITS-1 and ITS-2 genes are suggested for the molecular differentiation of Toxocara species and can enable a reliable tool for identification functions, especially at the larval and egg stages [33, 42, 43]. Our results in this study indicated that the sequence of ITS2 and COX1 region of T. canis isolates in Zabol city is not only different from each other but also from other parts of the world and genetic diversity within isolates of T. canis from Zabol is extremely low. Zhu et al. (2001) found that the level of sequence differences among species ranges from 26 to 50%, whereas there is almost no variation (0-0.6%) within the same species [21]. Also, a comparison of ITS sequences of T. canis from Poland with sequences deposited in GenBank showed that the scope of interspecies variability of the species did not exceed 0.4%, while in T. cati the differences did not exceed 2%. [42]. The study conducted by Borecka (2004) for the difference between Toxocara spp. eggs isolated from soil by molecular methods showed that ITS2 PCR products of T. canis and T. cati were similar in size. Therefore, this PCR approach alone could not differentiate the eggs of Toxocara spp. The authors used the PCR-RFLP technique, and this methodology gave the impression to be helpful for the characterization of the species level for Toxocara spp. [41]. In addition to nuclear ITS-1 and ITS-2 rDNA sequences, recent studies have shown that mitochondrial DNA such as the mitochondrial cytochrome c oxidase subunit (COX1) gene is a helpful genetic marker for the identification of ascaridoid nematodes and also identify parasites such as Ancylostoma ceylanicum, Spirometra spp., Echinococcus granulosus [25, 44-51]. Mitochondrial DNA has several benefits when studying investigating population genetic structures, and phylogeny of parasitic nematodes due to their higher mutation rates than nuclear genes and preserved genome structures. Mitochondrial DNA has necessary implications for various fundamental areas, as well as biochemistry, biology, and physiology [24, 52, 53]. Mikaeli et al. (2015) showed the diversity of COX1 and NAD1 mitochondrial gene sequences in dog and cat nematodes in several regions of Iran. Sequence and phylogenetic analysis of COX1 and NAD1 genes showed significant genetic diversity in and between 9 isolates of T. canis, 32 isolates of T. cati, and 19 isolates of Toxascaris leonina and these genes can be used to study the genetic variation of ascaridoid nematodes [54]. This study is the first phylogenetic analysis of T. canis from dog Zabol city, Southeast Iran.
Conclusion
This study incontestable the existence of genetic diversity in mitochondrial genes and ribosomal genes among isolates of T. Canis in Zabol, southeast Iran. In general, we conclude that the sequence of ITS2 and COX1 region of T. canis isolates in Zabol city differs not only from each other but also from other parts of the world. The ITS2 can be utilized for particular identification of Toxocara nematodes and specific primer design, although the COX1 might provide valuable data for future phylogenetic and diversity genetics studies on the Toxocara nematodes. For a deeper understanding of genetic diversity among populations of Toxocara, it is recommended to analyze more isolates from various geographical areas and variable genetic markers.
Conflict of Interest
The authors declare that there are no competing interests.
Acknowledgments
This paper was extracted from Esmaeil Sarani's MSc thesis, supported financially by a grant from Zabol University of Medical Sciences.
References
- Despommier D. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev. 2003; 16(2): 265-72.
- Rubinsky-Elefant G, Hirata CE, Yamamoto JH, Ferreira MU. Human toxocariasis: diagnosis, worldwide seroprevalences and clinical expression of the systemic and ocular forms. Ann Trop Med Parasitol. 2010; 104(1): 3-23.
- Schantz PM. Toxocara larva migrans now. Am J Trop Med Hyg. 1989; 41(S3): 21-34.
- Glickman LT, Schantz PM. Epidemiology and pathogenesis of zoonotic toxocariasis. Epidemiol Rev. 1981; 3: 230-50.
- Emamapour SR, Borji H, Nagibi A. An epidemiological survey on intestinal helminths of stray dogs in Mashhad, North-east of Iran. J Parasit Dis. 2015; 39(2): 266-71.
- Sardarian K, Maghsood AH, Ghiasian SA, Zahirnia AH. Prevalence of zoonotic intestinal parasites in household and stray dogs in rural areas of Hamadan, Western Iran. Trop Biomed. 2015; 32(2): 240-46.
- Vafae Eslahi A, Kia EB, Mobedi I, Sharifdini M, Badri M, Mowlavi G. Road Killed Carnivores illustrate the status of zoonotic helminthes in Caspian Sea Littoral of Iran. Iran J Parasitol. 2017;12(2):230-5.
- Sadjjadi S, Khosravi M, Mehrabani D, Oryan A. Seroprevalence of Toxocara infection in school children in Shiraz, Southern Iran. Journal of Tropical Pediatrics 2000; 46(6): 327-30.
- Akhlaghi L, Ourmazdi H, Sarafnia A, Vaziri S, Jadidian K, Leghaii Z. An investigation on the toxocariasis seroprevalence in children (2-12 years old) from mahidasht area of Kermanshah Province (2003-2004). Razi Journal of Medical Sciences 2006; 13(52): 41-8.
- Fallah M, Azimi A, Taherkhani H. Seroprevalence of toxocariasis in children aged 1-9 years in western Islamic Republic of Iran, 2003. Eastern Mediterranean Health Journal 2007; 13(5): 1073-77.
- Nourian A, Amiri M, Ataeian A, Haniloo A, Mosavinasab S, Badali H. Seroepidemiological study for toxocariasis among children in Zanjan-northwest of Iran. Pakistan Journal of Biological Sciences 2008; 11(14): 1844.
- Hosseini-Safa A, Mousavi SM, Badorani MBB, Samani MG, Mostafaei S, Darani HY. Seroepidemiology of toxocariasis in children (5-15 yr old) referred to the pediatric clinic of Imam Hossein Hospital, Isfahan, Iran. Iranian Journal of Parasitology 2015; 10(4): 632.
- Shahraki MK, Dabirzadeh M, Afshari M, Maroufi Y. Epidemiological study of Toxocar canis in children under 14-years-old and dogs in Zabol and Chabahar Districts, southeast of Iran. Iranian Journal of Parasitology 2017; 12(1): 101.
- Finsterer J, Auer H. Neurotoxocarosis. Rev Inst Med Trop Sao Paulo 2007; 49(5): 279-87.
- Ma G, Holland CV, Wang T, Hofmann A, Fan CK, Maizels RM, et al. Human toxocariasis. Lancet Infect Dis. 2018; 18(1): 14-24.
- Yamaguti S. Systema helminthum. Volume III. The nematodes of vertebrates. Systema helminthum Volume III The nematodes of vertebrates. New York & London: Interscience Publishers; 1961. p.1261.
- Borecka A, Gawor J, Niedworok M, Sordyl B. Occurrence of Toxocara spp. eggs in household environment of children with diagnosed toxocariasis in Łódź voivodeship. Wiadomosci Parazytologiczne 2010; 56(2): 141-44.
- Fahrion AS, Schnyder M, Wichert B, Deplazes P. Toxocara eggs shed by dogs and cats and their molecular and morphometric species-specific identification: is the finding of T. cati eggs shed by dogs of epidemiological relevance? Veterinary Parasitology 2011; 177(1-2): 186-89.
- Liu W, Liu G, Li F, He D, Wang T, Sheng X, et al. Sequence variability in three mitochondrial DNA regions of Spirometra erinaceieuropaei spargana of human and animal health significance. Journal of Helminthology 2012; 86(3): 271-75.
- Jacobs DE, Zhu X, Gasser RB, Chilton NB. PCR-based methods for identification of potentially zoonotic ascaridoid parasites of the dog, fox and cat. Acta Tropica 1997; 68(2): 191-200.
- Zhu X, Gasser R, Chilton N, Jacobs D. Molecular approaches for studying ascaridoid nematodes with zoonotic potential, with an emphasis on Toxocara species. Journal of Helminthology 2001; 75(2): 101-108.
- Fogt R. Molecular techniques applied in species identification of Toxocara. Wiadomosci Parazytologiczne 2006; 52(1): 31-5.
- Sultan K, Omar M, Desouky AY, El-Seify MA. Molecular and phylogenetic study on Toxocara vitulorum from cattle in the mid-Delta of Egypt. Journal of Parasitic Diseases 2015; 39(3): 584-87.
- Wickramasinghe S, Yatawara L, Rajapakse R, Agatsuma T. Toxocara vitulorum (Ascaridida: Nematoda): mitochondrial gene content, arrangement and composition compared with other Toxocara species. Molecular and Biochemical Parasitology. 2009; 166(1): 89-92.
- Wickramasinghe S, Yatawara L, Rajapakse R, Agatsuma T. T. canis and Toxocara vitulorum: molecular characterization, discrimination, and phylogenetic analysis based on mitochondrial (ATP synthase subunit 6 and 12S) and nuclear ribosomal (ITS-2 and 28S) genes. Parasitology Research 2009; 104(6): 1425-430.
- Fava NMN, Cury MC, Santos HA, Takeuchi-Storm N, Strube C, Zhu XQ, et al. Phylogenetic relationships among Toxocara spp. and Toxascaris sp. from different regions of the world. Vet Parasitol. 2020; 282: 109133.
- Wang ZQ, Li LZ, Jiang P, Liu LN, Cui J. Molecular identification and phylogenetic analysis of Trichinella isolates from different provinces in mainland China. Parasitol Res. 2012; 110(2): 753-57.
- Betson M, Nejsum P, Llewellyn-Hughes J, Griffin C, Atuhaire A, Arinaitwe M, et al. Genetic diversity of Ascaris in southwestern Uganda. Trans R Soc Trop Med Hyg. 2012; 106(2): 75-83.
- Gawor J, Borecka A, Marczyńska M, Dobosz S, Żarnowska-Prymek H. Risk of human toxocarosis in Poland due to Toxocara infection of dogs and cats. Acta Parasitologica 2014; 60(1): 99-104.
- Faust EC, Sawitz W, Tobie J, Odom V, Peres C, Lincicome DR. Comparative efficiency of various technics for the diagnosis of protozoa and helminths in feces. The Journal of Parasitology 1939; 25(3): 241-62.
- Borecka A, Gawor J. Modification of gDNA extraction from soil for PCR designed for the routine examination of soil samples contaminated with Toxocara spp. eggs. Journal of Helminthology 2008; 82(2): 119-22.
- Li MW, Lin RQ, Song HQ, Sani RA, Wu XY, Zhu XQ. Electrophoretic analysis of sequence variability in three mitochondrial DNA regions for ascaridoid parasites of human and animal health significance. Electrophoresis 2008; 29(13): 2912-917.
- Mikaeili F, Mathis A, Deplazes P, Mirhendi H, Barazesh A, Ebrahimi S, et al. Differentiation of Toxocara canis and Toxocara cati based on PCR-RFLP analyses of rDNA-ITS and mitochondrial cox1 and nad1 regions. Acta Parasitologica 2017; 62(3): 549-56.
- Li K, Lan Y, Luo H, Zhang H, Liu D, Zhang L, et al. Prevalence, associated risk factors, and phylogenetic analysis of Toxocara vitulorum infection in yaks on the Qinghai Tibetan plateau, China. The Korean Journal of Parasitology 2016; 54(5): 645.
- Minnaar WN, Krecek RC, Fourie LJ. Helminths in dogs from a peri-urban resource-limited community in Free State Province, South Africa. Vet Parasitol. 2002; 107(4): 343-49.
- Habluetzel A, Traldi G, Ruggieri S, Attili AR, Scuppa P, Marchetti R, et al. An estimation of Toxocara canis prevalence in dogs, environmental egg contamination and risk of human infection in the Marche region of Italy. Vet Parasitol. 2003; 113(3-4): 243-52.
- Zhu X, D'Amelio S, Hu M, Paggi L, Gasser RB. Electrophoretic detection of population variation within Contracaecum ogmorhini (Nematoda: Ascaridoidea: Anisakidae). Electrophoresis 2001; 22(10): 1930-934.
- Öge H, Öge S, Özbakiş-Beceriklisoy G. Detection and identification of Toxocara canis in infected dogs using PCR. Helminthologia 2019; 56(2): 118.
- Li M, Lin R, Chen H, Sani R, Song H, Zhu X. PCR tools for the verification of the specific identity of ascaridoid nematodes from dogs and cats. Molecular and Cellular Probes. 2007; 21(5-6): 349-54.
- Fava NM, Cury MC, Santos HA, Takeuchi-Storm N, Strube C, Zhu X-Q, et al. Phylo-genetic relationships among Toxocara spp. and Toxascaris sp. from different regions of the world. Veterinary Parasitology 2020; 282(6): 109133.
- Borecka A. Differentiation of Toxocara spp. eggs isolated from the soil by the PCR-linked RFLP method. Helminthologia 2004; 41(4): 185-87.
- Fogt-Wyrwas R, Mizgajska-Wiktor H, Pacoń J, Jarosz W. Intraspecific variation between the ITS sequences of Toxocara canis, Toxocara cati and Toxascaris leonina from different host species in south-western Poland. Journal of Helminthology 2013; 87(4): 432-42.
- Zhu X, Gasser RB, Jacobs DE, Hung G-C, Chilton NB. Relationships among some ascaridoid nematodes based on ribosomal DNA sequence data. Parasitology Research 2000; 86(9): 738-44.
- Gasser RB, Zhu X-Q, Jacobs D, Hu M, Chilton NB, Holland C, et al. Molecular genetic characterization of members of the genus Toxocara–taxonomic, population genetic and epidemiological considerations. Toxocara: the enigmatic parasite United Kingdom: Wallingford; 2006. pp. 18-31.
- Hu M, Chilton NB, Gasser RB. The mitochondrial genomics of parasitic nematodes of socio-economic importance: recent progress, and implications for population genetics and systematics. Advances in Parasitology 2004; 56(3): 133-212.
- Gasser RB. A perfect time to harness advanced molecular technologies to explore the fundamental biology of Toxocara species. Veterinary Parasitology 2013; 193(4): 353-64.
- Boubaker G, Macchiaroli N, Prada L, Cucher MA, Rosenzvit MC, Ziadinov I, et al. A multiplex PCR for the simultaneous detection and genotyping of the Echinococcus granulosus complex. PLoS Neglected Tropical Diseases. 2013; 7(1): 2017.
- He X, Lv MN, Liu GH, Lin RQ. Genetic analysis of Toxocara cati (Nematoda: Ascarididae) from Guangdong province, subtropical China. Mitochondrial DNA Part A 2018; 29(1): 132-35.
- Luo H, Zhang H, Li K, Lan Y, Shahzad M, Wang X, et al. Molecular characterization of ascaris from Tibetan pigs by three mitochondrial markers of nad1, cox1 and cox2. Tropical Biomedicine 2017; 34(3): 576-82.
- Ngui R, Mahdy MA, Chua KH, Traub R, Lim YA. Genetic characterization of the partial mitochondrial cytochrome oxidase c subunit I (cox 1) gene of the zoonotic parasitic nematode, Ancylostoma ceylanicum from humans, dogs and cats. Acta Tropica 2013; 128(1): 154-57.
- Petrigh R, Scioscia N, Denegri G, Fugassa M. Research note. Cox-1 gene sequence of Spirometra in Pampas foxes from Argentina. Helminthologia 2015; 52(4): 355.
- Oguz B. Genetic characterization of toxocara vitilorum in Turkey by mitochondrial gene markers (cox1). Acta Scientiae Veterinariae 2018; 46(1): 6.
- Chang QC, Gao JF, Sheng ZH, Lou Y, Zheng X, Wang CR. Sequence variability in three mitochondrial genes among four roundworm species from wild animals in China. Mitochondrial DNA 2015; 26(1): 75-8.
- Mikaeili F, Mirhendi H, Mohebali M, Hosseini M, Sharbatkhori M, Zarei Z, et al. Sequence variation in mitochondrial cox1 and nad1 genes of ascaridoid nematodes in cats and dogs from Iran. J Helminthol. 2015; 89(4): 496-501.