Sun, Feb 22, 2026
[Archive]
Volume 10, Issue 3 (August 2023)
IJML 2023, 10(3): 217-228 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Abbasi S, Fani M, Sayar S, Radmanesh E, Jelvay S, Pahlavanzadeh B, et al . The Effects of Vitamin D3 and N-Acetylcysteine Administration in Patients with COVID-19 Hospitalization in the Iranian Population. IJML 2023; 10 (3) :217-228
URL: http://ijml.ssu.ac.ir/article-1-472-en.html
URL: http://ijml.ssu.ac.ir/article-1-472-en.html
Samaneh Abbasi * 

 , Mona Fani
, Mona Fani 

 , Sara Sayar
, Sara Sayar 

 , Esmat Radmanesh
, Esmat Radmanesh 
 , Saeed Jelvay
, Saeed Jelvay 

 , Bagher Pahlavanzadeh
, Bagher Pahlavanzadeh 

 , Zahra Arizavi
, Zahra Arizavi 
 , Hani Esmaeelian
, Hani Esmaeelian 
 , Masoomeh Asadi
, Masoomeh Asadi 
 , Najmeh Babaeian
, Najmeh Babaeian 
 , Raheleh Pour Yoosefi
, Raheleh Pour Yoosefi 
 , Saeed Bitaraf
, Saeed Bitaraf 
 , Saeedeh Elhami
, Saeedeh Elhami 
 , Sara Mobarak
, Sara Mobarak 



 , Mona Fani
, Mona Fani 

 , Sara Sayar
, Sara Sayar 

 , Esmat Radmanesh
, Esmat Radmanesh 
 , Saeed Jelvay
, Saeed Jelvay 

 , Bagher Pahlavanzadeh
, Bagher Pahlavanzadeh 

 , Zahra Arizavi
, Zahra Arizavi 
 , Hani Esmaeelian
, Hani Esmaeelian 
 , Masoomeh Asadi
, Masoomeh Asadi 
 , Najmeh Babaeian
, Najmeh Babaeian 
 , Raheleh Pour Yoosefi
, Raheleh Pour Yoosefi 
 , Saeed Bitaraf
, Saeed Bitaraf 
 , Saeedeh Elhami
, Saeedeh Elhami 
 , Sara Mobarak
, Sara Mobarak 

Department of Microbiology, School of Medicine, Abadan University of Medical Sciences, Abadan, Iran.
Full-Text [PDF 346 kb]
(406 Downloads)
| Abstract (HTML) (1469 Views)
Introduction
At the end of 2019, a novel flu-like coronavirus related to the Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS) coronaviruses was found in China and the evidence of human-to-human transmission was confirmed in the case of close contacts [1]. This virus can be found in human respiratory epithelial cells, which firstly attacks the lungs and induces serous fluid, fibrin exudates, and hyaline membrane formation in the alveoli [2]. The spectrum of disease associated with this infection ranges from asymptomatic or mild self-limiting infection to rapidly progressing life-threatening disease, with higher mortality rates in older adults with underlying chronic diseases. Respiratory failure is common among critically ill patients, often requiring invasive mechanical ventilation. Given the poor outcome of patients progressing to critical disease, there is a desperate need to identify drugs that could potentially improve their prognosis [3].
Vitamin D is a steroid hormone that plays a chief function in the regulation of natural immunity. Respiratory epithelial cells constitutively stimulate vitamin D and are capable of creating a microenvironment that has high levels of the active form of the vitamin. Activation has downstream influences that contain up-regulation of the cathelicidin antimicrobial peptide gene, the Toll-like receptor (TLR), coreceptor, and CD14. Viral infection leads to raised activation of vitamin D and additional increases in cathelicidin mRNA. Cathelicidin is recognized to be a significant constituent of natural immunity in the lungs and thus restricted vitamin D activation might be a significant part of host defense [4]. Observational and supplementation trials have reported higher 25(OH)D concentrations associated with reduced risk of influenza, respiratory syncytial virus infections, and pneumonia. Results of a community field trial reported herein indicated that 25(OH)D concentrations above 50 ng/ml vs. <20 ng/ml were associated with a 27% reduction in influenza-like illnesses. The researcher hypothesizes that raising serum 25(OH)D concentrations through vitamin D supplementation could reduce the incidence, severity, and risk of death from influenza, pneumonia, and the current coronavirus disease 2019 (COVID-19) epidemic [5]. Some studies also emphasize that the addition of vitamin D supplements to the theorized [Minocycline, N-acetylcysteine (NAC) and Aspirin] triple combined therapy to COVID-19, may improve the therapeutic efficacy of the joint therapy. In vivo and in vitro laboratory experiments will confirm this theory about the COVID-19 crisis [4]. In addition, some evidence indicates that NAC may be used for prophylaxis or therapy of virus diseases. NAC prevents viral replication by inhibiting viral DNA polymerase, binding to specific cell-surface receptors inhibiting viral penetration or uncoating, inhibiting viral protein synthesis, or blocking late stages of virus assembly [6].
Since the outbreak of COVID‐19 infection has posed significant threats to international health and the economy, also in the absence of treatment for this virus, there is an urgent need to find alternative methods to control the disease. So this study aimed to compare the effects of vitamin D3 and NAC administration on clinical status indicators and recovery process in patients with COVID-19 hospitalization.
Materials and Methods
Participants
The present study is a pilot, double-blind, and placebo-controlled randomized clinical trial that was conducted to compare the effects of vitamin D3 and NAC administration on clinical status indicators and recovery process in patients with COVID-19 hospitalization in Abadan, Iran in 2020. We recruited hospitalized patients with COVID-19 infection from the Taleghani Hospital, Abadan, Iran. The patients were selected based on the inclusion and exclusion criteria and entered the study after obtaining informed consent. Inclusion criteria included: being at least 18 years old, being diagnosed with COVID-19, and not suffering from another serious and restrictive disease (such as a major psychological disorder, neural disease, musculoskeletal disease, cancer, and cardiac attack). COVID-19 infection was diagnosed via chest computed tomography scan and was confirmed using the real-time polymerase chain reaction test of the nasopharyngeal sample. Exclusion criteria include pregnant or lactating women, those having any life-threatening factors, and those who were not willing to continue the experiment (The study flowchart is shown in Fig. 1).
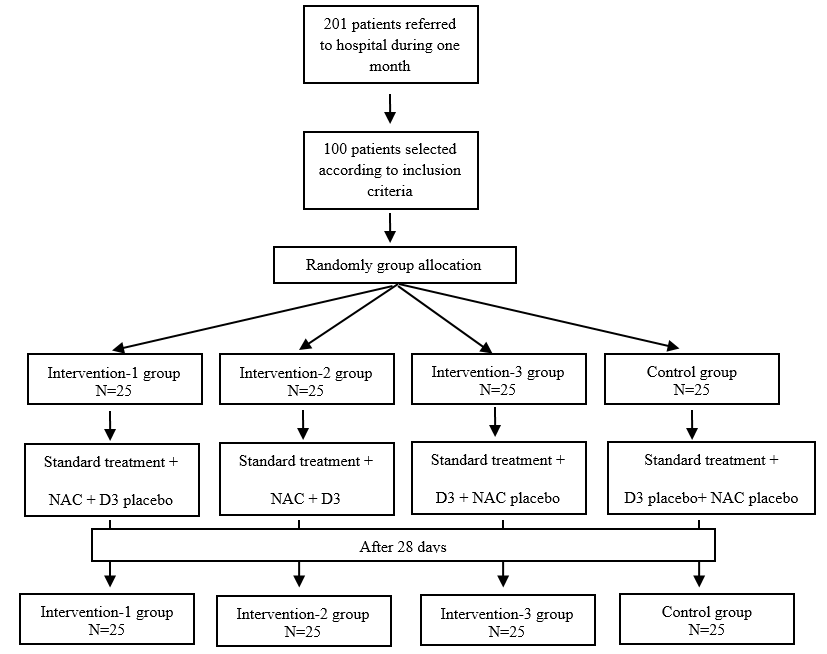
Sampling and study design
In this pilot study, the researcher attended the hospital from June 2020 to July 2020, and the patients with COVID-19 infection were invited to participate in the research. Among 201 patients, 100 patients who met the inclusion criteria were selected for participation in this study. After the patients' enrollment, they were randomly allocated to four groups (3 intervention groups and 1 control group) using block randomization and block sizes were octahedral and quaternary. Randomization sequence and concealment codes were created by www.sealedenvelope.com website. Sealed envelopes were used for allocation concealment. Enrollment was performed by one researcher and intervention was performed by another researcher. All participants agreed to participate in the study and completed an informed consent form. No payment was offered to the participants for their participation in the study. The initial plan of the study was approved by the Ethics Committee of the Abadan School of Medical Sciences (Ethic code: IR.ABADANUMS. REC.1398.118), and this study was recorded with randomized clinical trial code: IRCT20200324046850N1 in the Iranian Registry of Clinical Trials (www.irct.ir) on 2020-03-29.
Intervention
All patients received standard country protocol treatment for COVID-19 infection [Tab Hydroxychloroquine Sulfate 200 mg 2 tablets one dose (Isfahan-Amir Company) & Tab Lopinavir 50 mg-Ritonavir 200 mg 2 tablets every 12 hours until the patient's clinical symptoms improve (Emcure Company)]. In addition, patients in the intervention-1 group received NAC tablets 600 mg every 12 hours and vitamin D3 placebo ampoules once a week. The intervention-2 group received NAC tablets 600 mg every 12 hours and vitamin D3 ampoules 500,000 units once a week. The intervention-3 group received vitamin D3 ampoules 50,000 units once a week and NAC placebo tablets every 12 hours. The control group received vitamin D3 placebo ampoules once a week and NAC placebo tablets every 12 hours. The placebo tablets contained starch and the placebo ampoules contained sterile water. The placebo tablets were similar to the NAC tablets in terms of color and appearance. Vitamin D3 ampoules were purchased from the Osveh Company and NAC tablets were purchased from the Caspian Company in Iran.
Data collection
Demographic information (such as age, sex, marital status, level of education, length of hospitalization, and the outcome of the disease) and clinical parameters (namely, comorbid diseases, intensive care unit (ICU) hospitalization, severity of dyspnea at admission and discharge) were assessed for all patients through interviews and patients file documentations by a trained nurse. Besides, symptoms of COVID-19 infection, including fever, cough, dyspnea, otalgia, fatigue, headache, myalgia, taste disorder, chest pain, diarrhea, and nausea were evaluated at the baseline and on days 7, 14, 21, and 28 by a trained nurse. These assessments were done using clinical observation and examination by an interview with the patients.
Also, some clinical status indicators including Glasgow Coma Scale (GCS), Respiratory Rate (RR), O2 saturation (Sao2), Systolic Blood Pressure (SBP), and Diastolic Blood Pressure (DBP) were compared in groups at the baseline and days 7, 14, 21 and 28. It is necessary to explain that GCS was assessed by the Glasgow Coma Scale, RR was considered as the number of breaths per minute, Sao2 was measured using the pulse-oximeter, SBP, and DBP were measured at the right arm using a mercury barometer calibrated by the institute of standardization and industrial research.
The laboratory parameters such as blood urea nitrogen (BUN), creatinine (Cr), serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), alkaline phosphatase (ALKPH), sodium (Na), potassium (K), prothrombin time (PT), partial thromboplastin time (PTT), the international normalized ratio (INR), erythrocyte sedimentation rate (ESR), white blood cells (WBC), red blood cells (RBC), hemoglobin (Hgb), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), neutrophil, lymphocyte, platelet (PLT), and C-reactive protein (CRP) were measured at the baseline and the end of the intervention (day 28). For this measurement, a 10-ml venous blood sample was taken from each patient at baseline and the end of the intervention.
Statistical analysis
The collected data were entered into the SPSS statistical software v.23, and for descriptive statistics, we determined the frequency, percent, mean, and standard deviation. The chi-square test and Fisher's exact test were used to compare the distribution of qualitative variables between groups. Two sample t-tests were used to compare the distribution of quantitative variables and the Mann-Whitney test was used for abnormal variables. Generalized Linear Model analysis was used to compare the effect of 4 treatment regimens on response variables. In these analyses, for the variables with two-state qualitative responses, the odds ratio (95% confidence interval) was estimated in the three groups compared to the group with the least impact. For the variables with quantitative response, the difference between the mean and the group with the lowest mean was estimated. In addition to comparison between groups, the significance of changes in response variables during the study period was also examined. For laboratory variables in which two measurements of each variable were recorded for the patient, comparisons between the four groups were performed using generalized linear model analysis.
Results
Among 100 participants entering the study, 100 people completed the study. Analysis showed that there were no statistically significant differences between the four groups in demographic characteristics and outcomes (Table 1). In addition, there was no statistically significant difference between the four groups in comparison of comorbidities, ICU indicators, and dyspnea severity (Table 2). The ratio of changes in disease symptoms throughout the trial in the four groups was calculated considering baseline and days 7, 14, 21, and 28. The comparison of cough throughout the trial showed that the incidence of cough in the NAC-P1 group was lower than the other three groups and the chance of cough in the D3-P2 group was 2.85 times higher than the NAC-P1 group (p = 0.037). Also, it was found that in four groups, the chance of coughing was 0.5 times lower with each passing week (p < 0.001). A comparison of the incidence of dyspnea showed that the chance of dyspnea in four groups had a decreasing trend and with each passing week, the chance of dyspnea was 0.53 times lower than the previous week (p < 0.001). Also, it was found that the chance of dyspnea in the P1-P2 group was 2.7 times, and in the D3-P2 group was 2.8 times higher than the NAC-D3 group (p = 0.041 and p= 0.031, respectively). The comparison of otalgia occurrence was also significantly reduced in four groups (p = 0.008). The comparison of the incidence of fatigue and headache was significantly reduced in the four groups so that with each passing week the chance of fatigue was 0.54 times higher than the previous week (p < 0.001) and the chance of headache was 32 times higher than the previous week (p < 0.001). The incidence of myalgia decreased significantly over time in four groups (p < 0.001). The comparison between the groups showed that the chance of myalgia in the P1-P2 group was 5.59 times and in the NAC-P1 group was 4.63 times that of the D3-P1 group. Over time, the incidence of taste disorder decreased significantly in the four groups, and with each passing week, the chance of taste disorder was 0.38 times higher than the previous week (p = 0.004); however, there was no significant difference in the incidence of taste disorder between NAC-P1 group and any of the groups. The chance of chest pain was significantly reduced in four groups and it was found that the chance of chest pain with each passing week was 0.39 times higher than the previous week (p = 0.007). However, there was no statistically significant difference between the groups concerning the incidence of chest pain. Also, the chance of diarrhea decreased 0.23 times compared to the previous week (p = 0.002), but there was no significant difference in the chance of diarrhea between the groups. One week after starting the treatment, the chance of nausea in the four groups was 0.02 times that of the start of the study and a significant decrease was observed in the four groups (p< 0.001), but no statistically significant difference was observed between the groups. Mean changes in clinical status indexes such as GCS, RR, Sao2, SBP, and DBP throughout the trial in the four groups are illustrated in Table 3. Mean changes in these variables were calculated by considering baseline and days 7, 14, 21, and 28. Comparison of GCS mean changes showed that these changes were not significant over time. However, the comparison of each group with the P1-P2 group in the study period observed that the mean GCS of the NAC-D3 group was significantly higher than the P1-P2 group (p = 0.01). Comparing the RR changes during the treatment period, it was found that these changes were not significant over time between groups (p = 0.23). Comparing SPO2 changes also showed that changes in this variable during the study period in the P1-P2 group were associated with fluctuations, but the other three groups did not show much change (p=0.21). The mean changes in SBP in the groups before and after day 21 were different. In separate analyses before day 21, it was observed that the mean SBP in the groups decreased by 3.29 mmHg with each passing week (p=0.004). Analyzes for days 21 and 28 also showed that although the mean SBP in the groups had an increasing trend, this increase was not significant (p=0.98). Assessment of DBP changes during the treatment period showed that in NAC-D3 and NAC-P1 groups, mean DBP decreased and then increased until day 14, while in P1-P2 and D3-P2 groups in day 7. And the second increased and then decreased. But in general, changes in DBP during the study period between groups were not significant (p = 0.95) (Table 3). Also, a comparison of laboratory test results between the four groups showed that there was only a significant difference between the mean of SGPT in the four groups (p=0.024). In pairwise comparisons, it was found that the SGPT mean in the P1-P2 group was significantly higher than in the NAC-P1 group. Also, relative differences between PTT and MCHC were observed in the four groups, although these differences were not significant (p=0.06, p=0.08).
Discussion
The outbreak of COVID‐19 infection has posed significant threats to international health. In the absence of treatment for this virus, there is an urgent need to find alternative methods to control the disease. Until now, many proposed protocols and therapeutic methods could not achieve this goal. Thus, this research aimed to investigate the effects of vitamin D3 and NAC administration on clinical status indicators and recovery processes in patients with COVID-19 hospitalization. According to Grant et al.'s review, the human immune system requires particular micronutrients, such as vitamins A, B6, B12, C, D and E. Micronutrients with the strongest evidence for immune support are vitamins C and D and zinc. The evidence reviewed here supports the role of higher 25(OH)D concentrations in reducing the risk of infection and death from acute respiratory tract infections (ARTIs), including those from influenza, coronaviruses, and pneumonia. Vitamin D supplementation also enhances the expression of genes related to antioxidation (glutathione reductase and glutamate–cysteine ligase modifier subunit [5]. Despite the lack of direct evidence of the effect of vitamin D on SARS-CoV-2 infections, it has been suggested that vitamin D supplementation could be a safe and inexpensive intervention for COVID-19 [7]. Other studies suggest that serum vitamin D levels could be valuable in preventing SARS-CoV-2 infection [8]. However, there is not enough evidence on the association between vitamin D levels and COVID-19 severity and mortality [9]. Furthermore, NAC, has been used to loosen thick mucus in the lungs and treat acetaminophen overdose for decades. However, NAC can improve the immune system function, suppress viral replication, and reduce inflammation.
Previous studies have shown that oral NAC treatment significantly reduced the frequency of influenza and pneumonia and it has also been effective in reducing the severity and duration of most symptoms [10]. In addition, Sharaf-Khah et al. have demonstrated that NAC is safe and effective in preventing and delaying ventilator-associated pneumonia in the ICU population [11].
The results of this study showed that there is a significant difference in the length of hospitalization between the four groups. Furthermore, the mean length of hospitalization was increased in the D3-P2 group and this data is parallel to the results in previous studies. Another study represented that among hospitalized patients with COVID-19, a single high dose of vitamin D3 did not significantly reduce the length of hospitalization [12]. Surprisingly, the length of hospitalization was decreased in NAC-D3 which may indicate the role of oral administration of NAC in decreasing the disease symptoms such as pain severity, dyspnea, and cough. According to our results, during the study, the chance of shortness of breath was decreased in all treatment groups. In general, the incidence of shortness of breath in NAC-D3 patients was lower than in the other three groups. Studies have shown that higher concentrations of serum 25(OH)D are significantly associated with a lower extent of lung involvement and better outcomes in patients with COVID-19 [13, 14].
Another clinical factor that was examined was the presence of cough in patients. Throughout the study, the rate of cough in patients in three treatment groups was decreased However, this decrease was more significant in the NAC-P1 group compared to other groups.
The researchers classified the probable effects of NAC as oxidative-regulatory and apoptotic-regulatory roles, antiviral activities, anti-inflammatory roles, and preventive and therapeutic roles in lung disorders. So, based on the different beneficial effects of NAC, it could be administered as a potential adjuvant therapy for COVID-19 [15]. In addition, previous research showed that oral and Intravenous (IV) glutathione (N-acetyl-cysteine) and alpha lipoic acid may represent a novel treatment approach for blocking Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and addressing “cytokine storm syndrome” and respiratory distress in patients with COVID-19 pneumonia [16].
Abnormal liver function has been observed in patients after SARS-CoV2 infection since initial contact with the medical system Which indicates the direct effect of the virus [17] due to the presence of the angiotensin-converting enzyme 2 receptor in the liver [18]. Due to the high prevalence of liver function abnormality in patients with COVID-19, it is important to monitor hepatic enzymes during the course of the disease to prevent additional injury. In our study, there was a significant difference between the mean of SGPT in the four groups and it was found that the SGPT mean in the P1-P2 group was significantly higher than the NAC-P1 group. Previous research showed that N-acetylcysteine can improve liver function in patients with non-alcoholic fatty liver disease [19]. Feketea's study expressed that vitamin D levels could be valuable in predicting severe forms of multisystem inflammatory syndrome in children is a rare complication of COVID-19 [20]. Ilie et al. assessed the potential association between mean levels of vitamin D in 20 European countries and showed that the higher vitamin D levels were associated not only with the lower number of cases diagnosed with COVID-19 but also with lower mortality per million population [21].
In this study, other clinical factors were also assessed that were including pain severity, sore throat, ear pain, joint pain, fever, fatigue, headache, myalgia, taste disorder, night sweats, chest pain, anorexia, diarrhea, stomachache and nausea but no significant difference was observed between the three treatment groups compared to the control group. Furthermore, there was not seen significant difference in evaluating the GCS mean score, RR and BP changes, and SPO2 percentage in treatment groups.
Conclusion
Based on the result, the addition of vitamin D supplements and NAC will improve some outcomes in COVID-19 patients treated but more randomized controlled trial studies are needed in this field. In the present study, no RCT has used NAC+Vitamin D in COVID-19 patients. The main limitation of our study is the small number of patients enrolled. This protocol has been proposed as a preparatory stage before a larger multi-centric trial.
Some clinical and therapeutic features of COVID-19 and its outcome may become more evident over time, especially about non-respiratory symptoms such as gastrointestinal, musculoskeletal, and cutaneous signs and some of them could be targeted by NAC such as severe adverse cutaneous reactions that need more focus in future studies.
Conflict of Interest
The authors declare that there is no conflict of interest.
Acknowledgments
The authors would like to thank the Abadan University of Medical Sciences for funding this project.
References
Full-Text: (1077 Views)
Introduction
At the end of 2019, a novel flu-like coronavirus related to the Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS) coronaviruses was found in China and the evidence of human-to-human transmission was confirmed in the case of close contacts [1]. This virus can be found in human respiratory epithelial cells, which firstly attacks the lungs and induces serous fluid, fibrin exudates, and hyaline membrane formation in the alveoli [2]. The spectrum of disease associated with this infection ranges from asymptomatic or mild self-limiting infection to rapidly progressing life-threatening disease, with higher mortality rates in older adults with underlying chronic diseases. Respiratory failure is common among critically ill patients, often requiring invasive mechanical ventilation. Given the poor outcome of patients progressing to critical disease, there is a desperate need to identify drugs that could potentially improve their prognosis [3].
Vitamin D is a steroid hormone that plays a chief function in the regulation of natural immunity. Respiratory epithelial cells constitutively stimulate vitamin D and are capable of creating a microenvironment that has high levels of the active form of the vitamin. Activation has downstream influences that contain up-regulation of the cathelicidin antimicrobial peptide gene, the Toll-like receptor (TLR), coreceptor, and CD14. Viral infection leads to raised activation of vitamin D and additional increases in cathelicidin mRNA. Cathelicidin is recognized to be a significant constituent of natural immunity in the lungs and thus restricted vitamin D activation might be a significant part of host defense [4]. Observational and supplementation trials have reported higher 25(OH)D concentrations associated with reduced risk of influenza, respiratory syncytial virus infections, and pneumonia. Results of a community field trial reported herein indicated that 25(OH)D concentrations above 50 ng/ml vs. <20 ng/ml were associated with a 27% reduction in influenza-like illnesses. The researcher hypothesizes that raising serum 25(OH)D concentrations through vitamin D supplementation could reduce the incidence, severity, and risk of death from influenza, pneumonia, and the current coronavirus disease 2019 (COVID-19) epidemic [5]. Some studies also emphasize that the addition of vitamin D supplements to the theorized [Minocycline, N-acetylcysteine (NAC) and Aspirin] triple combined therapy to COVID-19, may improve the therapeutic efficacy of the joint therapy. In vivo and in vitro laboratory experiments will confirm this theory about the COVID-19 crisis [4]. In addition, some evidence indicates that NAC may be used for prophylaxis or therapy of virus diseases. NAC prevents viral replication by inhibiting viral DNA polymerase, binding to specific cell-surface receptors inhibiting viral penetration or uncoating, inhibiting viral protein synthesis, or blocking late stages of virus assembly [6].
Since the outbreak of COVID‐19 infection has posed significant threats to international health and the economy, also in the absence of treatment for this virus, there is an urgent need to find alternative methods to control the disease. So this study aimed to compare the effects of vitamin D3 and NAC administration on clinical status indicators and recovery process in patients with COVID-19 hospitalization.
Materials and Methods
Participants
The present study is a pilot, double-blind, and placebo-controlled randomized clinical trial that was conducted to compare the effects of vitamin D3 and NAC administration on clinical status indicators and recovery process in patients with COVID-19 hospitalization in Abadan, Iran in 2020. We recruited hospitalized patients with COVID-19 infection from the Taleghani Hospital, Abadan, Iran. The patients were selected based on the inclusion and exclusion criteria and entered the study after obtaining informed consent. Inclusion criteria included: being at least 18 years old, being diagnosed with COVID-19, and not suffering from another serious and restrictive disease (such as a major psychological disorder, neural disease, musculoskeletal disease, cancer, and cardiac attack). COVID-19 infection was diagnosed via chest computed tomography scan and was confirmed using the real-time polymerase chain reaction test of the nasopharyngeal sample. Exclusion criteria include pregnant or lactating women, those having any life-threatening factors, and those who were not willing to continue the experiment (The study flowchart is shown in Fig. 1).

Fig. 1. Flowchart of recruitment
Sampling and study design
In this pilot study, the researcher attended the hospital from June 2020 to July 2020, and the patients with COVID-19 infection were invited to participate in the research. Among 201 patients, 100 patients who met the inclusion criteria were selected for participation in this study. After the patients' enrollment, they were randomly allocated to four groups (3 intervention groups and 1 control group) using block randomization and block sizes were octahedral and quaternary. Randomization sequence and concealment codes were created by www.sealedenvelope.com website. Sealed envelopes were used for allocation concealment. Enrollment was performed by one researcher and intervention was performed by another researcher. All participants agreed to participate in the study and completed an informed consent form. No payment was offered to the participants for their participation in the study. The initial plan of the study was approved by the Ethics Committee of the Abadan School of Medical Sciences (Ethic code: IR.ABADANUMS. REC.1398.118), and this study was recorded with randomized clinical trial code: IRCT20200324046850N1 in the Iranian Registry of Clinical Trials (www.irct.ir) on 2020-03-29.
Intervention
All patients received standard country protocol treatment for COVID-19 infection [Tab Hydroxychloroquine Sulfate 200 mg 2 tablets one dose (Isfahan-Amir Company) & Tab Lopinavir 50 mg-Ritonavir 200 mg 2 tablets every 12 hours until the patient's clinical symptoms improve (Emcure Company)]. In addition, patients in the intervention-1 group received NAC tablets 600 mg every 12 hours and vitamin D3 placebo ampoules once a week. The intervention-2 group received NAC tablets 600 mg every 12 hours and vitamin D3 ampoules 500,000 units once a week. The intervention-3 group received vitamin D3 ampoules 50,000 units once a week and NAC placebo tablets every 12 hours. The control group received vitamin D3 placebo ampoules once a week and NAC placebo tablets every 12 hours. The placebo tablets contained starch and the placebo ampoules contained sterile water. The placebo tablets were similar to the NAC tablets in terms of color and appearance. Vitamin D3 ampoules were purchased from the Osveh Company and NAC tablets were purchased from the Caspian Company in Iran.
Data collection
Demographic information (such as age, sex, marital status, level of education, length of hospitalization, and the outcome of the disease) and clinical parameters (namely, comorbid diseases, intensive care unit (ICU) hospitalization, severity of dyspnea at admission and discharge) were assessed for all patients through interviews and patients file documentations by a trained nurse. Besides, symptoms of COVID-19 infection, including fever, cough, dyspnea, otalgia, fatigue, headache, myalgia, taste disorder, chest pain, diarrhea, and nausea were evaluated at the baseline and on days 7, 14, 21, and 28 by a trained nurse. These assessments were done using clinical observation and examination by an interview with the patients.
Also, some clinical status indicators including Glasgow Coma Scale (GCS), Respiratory Rate (RR), O2 saturation (Sao2), Systolic Blood Pressure (SBP), and Diastolic Blood Pressure (DBP) were compared in groups at the baseline and days 7, 14, 21 and 28. It is necessary to explain that GCS was assessed by the Glasgow Coma Scale, RR was considered as the number of breaths per minute, Sao2 was measured using the pulse-oximeter, SBP, and DBP were measured at the right arm using a mercury barometer calibrated by the institute of standardization and industrial research.
The laboratory parameters such as blood urea nitrogen (BUN), creatinine (Cr), serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), alkaline phosphatase (ALKPH), sodium (Na), potassium (K), prothrombin time (PT), partial thromboplastin time (PTT), the international normalized ratio (INR), erythrocyte sedimentation rate (ESR), white blood cells (WBC), red blood cells (RBC), hemoglobin (Hgb), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), neutrophil, lymphocyte, platelet (PLT), and C-reactive protein (CRP) were measured at the baseline and the end of the intervention (day 28). For this measurement, a 10-ml venous blood sample was taken from each patient at baseline and the end of the intervention.
Statistical analysis
The collected data were entered into the SPSS statistical software v.23, and for descriptive statistics, we determined the frequency, percent, mean, and standard deviation. The chi-square test and Fisher's exact test were used to compare the distribution of qualitative variables between groups. Two sample t-tests were used to compare the distribution of quantitative variables and the Mann-Whitney test was used for abnormal variables. Generalized Linear Model analysis was used to compare the effect of 4 treatment regimens on response variables. In these analyses, for the variables with two-state qualitative responses, the odds ratio (95% confidence interval) was estimated in the three groups compared to the group with the least impact. For the variables with quantitative response, the difference between the mean and the group with the lowest mean was estimated. In addition to comparison between groups, the significance of changes in response variables during the study period was also examined. For laboratory variables in which two measurements of each variable were recorded for the patient, comparisons between the four groups were performed using generalized linear model analysis.
Results
Among 100 participants entering the study, 100 people completed the study. Analysis showed that there were no statistically significant differences between the four groups in demographic characteristics and outcomes (Table 1). In addition, there was no statistically significant difference between the four groups in comparison of comorbidities, ICU indicators, and dyspnea severity (Table 2). The ratio of changes in disease symptoms throughout the trial in the four groups was calculated considering baseline and days 7, 14, 21, and 28. The comparison of cough throughout the trial showed that the incidence of cough in the NAC-P1 group was lower than the other three groups and the chance of cough in the D3-P2 group was 2.85 times higher than the NAC-P1 group (p = 0.037). Also, it was found that in four groups, the chance of coughing was 0.5 times lower with each passing week (p < 0.001). A comparison of the incidence of dyspnea showed that the chance of dyspnea in four groups had a decreasing trend and with each passing week, the chance of dyspnea was 0.53 times lower than the previous week (p < 0.001). Also, it was found that the chance of dyspnea in the P1-P2 group was 2.7 times, and in the D3-P2 group was 2.8 times higher than the NAC-D3 group (p = 0.041 and p= 0.031, respectively). The comparison of otalgia occurrence was also significantly reduced in four groups (p = 0.008). The comparison of the incidence of fatigue and headache was significantly reduced in the four groups so that with each passing week the chance of fatigue was 0.54 times higher than the previous week (p < 0.001) and the chance of headache was 32 times higher than the previous week (p < 0.001). The incidence of myalgia decreased significantly over time in four groups (p < 0.001). The comparison between the groups showed that the chance of myalgia in the P1-P2 group was 5.59 times and in the NAC-P1 group was 4.63 times that of the D3-P1 group. Over time, the incidence of taste disorder decreased significantly in the four groups, and with each passing week, the chance of taste disorder was 0.38 times higher than the previous week (p = 0.004); however, there was no significant difference in the incidence of taste disorder between NAC-P1 group and any of the groups. The chance of chest pain was significantly reduced in four groups and it was found that the chance of chest pain with each passing week was 0.39 times higher than the previous week (p = 0.007). However, there was no statistically significant difference between the groups concerning the incidence of chest pain. Also, the chance of diarrhea decreased 0.23 times compared to the previous week (p = 0.002), but there was no significant difference in the chance of diarrhea between the groups. One week after starting the treatment, the chance of nausea in the four groups was 0.02 times that of the start of the study and a significant decrease was observed in the four groups (p< 0.001), but no statistically significant difference was observed between the groups. Mean changes in clinical status indexes such as GCS, RR, Sao2, SBP, and DBP throughout the trial in the four groups are illustrated in Table 3. Mean changes in these variables were calculated by considering baseline and days 7, 14, 21, and 28. Comparison of GCS mean changes showed that these changes were not significant over time. However, the comparison of each group with the P1-P2 group in the study period observed that the mean GCS of the NAC-D3 group was significantly higher than the P1-P2 group (p = 0.01). Comparing the RR changes during the treatment period, it was found that these changes were not significant over time between groups (p = 0.23). Comparing SPO2 changes also showed that changes in this variable during the study period in the P1-P2 group were associated with fluctuations, but the other three groups did not show much change (p=0.21). The mean changes in SBP in the groups before and after day 21 were different. In separate analyses before day 21, it was observed that the mean SBP in the groups decreased by 3.29 mmHg with each passing week (p=0.004). Analyzes for days 21 and 28 also showed that although the mean SBP in the groups had an increasing trend, this increase was not significant (p=0.98). Assessment of DBP changes during the treatment period showed that in NAC-D3 and NAC-P1 groups, mean DBP decreased and then increased until day 14, while in P1-P2 and D3-P2 groups in day 7. And the second increased and then decreased. But in general, changes in DBP during the study period between groups were not significant (p = 0.95) (Table 3). Also, a comparison of laboratory test results between the four groups showed that there was only a significant difference between the mean of SGPT in the four groups (p=0.024). In pairwise comparisons, it was found that the SGPT mean in the P1-P2 group was significantly higher than in the NAC-P1 group. Also, relative differences between PTT and MCHC were observed in the four groups, although these differences were not significant (p=0.06, p=0.08).
Table 1. Demographic characteristics and final outcome of participants
Significant difference (P<0.05)
Table 2. Comparison of comorbidities, ICU indicators, dyspnea, and pain severity between groups
Significant difference (P<0.05); ICU= Intensive care unit
Table 3. Comparison of Clinical status indicators means between groups at baseline and days 7, 14, 21, and 28
Significant difference (p < 0.05)
| Variable | Groups | P-Value | ||||
| Control (P1-P2) (N=25) |
Intervention-1 NAC-P1 (N=25) |
Intervention-2 NAC-D3 (N=25) |
Intervention-3 D3-P2 (N=25) |
|||
| Age (yr) (Mean±SD) |
49.2±13.84 | 53.2±9.8 | 53.5±14.36 | 53.4±16.41 | 0.55 | |
| Sex N (%) |
Male Female |
14(56) 11(44) |
14(56) 11(44) |
13(48) 13(52) |
13(52) 12(48) |
0.96 |
| Marital status N (%) |
Unmarried Married |
2(8) 23(92) |
4(16) 23(84) |
3(12) 23(88) |
2(8) 23(92) |
0.9 |
| Education N (%) |
Illiterate Primary Upper |
1(4) 20(80) 4(16) |
4(16) 20(80) 1(4) |
2(8) 21(80) 3(12) |
0(0.0) 24(96) 1(4) |
0.22 |
| Final outcome N (%) |
Recovery Death |
20(80) 5(20) |
17(68) 8(32) |
22(84) 4(16) |
18(72) 7(28) |
0.53 |
| Duration of hospitalization (day) (Mean±SD) |
8.4±3.72 | 7±3.85 | 6.42±4.8 | 8.8±4.6 | .039 | |
Table 2. Comparison of comorbidities, ICU indicators, dyspnea, and pain severity between groups
| Variable Frequency (%) |
Groups | P-Value | |||
| Control (P1-P2) (N=25) |
Intervention-1 NAC-P1(N=25) |
Intervention-2 NAC-D3 (N=25) |
Intervention-3 D3-P2 (N=25) |
||
| Hypertension | 10(40) | 10(40) | 9(34.6) | 13(52) | 0.7 |
| Diabetes | 8(32) | 12(48) | 11(42.3) | 8(32) | 0.54 |
| Chronic pulmonary disease | 3(12) | 2(8) | 1(3.8) | 4(16) | 0.68 |
| Chronic kidney disease | 5(20) | 3(12) | 0(0) | 3(12) | 0.11 |
| Chronic cardiac disease | 4(16) | 3(12) | 3(11.5) | 2(8) | 0.97 |
| Chronic neurological disease | 1(4) | 1(4) | 1(3.8) | 1(4) | 1.00 |
| Chronic hematologic disease | 0(0) | 1(4) | 0(0) | 0(0) | 1.00 |
| Human immunodeficiency virus | 0(0) | 1(4) | 0(0) | 0(0) | 1.00 |
| Malnutrition | 0(0) | 1(4) | 0(0) | 0(0) | 1.00 |
| Other risk factors | 2(8) | 6(24) | 3(11.5) | 2(8) | 0.45 |
| Admission to ICU (entry) | 6(24) | 7(28) | 1(4) | 5(20) | 0.11 |
| Duration of ICU (Mean±SD) | 1.42±3.57 | 2.04±4.22 | 0.2±1 | 1.56±4.24 | 0.14 |
| Mild dyspnea (Admission) | 10(40) | 5(20) | 8(32) | 9(36) | 0.54 |
| Moderate dyspnea (Admission) | 8(32) | 12(48) | 10(40) | 9(36) | 0.74 |
| Sever dyspnea (Admission) | 5(20) | 7(28) | 6(24) | 7(28) | 0.96 |
| Mild dyspnea (Discharge) | 7(28) | 5(20) | 10(40) | 10(40) | 0.39 |
| Moderate dyspnea (Discharge) | 1(4) | 1(4) | 0(0) | 0(0) | 1.00 |
| Sever dyspnea (Discharge) | 0(0) | 0(0) | 0(0) | 0(0) | - |
Table 3. Comparison of Clinical status indicators means between groups at baseline and days 7, 14, 21, and 28
| Variable Mean(SD) |
Groups | P-Value | ||||
| Control (P1-P2) (N=25) |
Intervention-1 NAC-P1 (N=25) |
Intervention-2 NAC-D3 (N=25) |
Intervention-3 D3-P2 (N=25) |
|||
| Glasgow coma scale | Baseline | 14.36(1.65) | 14.68(1.14) | 15(0) | 15(0) | 0.2 |
| Day 7 | 14(2.35) | 14.6(1.38) | 15(0) | 14.92(0.4) | ||
| Day 14 | 14.04(2.44) | 14.54(1.59) | 15(0) | 14.68(1.14) | ||
| Day 21 | 13.58(3.37) | 14.52(1.63) | 15(1.04) | 14.5(1.71) | ||
| Day 28 | 13.58(3.37) | 14.36(1.92) | 15(1.49) | 14.77(1.06) | ||
Respiratory rate |
Baseline | 21.52(2.19) | 21.48(1.88) | 20.77(1.24) | 21(1.63) | 0.23 |
| Day 7 | 22.23(2.36) | 22.43(3.02) | 25.29(15.17) | 23.6(7.85) | ||
| Day 14 | 24.05(5.47) | 22.32(4.23) | 25.17(16.14) | 22.28(7.98) | ||
| Day 21 | 30.43(43.54) | 21.61(2.68) | 24.36(13.25) | 21.35(1.49) | ||
| Day 28 | 21.21(5.58) | 21.21(1.67) | 26.6(24.37) | 21.64(2.62) | ||
| O2 Saturation |
Baseline | 95.56(3.06) | 93.76(5.09) | 93.46(5.55) | 93.48(4.57) | 0.21 |
| Day 7 | 95.96(2.79) | 92.56(5.55) | 94.36(5.15) | 94(3.88) | ||
| Day 14 | 90.83(17.92) | 91.95(7.87) | 93.68(9.53) | 93.6(5.4) | ||
| Day 21 | 94.83(3.34) | 92.91(5.04) | 95.7(9.67) | 93(5.95) | ||
| Day 28 | 92.42(8.35) | 93.05(5.35) | 95.24(2.9) | 91.09(10.09) | ||
| Systolic blood pressure | Baseline | 123.16(16.75) | 118.2(15.34) | 120.44(15.68) | 121.67(19.26) | 0.98 |
| Day 7 | 116.46(21.29) | 117(12.91) | 117.92(19.78) | 114.17(11.76) | ||
| Day 14 | 116.54(17.55) | 100.32(32.93) | 113.33(13.41) | 113.67(19.67) | ||
| Day 21 | 123.17(20.36) | 113.18(13.23) | 114.55(12.24) | 165.65(225.95) | ||
| Day 28 | 115.33(28.02) | 116.05(13.18) | 119.47(15.08) | 115.71(15.68) | ||
| Diastolic blood pressure | Baseline | 71.36(8.73) | 70.2(8.95) | 72.16(11.59) | 70(10.63) | 0.95 |
| Day 7 | 71.25(9.47) | 71(9.79) | 69.58(9.99) | 73.75(10.13) | ||
| Day 14 | 73.33(9.63) | 68.41(12.48) | 68.33(8.16) | 72.21(9.49) | ||
| Day 21 | 71.13(9.18) | 69.09(7.5) | 69.55(7.22) | 70(9.05) | ||
| Day 28 | 70.54(8.75) | 71.58(8.98) | 72.11(7.87) | 72.86(9.56) | ||
Discussion
The outbreak of COVID‐19 infection has posed significant threats to international health. In the absence of treatment for this virus, there is an urgent need to find alternative methods to control the disease. Until now, many proposed protocols and therapeutic methods could not achieve this goal. Thus, this research aimed to investigate the effects of vitamin D3 and NAC administration on clinical status indicators and recovery processes in patients with COVID-19 hospitalization. According to Grant et al.'s review, the human immune system requires particular micronutrients, such as vitamins A, B6, B12, C, D and E. Micronutrients with the strongest evidence for immune support are vitamins C and D and zinc. The evidence reviewed here supports the role of higher 25(OH)D concentrations in reducing the risk of infection and death from acute respiratory tract infections (ARTIs), including those from influenza, coronaviruses, and pneumonia. Vitamin D supplementation also enhances the expression of genes related to antioxidation (glutathione reductase and glutamate–cysteine ligase modifier subunit [5]. Despite the lack of direct evidence of the effect of vitamin D on SARS-CoV-2 infections, it has been suggested that vitamin D supplementation could be a safe and inexpensive intervention for COVID-19 [7]. Other studies suggest that serum vitamin D levels could be valuable in preventing SARS-CoV-2 infection [8]. However, there is not enough evidence on the association between vitamin D levels and COVID-19 severity and mortality [9]. Furthermore, NAC, has been used to loosen thick mucus in the lungs and treat acetaminophen overdose for decades. However, NAC can improve the immune system function, suppress viral replication, and reduce inflammation.
Previous studies have shown that oral NAC treatment significantly reduced the frequency of influenza and pneumonia and it has also been effective in reducing the severity and duration of most symptoms [10]. In addition, Sharaf-Khah et al. have demonstrated that NAC is safe and effective in preventing and delaying ventilator-associated pneumonia in the ICU population [11].
The results of this study showed that there is a significant difference in the length of hospitalization between the four groups. Furthermore, the mean length of hospitalization was increased in the D3-P2 group and this data is parallel to the results in previous studies. Another study represented that among hospitalized patients with COVID-19, a single high dose of vitamin D3 did not significantly reduce the length of hospitalization [12]. Surprisingly, the length of hospitalization was decreased in NAC-D3 which may indicate the role of oral administration of NAC in decreasing the disease symptoms such as pain severity, dyspnea, and cough. According to our results, during the study, the chance of shortness of breath was decreased in all treatment groups. In general, the incidence of shortness of breath in NAC-D3 patients was lower than in the other three groups. Studies have shown that higher concentrations of serum 25(OH)D are significantly associated with a lower extent of lung involvement and better outcomes in patients with COVID-19 [13, 14].
Another clinical factor that was examined was the presence of cough in patients. Throughout the study, the rate of cough in patients in three treatment groups was decreased However, this decrease was more significant in the NAC-P1 group compared to other groups.
The researchers classified the probable effects of NAC as oxidative-regulatory and apoptotic-regulatory roles, antiviral activities, anti-inflammatory roles, and preventive and therapeutic roles in lung disorders. So, based on the different beneficial effects of NAC, it could be administered as a potential adjuvant therapy for COVID-19 [15]. In addition, previous research showed that oral and Intravenous (IV) glutathione (N-acetyl-cysteine) and alpha lipoic acid may represent a novel treatment approach for blocking Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and addressing “cytokine storm syndrome” and respiratory distress in patients with COVID-19 pneumonia [16].
Abnormal liver function has been observed in patients after SARS-CoV2 infection since initial contact with the medical system Which indicates the direct effect of the virus [17] due to the presence of the angiotensin-converting enzyme 2 receptor in the liver [18]. Due to the high prevalence of liver function abnormality in patients with COVID-19, it is important to monitor hepatic enzymes during the course of the disease to prevent additional injury. In our study, there was a significant difference between the mean of SGPT in the four groups and it was found that the SGPT mean in the P1-P2 group was significantly higher than the NAC-P1 group. Previous research showed that N-acetylcysteine can improve liver function in patients with non-alcoholic fatty liver disease [19]. Feketea's study expressed that vitamin D levels could be valuable in predicting severe forms of multisystem inflammatory syndrome in children is a rare complication of COVID-19 [20]. Ilie et al. assessed the potential association between mean levels of vitamin D in 20 European countries and showed that the higher vitamin D levels were associated not only with the lower number of cases diagnosed with COVID-19 but also with lower mortality per million population [21].
In this study, other clinical factors were also assessed that were including pain severity, sore throat, ear pain, joint pain, fever, fatigue, headache, myalgia, taste disorder, night sweats, chest pain, anorexia, diarrhea, stomachache and nausea but no significant difference was observed between the three treatment groups compared to the control group. Furthermore, there was not seen significant difference in evaluating the GCS mean score, RR and BP changes, and SPO2 percentage in treatment groups.
Conclusion
Based on the result, the addition of vitamin D supplements and NAC will improve some outcomes in COVID-19 patients treated but more randomized controlled trial studies are needed in this field. In the present study, no RCT has used NAC+Vitamin D in COVID-19 patients. The main limitation of our study is the small number of patients enrolled. This protocol has been proposed as a preparatory stage before a larger multi-centric trial.
Some clinical and therapeutic features of COVID-19 and its outcome may become more evident over time, especially about non-respiratory symptoms such as gastrointestinal, musculoskeletal, and cutaneous signs and some of them could be targeted by NAC such as severe adverse cutaneous reactions that need more focus in future studies.
Conflict of Interest
The authors declare that there is no conflict of interest.
Acknowledgments
The authors would like to thank the Abadan University of Medical Sciences for funding this project.
References
- Fani M, Zandi M, Ebrahimi S, Soltani S, Abbasi S. The role of miRNAs in COVID-19 disease. Future Virol. 2021; 16(4): 301-306.
- Liu K, Zhang W, Yang Y, Zhang J, Li Y, Chen Y. Respiratory rehabilitation in elderly patients with COVID-19: A randomized controlled study. Complementary Therapies in Clinical Practice 2020; 39: 101166.
- Jeronimo CMP, Farias MEL, Val FFA, Sampaio VS, Alexandre MA, Melo GC, et al. Methylprednisolone as adjunctive therapy for patients hospitalized with coronavirus disease 2019 (COVID-19; Metcovid): A randomized, double-blind, phase iib, placebo-controlled trial. Clin Infect Dis. 2021; 72(9): 373-81.
- Mohammed Hamad MN, Vitamin D supplements improve efficacy of minocycline, n-acetylcysteine and aspirin triple therapy to COVID-19 infection. Saudi J Biomed Res. 2020; 5(4): 59-60.
- Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 2020; 12: 988.
- Calzetta L, Matera MG, Rogliani P, Cazzola M. Multifaceted activity of N-acetyl-l-cysteine in chronic obstructive pulmonary disease. Expert Review of Respiratory Medicine 2018; 12(8): 693-708.
- Zemb P, Bergmanb P, Camargojr CA, Cavalier E, Cormier C, Courbebaisse M, et al. Vitamin D deficiency and the COVID-19 pandemic. Journal Of Global Antimicrobial Resistance 2020; 22: p.133-34.
- Benskin LL. A basic review of the preliminary evidence that COVID-19 risk and severity is increased in vitamin D deficiency. Front Public Health 2020; 8: 513.
- Ali N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. J Infect Public Health. 2020; 13(13): 1373-380.
- Liu Y, Wang M, Luo G, Qian X, Wu C, Zhang Y, et al. Experience of n-acetylcysteine airway management in the successful treatment of one case of critical condition with COVID-19: A case report. Medicine 2020; 99: 42: 22577.
- Sharafkhah M, Abdolrazaghnejad A, Zarinfar N, Mohammadbeigi A, Massoudifar A, Abaszadeh S. Safety andefficacy of n-acetyl-cysteine for prophylaxis of ventilator-associated pneumonia: A randomized, double blind, placebo-controlled clinical trial. Med Gas Res. 2018; 8(1): 19-23.
- Murai IH, Fernandes AL, Sales LP, Pinto A, Goessler K, Duran C, et al. Effect of a single high dose of vitamin D3 on hospital length of stayin patients with moderate to severe COVID-19: A randomized clinical Trial. JAMA 2021; 325( 11): 1053-1060.
- Abrishami A, Dalili N, Mohammadi Torbati P, Asgari R, Arab Ahmadi M, Behnam B, et al. Possible association of vitamin D status with lung involvement and outcome in patients with COVID-19: A retrospective study. European Journal of Nutrition 2021; 60: 2249-257.
- Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia Jf, Bergman P, et al. vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017; 356: I6583.
- Atefi N, Behrangi E, Mozafarpoor S, Seirafianpour F, Peighambari S, Goodarzi A. N-acetylcysteine and coronavirus disease 2019: may it work as a beneficial preventive and adjuvant therapy? A comprehensive review study. J Res Med Sci. 2020; 25: 109.
- Horowitz RI, Freeman PH, Bruzzese J. Efficacy of glutathione therapy in relieving dyspnea associated with COVID-19 pneumonia: A report of 2 cases. Respiratory Medicine Case Reports 2020; 30: 101063.
- Schaefer E, Arvind A, Bloom P, Chung R. Nterrelationship between coronavirus infection and liver disease. Clinical Liver Disease 2020; 15(5): 175-80
- Yang J, Han B, Wang J. COVID-19: Gastrointestinal manifestations and potential fecal–oral transmission. Gastroenterology 2020; 158: 1518-519.
- Khoshbaten M, Aliasgarzadeh A, Masnadi K, Tarzamani M, Farhang S, Babaei H, et al. N Acetylcysteine improves liver function in patients with non-alcoholic fatty liver disease. Hepatitis Monthly 2010; 10(1): 12-16.
- Feketea G, Vlacha V, Bocsan IC, Vassilopoulou E, Stanciu LA, Zdrenghea M. Vitamin D in corona virus disease 2019 (COVID-19) related multisystem inflammatory syndrome in children (MIS-C). Front Immunol. 2021; 12: 648546.
- Cristian Ilie P, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020; 32(7): 1195-198.
Type of Study: Research |
Subject:
General
Received: 2023/01/24 | Accepted: 2023/07/23 | Published: 2023/10/2
Received: 2023/01/24 | Accepted: 2023/07/23 | Published: 2023/10/2
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




