Wed, Feb 4, 2026
[Archive]
Volume 10, Issue 3 (August 2023)
IJML 2023, 10(3): 229-237 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Safaie Z, Ghorbani S, Salavatiha Z, Tavakoli A, Kiani S J, Monavari S H. The Molecular Prevalence of Varicella-Zoster Virus and Human Herpesvirus Types 6 and 7 in Sperm Samples of Infertile Men. IJML 2023; 10 (3) :229-237
URL: http://ijml.ssu.ac.ir/article-1-479-en.html
URL: http://ijml.ssu.ac.ir/article-1-479-en.html
Zahra Safaie 
 , Saied Ghorbani
, Saied Ghorbani 
 , Zahra Salavatiha
, Zahra Salavatiha 
 , Ahmad Tavakoli
, Ahmad Tavakoli 

 , Seyed Jalal Kiani
, Seyed Jalal Kiani 

 , Seyed Hamidreza Monavari *
, Seyed Hamidreza Monavari * 



 , Saied Ghorbani
, Saied Ghorbani 
 , Zahra Salavatiha
, Zahra Salavatiha 
 , Ahmad Tavakoli
, Ahmad Tavakoli 

 , Seyed Jalal Kiani
, Seyed Jalal Kiani 

 , Seyed Hamidreza Monavari *
, Seyed Hamidreza Monavari * 


Department of Medical Virology, Faculty of Medicine, Iran University of Medical Sciences, Tehran, Iran
Full-Text [PDF 205 kb]
(766 Downloads)
| Abstract (HTML) (1086 Views)
Table 2. DNA fragmentation index specifications for patients under study
Table 4. Distribution of cytologic specifications in sperm samples based on sperm specifications and Varicella-Zoster virus (VZV) infection
Table 6. Human herpes virus (HHV)-6 diagnosis based on different sperm morphology
References
[1]. Wortley PM, Hammett TA, Fleming PL. Donor insemination and human immunodeficiency virus transmission. Obstet Gynecol. 1998;91(4): 515-18.
[2]. Berry WR, Gottesfeld RL, Alter HJ, Vierling JM. Transmission of hepatitis B virus by artificial insemination. JAMA. 1987;257(8): 1079-1081.
[3]. Kapranos N, Petrakou E, Anastasiadou C, Kotronias D. Detection of herpes simplex virus, cytomegalovirus, and Epstein-Barr virus in the semen of men attending an infertility clinic. Fertil Steril. 2003;79(Suppl 3): 1566-570.
[4]. Neofytou E, Sourvinos G, Asmarianaki M, Spandidos DA, Makrigiannakis A. Prevalence of human herpes virus types 1-7 in the semen of men attending an infertility clinic and correlation with semen parameters. Fertil Steril. 2009;91(6): 2487-494.
[5]. Naumenko V, Tyulenev Y, Kurilo L, Shileiko L, Sorokina T, Evdokimov V, et al. Detection and quantification of human herpes viruses types 4-6 in sperm samples of patients with fertility disorders and chronic inflammatory urogenital tract diseases. Andrology 2014;2(5): 687-94.
[6]. Chen M, Cai LY, Kanno N, Kato T, Lu J, Jin F, et al. Detection of human herpesviruses (HHVs) in semen of human male infertile patients. J Reprod Dev. 2013;59(5): 457-62.
[7]. World Health Organisation. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th ed, UK: Cambridge university press;1999.
[8]. Skakkebæk NE, Giwercman A, de Kretser D. Pathogenesis and management of male infertility. Lancet. 1994;343(8911):1473-479.
[9]. Garolla A, Pizzol D, Bertoldo A, Menegazzo M, Barzon L, Foresta C. Sperm viral infection and male infertility: Focus on HBV, HCV, HIV, HPV, HSV, HCMV, and AAV. J Reprod Immunol. 2013;100(1): 20-29.
[10]. Oliva A, Spira A, Multigner L. Contribution of environmental factors to the risk of male infertility. Hum Reprod. 2001;16(8): 1768-776.
[11]. Schlehofer JR, Boeke C, Reuland M, Eggert-Kruse W. Presence of DNA of adeno-associated virus in subfertile couples, but no association with fertility factors. Hum Reprod. 2012; 27(3):770-78.
[12]. Bezold G, Politch JA, Kiviat NB, Kuypers JM, Wolff H, Anderson DJ. Prevalence of sexually transmissible pathogens in semen from asymptomatic male infertility patients with and without leukocytospermia. Fertil Steril. 2007;87(5): 1087-1097.
[13]. Kaspersen MD, Höllsberg P. Seminal shedding of human herpesviruses. Virol J. 2013;10(1): 1-8.
[14]. Kaspersen MD, Larsen PB, Kofod-Olsen E, Fedder J, Bonde J, Höllsberg P. Human Herpesvirus-6A/B binds to spermatozoa acrosome and is the most prevalent herpesvirus in semen from sperm donors. PLoS One. 2012;7(11): 48810.
[15]. Wyatt LS, Rodriguez WJ, Balachandran N, Frenkel N. Human herpesvirus 7: antigenic properties and prevalence in children and adults. J Virol. 1991;65(11): 6260-265.
[16]. Caserta MT, Hall CB, Schnabel K, Lofthus G, McDermott MP. Human herpesvirus (HHV)-6 and HHV-7 infections in pregnant women. J Infect Dis. 2007;196(9): 1296-303.
[17]. Pallier C, Tebourbi L, Chopineau-Proust S, Schoevaert D, Nordmann P, Testart J, et al. Herpesvirus, cytomegalovirus, human sperm and assisted fertilization. Hum Reprod. 2002;17(5): 1281-287.
[18]. Volpi A. Severe complications of herpes zoster. Herpes. 2007;14 (Suppl 2): 35-9.
[19]. Bezold G, Schuster-Grusser A, Lange M, Gall H, Wolff H, Peter RU. Prevalence of human herpesvirus types 1-8 in the semen of infertility patients and correlation with semen parameters. Fertil Steril. 2001;76(2): 416-18.
Full-Text: (1406 Views)
Introduction
Sexually transmitted pathogens are a major global cause of infertility, and many of them have been associated with deadly or incurable diseases [1, 2]. Studies showed that men could harbor sexually transmitted pathogens in their genital tract for a long time. Furthermore, the possible role of members of the Herpesviridae family in causing infertility has been studied [3–5]. Previous studies showed that 15%-25% of couples struggle with infertility problems. In 20%-50% of the cases, infertility is attributable to males [4, 6]. Various studies have shown that a wide range of issues affect fertility.
Varicocele is the most common male infertility-related disease, accounting for 35% of the cases. In addition, other less prevalent factors that contribute to infertility include endocrine gland dysfunction, genital tract disorders, antibodies against sperm, drugs with specific toxicity, infections, ejaculation problems, and reduction in sperm production. Moreover, in 50% of men's infertility cases, the reason was unknown, referred to as idiopathic infertility [4, 5, 7, 8].
Sexually transmitted infections, such as bacterial and viral infections, are the most important causes of idiopathic infertility. Viral infections can cause infertility either directly or indirectly [5, 9, 10]. The effect on the genital tract epithelial cells is an instance of the direct impact of the virus on spermatogenesis, and the immune system is an example of the indirect effect [4]. In sperm examinations, quantities, including shape, mobility, and sperm counts, are affected by viral infections [11]. Several studies have detected viral infection in men with infertility symptoms, which decreased sperm quality [9,12].
Varicella-Zoster virus (VZV) is among the Alphaherpesviruses that infects most people during childhood. Primary infection during childhood is benign and self-limiting and causes chickenpox, whereas its reactivation during adulthood leads to shingles or herpes zoster. Studies on the effects of herpesviruses on infertility have been mainly focused on herpes simplex (HSV) and Epstein-Barr virus (EBV). In the meantime, several molecular studies showed the presence of the VZV genome in sperm samples [4].
Human herpes virus types (HHV)-6 is closely correlated with human cytomegalovirus (HCMV). HHV-6 infections commonly occur in the early stages of life, showing symptoms of exanthema subitum. Consequently, most adults are seropositive for this virus [13]. There are limited studies concerning the prevalence of HHV-6 infection among infertility patients; therefore, more research is required in this area. The reported prevalence in the general population ranges from 0.4% to 66% [5]. Although HHV-6 cannot alter sperm-related parameters directly, it showed a significant correlation with sperm acrosome. The ability of HHV-6 to attach to acrosomes indicates that this virus can enter the uterus through sperm [14]. Overall, no significant results or sufficient information can be observed on the role of HHV-6 in sperm performance or other aspects of the reproduction system. Also, the potential of HHV-6 to enter the uterus endometrium requires epidemiologic research. HHV-7 has a close genetic correlation with HHV-6. Infection by HHV-7 typically happens before age five, and approximately 96% of adults are seropositive for this virus. Primary infection with HHV-7 is usually asymptomatic. However, it can also cause symptoms of exanthema subitum [15]. Similar to HHV-6, only a few studies have investigated the prevalence of HHV-7 in sperm samples. Overall, the prevalence of HHV-7 was reported between 0.4% and 6% in pregnant women, which is lower than HHV-6. There has been no correlation between HHV-7 and sperm parameters and pathogenesis in infertility. Although currently, there is no direct evidence to support the role of these viruses in infertility and reproduction disorders, it is noteworthy that these viruses can be sexually transmitted and latent in cells and reactivate later. Therefore, a significant proportion of herpesvirus infection is still unknown regarding the transmission and replication of these viruses [16]. Since 50% of infertility cases in men have unknown etiology, finding a reason for this is vital. Regarding the high prevalence of herpesviruses in human populations worldwide and their transmission through sexual activities and being detected in sexual discharge in several studies, this study aimed to investigate the prevalence of these viruses in males with fertility problems.
Materials and Methods
Study population and sample preparation
Patients in this cross-sectional research were asymptomatic infertile males admitted to infertility institutions associated with the Iran University of Medical Sciences. The Ethical Committee of the Iran University of Medical Sciences approved this research (IR.IUMS. FMD.REC.1398.452). Additionally, enrolled patients provided written consent. Semen samples were gathered in tubes and kept at -20 °C for further examination. The sperm DNA fragmentation assay kit (SDFA, ACECR, Academic Center for Education, Culture, and Research, Tehran, Iran) procedures were used to calculate the DNA fragmentation index (DFI) according to the manufacturer's protocol. Other sperm analysis factors, such as motility, count, and morphology, were determined using World Health Organization (WHO) guidelines [7].
Nucleic acid extraction
According to the manufacturer's methodology, a tissue genomic DNA extraction kit (Favorgene, Taiwan) was used to extract DNA from samples. The amount of isolated DNA was measured using the NanoDrop ND-1000® spectrophotometry (Thermo Fisher Scientific Inc., USA). A conventional polymerase chain reaction (PCR) assay for the beta-globin gene was also done to approve the human spermatozoid genome isolation processes in samples.
PCR for beta-globin gene
A conventional PCR test for the beta-globin gene was carried out in the Bio-Rad thermocycler (T100TM Thermal Cycler) under the following thermal conditions: Initial denaturation was performed for 10 minutes at 95 °C, followed by 35 cycles of 1 minute at 94 °C, 30 seconds at 55 °C, 40 seconds at 72 °C, and a final extension step of 10 minutes at 72 °C. Gel electrophoresis in 1.5% agarose and ethidium bromide staining was employed to visualize PCR products. The following was the reaction mixture: 12.5 µl 2X Amplicon master mix (Amplicon, Denmark), 25-50 ng template DNA, 1 µl of each primer (10 pmol/µl), and sterilized deionized water to make a total of 25 µl for each test.
PCR assay for HHV-6, HHV-7, and VZV
PCR assay was used to investigate the presence of HHV-6 and HHV-7 DNA in specimens using specific primers (Table 1). For each PCR reaction, 20 µl PCR reaction volume was used, which contained 1 µl of extracted DNA from each sample, 10 μl master mix (Super PCR Mix, Yekta Tajhiz Azma, Iran), 1 μL of each primer (forward and reverse) (10 pmol/μl), and 7 μL of distilled water. PCR was performed using a Bio-Rad thermocycler with the following program: 10 min at 95 °C as initial denaturation, then 35 cycles of 1 min at 94 °C, 20 s at 60 °C as the annealing step, and 30 s at 72 °C for extension.
A pair of primers were designed to detect the orf 38 gene of the VZV. For each PCR reaction, 25 µl PCR reaction volume was used, which contained 25-50 ng of extracted DNA from each sample, 12.5 μl master mix (Super PCR Mix, Yekta Tajhiz Azma, Iran), 1 μL of each primer (forward and reverse) (15 pmol/μl), and distilled water. PCR was performed using a Bio-Rad thermocycler with the following program: 1 cycle for 2 min at 95° C, followed by 45 cycles for 1 min at 94 °C, 1 min at 56.7 °C as the annealing step. Finally, it remained at 72 °C for 5 min to produce DNA strands at complete length. For PCR product visualization, gel electrophoresis in 1% agarose was performed to detect 670 bp in the PCR product.
Statistical analysis
SPSS version 22 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis, and Fisher's exact and chi-square tests were used to evaluate the results. In this investigation, P-values less than 0.05 were deemed statistically significant.
Results
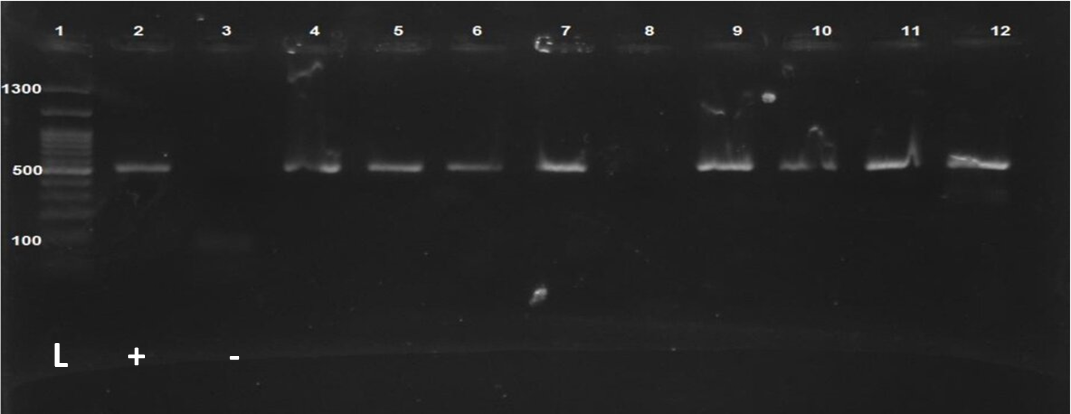
Eighty-two men with the mean age of 37.3 ± 6.1 years participated in this study. The mean sperm count in each milliliter was 21.5 ± 13 milion in each milimetr sperm, according to sperm parameter analysis. Furthermore, the mean sperm motility was 33.6 ± 20.1%. The morphology of the sperms was found to be normal in 49.2% of the patients. Furthermore, the mean DFI was 27.2 ± 1.2, and 26.8% of the recruited patients had poor DFI (Table 2). The beta-globin gene PCR results demonstrated effective extraction of genomic DNA from sperm samples (Fig. 1).
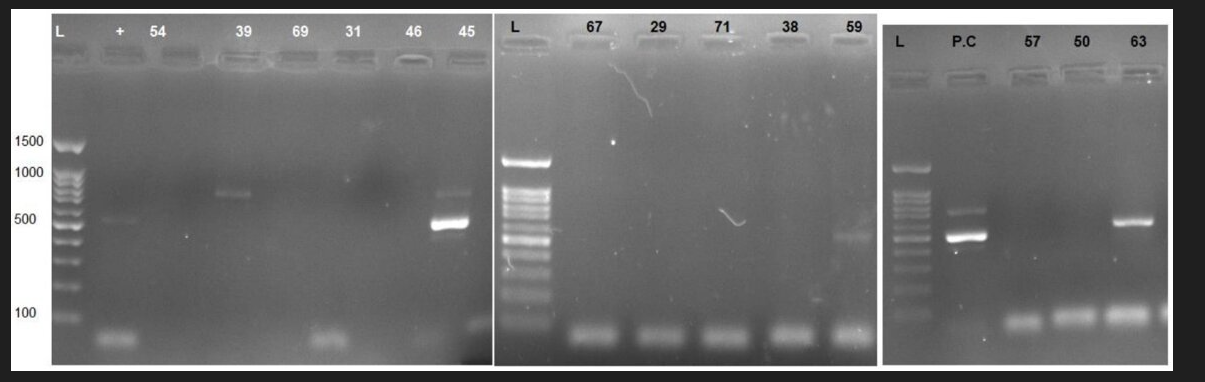
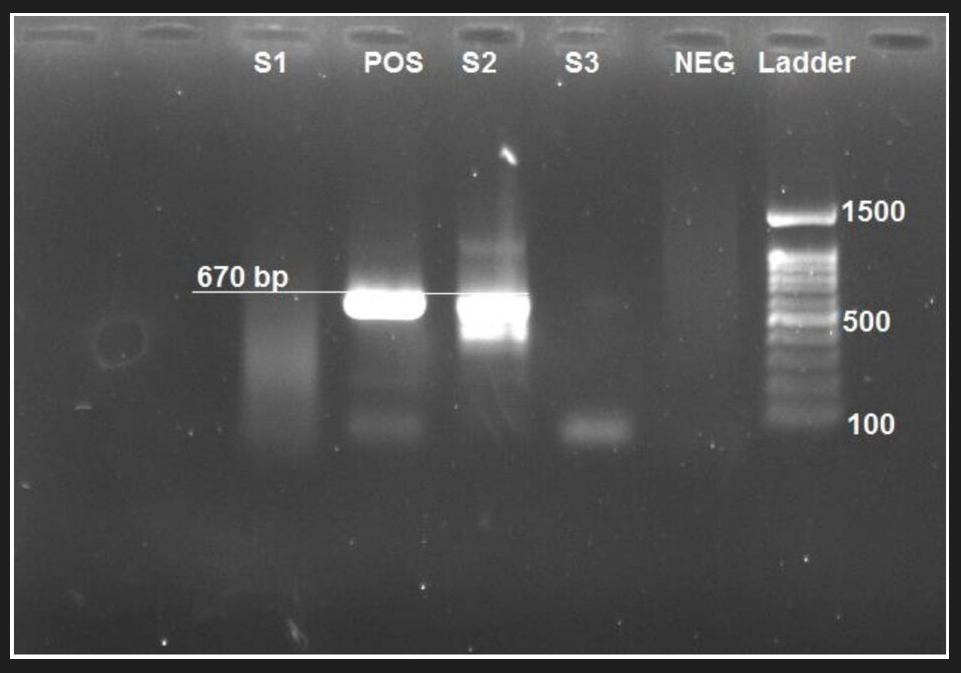
The results of the PCR assay showed that 6 out of 82 samples (7.3%) were positive for HHV-6 (Fig. 2). However, there was no positive case for HHV-7. The mean age of HHV-6-positive cases was 35.7 ± 8.3 years, which was lower than HHV-6-negative cases, and the difference was not statistically significant (Table 3). The results of the PCR assay showed that 4 out of 82 samples (4.9%) from infertile males were positive for VZV (Fig. 3). The mean age of VZV-positive cases was 34.3±4.7 years, which was lower than VZV-negative cases. However, this difference was not statistically significant. Concerning DFI, spermatozoid count, and sperm motility, there was no significant difference between VZV-positive and negative cases (Table 4).
In Table 5, HHV-6 prevalence in different DFIs is reported. The results of the chi-square test indicated that there is no significant difference in HHV-6 prevalence among different DFIs (p =0.364). The morphology index of four and more than four shows that the sperms are normal. Morphology assessment of the sperm showed that 48.8% of samples had normal morphology, among which HHV-6 prevalence was 35% (Table 6). The results of the Fisher test showed that HHV-6 infection has no significant correlation with sperm morphology (p = 0.128).
Varicocele is the most common male infertility-related disease, accounting for 35% of the cases. In addition, other less prevalent factors that contribute to infertility include endocrine gland dysfunction, genital tract disorders, antibodies against sperm, drugs with specific toxicity, infections, ejaculation problems, and reduction in sperm production. Moreover, in 50% of men's infertility cases, the reason was unknown, referred to as idiopathic infertility [4, 5, 7, 8].
Sexually transmitted infections, such as bacterial and viral infections, are the most important causes of idiopathic infertility. Viral infections can cause infertility either directly or indirectly [5, 9, 10]. The effect on the genital tract epithelial cells is an instance of the direct impact of the virus on spermatogenesis, and the immune system is an example of the indirect effect [4]. In sperm examinations, quantities, including shape, mobility, and sperm counts, are affected by viral infections [11]. Several studies have detected viral infection in men with infertility symptoms, which decreased sperm quality [9,12].
Varicella-Zoster virus (VZV) is among the Alphaherpesviruses that infects most people during childhood. Primary infection during childhood is benign and self-limiting and causes chickenpox, whereas its reactivation during adulthood leads to shingles or herpes zoster. Studies on the effects of herpesviruses on infertility have been mainly focused on herpes simplex (HSV) and Epstein-Barr virus (EBV). In the meantime, several molecular studies showed the presence of the VZV genome in sperm samples [4].
Human herpes virus types (HHV)-6 is closely correlated with human cytomegalovirus (HCMV). HHV-6 infections commonly occur in the early stages of life, showing symptoms of exanthema subitum. Consequently, most adults are seropositive for this virus [13]. There are limited studies concerning the prevalence of HHV-6 infection among infertility patients; therefore, more research is required in this area. The reported prevalence in the general population ranges from 0.4% to 66% [5]. Although HHV-6 cannot alter sperm-related parameters directly, it showed a significant correlation with sperm acrosome. The ability of HHV-6 to attach to acrosomes indicates that this virus can enter the uterus through sperm [14]. Overall, no significant results or sufficient information can be observed on the role of HHV-6 in sperm performance or other aspects of the reproduction system. Also, the potential of HHV-6 to enter the uterus endometrium requires epidemiologic research. HHV-7 has a close genetic correlation with HHV-6. Infection by HHV-7 typically happens before age five, and approximately 96% of adults are seropositive for this virus. Primary infection with HHV-7 is usually asymptomatic. However, it can also cause symptoms of exanthema subitum [15]. Similar to HHV-6, only a few studies have investigated the prevalence of HHV-7 in sperm samples. Overall, the prevalence of HHV-7 was reported between 0.4% and 6% in pregnant women, which is lower than HHV-6. There has been no correlation between HHV-7 and sperm parameters and pathogenesis in infertility. Although currently, there is no direct evidence to support the role of these viruses in infertility and reproduction disorders, it is noteworthy that these viruses can be sexually transmitted and latent in cells and reactivate later. Therefore, a significant proportion of herpesvirus infection is still unknown regarding the transmission and replication of these viruses [16]. Since 50% of infertility cases in men have unknown etiology, finding a reason for this is vital. Regarding the high prevalence of herpesviruses in human populations worldwide and their transmission through sexual activities and being detected in sexual discharge in several studies, this study aimed to investigate the prevalence of these viruses in males with fertility problems.
Materials and Methods
Study population and sample preparation
Patients in this cross-sectional research were asymptomatic infertile males admitted to infertility institutions associated with the Iran University of Medical Sciences. The Ethical Committee of the Iran University of Medical Sciences approved this research (IR.IUMS. FMD.REC.1398.452). Additionally, enrolled patients provided written consent. Semen samples were gathered in tubes and kept at -20 °C for further examination. The sperm DNA fragmentation assay kit (SDFA, ACECR, Academic Center for Education, Culture, and Research, Tehran, Iran) procedures were used to calculate the DNA fragmentation index (DFI) according to the manufacturer's protocol. Other sperm analysis factors, such as motility, count, and morphology, were determined using World Health Organization (WHO) guidelines [7].
Nucleic acid extraction
According to the manufacturer's methodology, a tissue genomic DNA extraction kit (Favorgene, Taiwan) was used to extract DNA from samples. The amount of isolated DNA was measured using the NanoDrop ND-1000® spectrophotometry (Thermo Fisher Scientific Inc., USA). A conventional polymerase chain reaction (PCR) assay for the beta-globin gene was also done to approve the human spermatozoid genome isolation processes in samples.
PCR for beta-globin gene
A conventional PCR test for the beta-globin gene was carried out in the Bio-Rad thermocycler (T100TM Thermal Cycler) under the following thermal conditions: Initial denaturation was performed for 10 minutes at 95 °C, followed by 35 cycles of 1 minute at 94 °C, 30 seconds at 55 °C, 40 seconds at 72 °C, and a final extension step of 10 minutes at 72 °C. Gel electrophoresis in 1.5% agarose and ethidium bromide staining was employed to visualize PCR products. The following was the reaction mixture: 12.5 µl 2X Amplicon master mix (Amplicon, Denmark), 25-50 ng template DNA, 1 µl of each primer (10 pmol/µl), and sterilized deionized water to make a total of 25 µl for each test.
PCR assay for HHV-6, HHV-7, and VZV
PCR assay was used to investigate the presence of HHV-6 and HHV-7 DNA in specimens using specific primers (Table 1). For each PCR reaction, 20 µl PCR reaction volume was used, which contained 1 µl of extracted DNA from each sample, 10 μl master mix (Super PCR Mix, Yekta Tajhiz Azma, Iran), 1 μL of each primer (forward and reverse) (10 pmol/μl), and 7 μL of distilled water. PCR was performed using a Bio-Rad thermocycler with the following program: 10 min at 95 °C as initial denaturation, then 35 cycles of 1 min at 94 °C, 20 s at 60 °C as the annealing step, and 30 s at 72 °C for extension.
A pair of primers were designed to detect the orf 38 gene of the VZV. For each PCR reaction, 25 µl PCR reaction volume was used, which contained 25-50 ng of extracted DNA from each sample, 12.5 μl master mix (Super PCR Mix, Yekta Tajhiz Azma, Iran), 1 μL of each primer (forward and reverse) (15 pmol/μl), and distilled water. PCR was performed using a Bio-Rad thermocycler with the following program: 1 cycle for 2 min at 95° C, followed by 45 cycles for 1 min at 94 °C, 1 min at 56.7 °C as the annealing step. Finally, it remained at 72 °C for 5 min to produce DNA strands at complete length. For PCR product visualization, gel electrophoresis in 1% agarose was performed to detect 670 bp in the PCR product.
Statistical analysis
SPSS version 22 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis, and Fisher's exact and chi-square tests were used to evaluate the results. In this investigation, P-values less than 0.05 were deemed statistically significant.
Results
Eighty-two men with the mean age of 37.3 ± 6.1 years participated in this study. The mean sperm count in each milliliter was 21.5 ± 13 milion in each milimetr sperm, according to sperm parameter analysis. Furthermore, the mean sperm motility was 33.6 ± 20.1%. The morphology of the sperms was found to be normal in 49.2% of the patients. Furthermore, the mean DFI was 27.2 ± 1.2, and 26.8% of the recruited patients had poor DFI (Table 2). The beta-globin gene PCR results demonstrated effective extraction of genomic DNA from sperm samples (Fig. 1).
The results of the PCR assay showed that 6 out of 82 samples (7.3%) were positive for HHV-6 (Fig. 2). However, there was no positive case for HHV-7. The mean age of HHV-6-positive cases was 35.7 ± 8.3 years, which was lower than HHV-6-negative cases, and the difference was not statistically significant (Table 3). The results of the PCR assay showed that 4 out of 82 samples (4.9%) from infertile males were positive for VZV (Fig. 3). The mean age of VZV-positive cases was 34.3±4.7 years, which was lower than VZV-negative cases. However, this difference was not statistically significant. Concerning DFI, spermatozoid count, and sperm motility, there was no significant difference between VZV-positive and negative cases (Table 4).
In Table 5, HHV-6 prevalence in different DFIs is reported. The results of the chi-square test indicated that there is no significant difference in HHV-6 prevalence among different DFIs (p =0.364). The morphology index of four and more than four shows that the sperms are normal. Morphology assessment of the sperm showed that 48.8% of samples had normal morphology, among which HHV-6 prevalence was 35% (Table 6). The results of the Fisher test showed that HHV-6 infection has no significant correlation with sperm morphology (p = 0.128).
Table 1. List of primers used in this study
| Primer | Sequences | Product size |
| Beta 1 | TCAACCCTACAGTCACCCAT | 500 |
| Beta 2 | CTAACAATTACGAACAGCAATGAG | |
| H6 F | ACAGTGATGTTGGGTCGAATC | 539 |
| H6 R | GAAAACGCCATTGATCAAGAA | |
| H7 F | AAATACGGCGCAACGAATAG | 694 |
| H7 R | TGAATGTCTAAGCCAAAATGGA | |
| H3 F | CTGGGGGAGTGGTAAGAACA | 670 |
| H3 R | AGACGCGCTTAACGGAAGTA |
Table 2. DNA fragmentation index specifications for patients under study
| Sperm chromatin dispersion | Chance of conception | Number | Frequency |
| DFI < 15 | Excellent | 15 | 18.4 |
| 15 - 25 | Good | 32 | 39 |
| 25 - 30 | Fair | 13 | 15.8 |
| DNA fragmentation index > 30 | Poor | 22 | 26.8 |
Table 3. Human herpes virus (HHV)-6 prevalence in sperm samples based on sperm specifications
| Mean age (year) | DNA fragmentation index (%) | Count (million/ml) |
Motility (%) | |
| Positive HHV-6 | 35.7 ± 8.3 | 24.5 ± 1.3 | 21.8 ± 12.5 | 29.1 ± 2.1 |
| Negative HHV-6 | 37.6 ± 5.3 | 26.8 ± 1.3 | 28.7 ± 9.1 | 48.5 ± 1.1 |
| P-value | 0.523 | 0.354 | 0.114 | 0.151 |
Table 4. Distribution of cytologic specifications in sperm samples based on sperm specifications and Varicella-Zoster virus (VZV) infection
| Mean age (year) | DNA fragmentation index (%) | Count (million/ml) | Motility (%) | |
| Positive VZV | 34.3 ± 4.7 | 22.5 ± 3.3 | 26.2 ± 11.2 | 35.1 ± 5.8 |
| Negative VZV | 37.2 ± 6.8 | 24.8 ± 7.2 | 28.3 ± 3.7 | 41.6 ± 5.1 |
| P-value | 0.116 | 0.325 | 0.263 | 0.258 |
Table 5. Human herpes virus types 6 prevalence based on different DFIs
| DFI category | Negative N (%) |
Positive N (%) |
Total |
| Excellent. Lower 15 | 15 (100) | 0 | 15 |
| Good. 15-25 | 31 (96.9) | 1 (3.1) | 32 |
| Fair. 25-30 | 11 (84.6) | 2 (15.4) | 13 |
| Poor. More 30 | 19 (86.4) | 3 (13.6) | 22 |
| Total | 76 (92.7) | 6 (7.3) | 82 |
DFI= DNA fragmentation index
Table 6. Human herpes virus (HHV)-6 diagnosis based on different sperm morphology
| Morphology grade | Negative N (%) |
Positive N (%) |
Total |
| 4 and more than 4 | 40 (95.3) | 2 (4.7) | 42 |
| Lower than 4 | 36 (90) | 4 (10) | 40 |
| Total | 76 (92.7) | 6 (7.3) | 82 |

Fig. 1. Observation of PCR products of the beta-globin gene. Lane 1: 1300 bp DNA ladder; Lane 2: Positive control; Lane 3: Negative control; Other lanes (4-12): Extracted samples

Fig. 2. Analysis of the image of electrophoresis gel that shows the proliferation of the target HHV-6 gene.

Fig. 3. Gel doc obtained from PCR showing orf 63 of the chickenpox virus in weight of 670 per bases.
Pos= positive control; and Neg= negative control; S= sample
Discussion
Despite the advances in reproduction technology, infertility is still a societal problem, and 20% of couples deal with it. 40-50% of infertility causes are related to men [4, 6]. Significantly, the cause of infertility has not been found in more than 50% of men [5, 8]. One of the reasons for infertility in men is sexually transmitted diseases without clinical symptoms. New studies have discussed the possibility of the role of Sexually transmitted diseases (STDs) in infertility. These STDs can cause fertility problems with different mechanisms, including spermatogenesis disorder, sperm functionality disorder, and seminal tract blockage. In addition, other less prevalent factors contributing to infertility include endocrine gland dysfunction, genital tract disorders, antibodies against sperm, drug-specific toxicity (e.g., drugs for chemotherapy and radiotherapy), STDs, ejaculation problems, and reduction in sperm production. Moreover, in 50% of men's infertility cases, the cause was unknown and was referred to as idiopathic infertility [4,7]. Various viruses can infect the genital system; their genomes are extracted from semen. Regarding the high prevalence of herpesviruses in society and their transmission through sexual activities and being detected in sexual discharge in several studies, a decision to study the prevalence of HHV-6, HHV-7, and VZV in individuals with infertility problems has been made.
In this study, 82 samples from patients were studied. The results of the PCR assay showed that 6 out of 82 samples (7.3%) were infected with HHV-6. Also, the HHV-7 genome was not detected in any of the samples. In 2013, Chen et al. studied the correlation between infertility and the prevalence of the Herpesviridae family. PCR detected viral DNA in 59.7 (39%) of 153 semen samples for at least one member of the Herpesviridae family. The results from the detection of viral DNA for all 153 samples are available as follows: HHV-6= 2%, HSV= 25%, CMV= 22%, and EBV= 4% [6]. Also, VZV was detected in 4 (4.9%) of 82 samples. In 2009, Neofytou et al. reported a prevalence of 83% for herpesviruses in sperm samples. PCR detected viral DNA for one member of the Herpesviridae family. The results from the detection of viral DNA are as follows: HSV=2.5%, HHV-6= 70%, CMV= 62%, EBV= 45%, HHV-7= 0%, and VZV= 1.2% [4]. Also, the results showed that 48.8% of samples did not have normal morphology, and the prevalence of HHV-6 was 35%. The results of the Chi-square showed that there is no significant difference in HHV-6 prevalence between different DFIs. Pallier et al. studied the effect of infection with herpesviruses on sperm motility. The results of this study did not show a significant correlation between infection with the Herpesviridae family and a reduction in sperm motility patterns [17]. In the study performed by Neofytou et al., the prevalence of chicken pox virus in sperm samples from patients was reported [4]. VZV can affect male infertility in two ways: 1: High fever and disease can temporarily decrease semen. Normally, semen production returns to normal within 90 days after the fever has stopped. 2: Infectious diseases such as chicken pox can cause orchitis. It can result in atrophy and infertility [18]. This study detected VZV DNA in 1 (1.2%) of 80 natural samples and 3 (3.2%) of 92 unnatural samples. The fact that there was a statistically significant correlation between VZV and teratospermia shows a need for more research into its effects on sperm morphology, and such results should be carefully evaluated. In the study performed by Bezold et al., the prevalence of chicken pox virus, HHV-6, and HHV-7 was reported in sperm samples from patients. The prevalence of HHV-6 was 4%, and HHV-7 was 0.4%. The result for VZV and HHV-8 was negative [19].
There were some limitations in our study. First, the sample size was small. A larger sample size might give better information about the role of the mentioned viruses. Second, all samples were obtained from the infertility centers in Tehran. Sampling from infertility centers and hospitals in different geographical regions of Iran can be useful for a more accurate diagnosis of viruses. Further studies with larger sample sizes and sampling from various regions within the country are recommended.
Conclusion
Our findings revealed that the prevalence of HHV-6 and VZV were 7.3% and 4.9%, respectively. Also, HHV-7 was not detected in sperm samples. Although the prevalence of herpesviruses seems low, further studies with larger sample sizes and sampling from various regions within the country are required to obtain better results.
Conflict of Interest
The authors have no conflict of interest.
Acknowledgments
The Iran University of Medical Sciences financially supported this study, Grants Number: 98-2-4-14394
Despite the advances in reproduction technology, infertility is still a societal problem, and 20% of couples deal with it. 40-50% of infertility causes are related to men [4, 6]. Significantly, the cause of infertility has not been found in more than 50% of men [5, 8]. One of the reasons for infertility in men is sexually transmitted diseases without clinical symptoms. New studies have discussed the possibility of the role of Sexually transmitted diseases (STDs) in infertility. These STDs can cause fertility problems with different mechanisms, including spermatogenesis disorder, sperm functionality disorder, and seminal tract blockage. In addition, other less prevalent factors contributing to infertility include endocrine gland dysfunction, genital tract disorders, antibodies against sperm, drug-specific toxicity (e.g., drugs for chemotherapy and radiotherapy), STDs, ejaculation problems, and reduction in sperm production. Moreover, in 50% of men's infertility cases, the cause was unknown and was referred to as idiopathic infertility [4,7]. Various viruses can infect the genital system; their genomes are extracted from semen. Regarding the high prevalence of herpesviruses in society and their transmission through sexual activities and being detected in sexual discharge in several studies, a decision to study the prevalence of HHV-6, HHV-7, and VZV in individuals with infertility problems has been made.
In this study, 82 samples from patients were studied. The results of the PCR assay showed that 6 out of 82 samples (7.3%) were infected with HHV-6. Also, the HHV-7 genome was not detected in any of the samples. In 2013, Chen et al. studied the correlation between infertility and the prevalence of the Herpesviridae family. PCR detected viral DNA in 59.7 (39%) of 153 semen samples for at least one member of the Herpesviridae family. The results from the detection of viral DNA for all 153 samples are available as follows: HHV-6= 2%, HSV= 25%, CMV= 22%, and EBV= 4% [6]. Also, VZV was detected in 4 (4.9%) of 82 samples. In 2009, Neofytou et al. reported a prevalence of 83% for herpesviruses in sperm samples. PCR detected viral DNA for one member of the Herpesviridae family. The results from the detection of viral DNA are as follows: HSV=2.5%, HHV-6= 70%, CMV= 62%, EBV= 45%, HHV-7= 0%, and VZV= 1.2% [4]. Also, the results showed that 48.8% of samples did not have normal morphology, and the prevalence of HHV-6 was 35%. The results of the Chi-square showed that there is no significant difference in HHV-6 prevalence between different DFIs. Pallier et al. studied the effect of infection with herpesviruses on sperm motility. The results of this study did not show a significant correlation between infection with the Herpesviridae family and a reduction in sperm motility patterns [17]. In the study performed by Neofytou et al., the prevalence of chicken pox virus in sperm samples from patients was reported [4]. VZV can affect male infertility in two ways: 1: High fever and disease can temporarily decrease semen. Normally, semen production returns to normal within 90 days after the fever has stopped. 2: Infectious diseases such as chicken pox can cause orchitis. It can result in atrophy and infertility [18]. This study detected VZV DNA in 1 (1.2%) of 80 natural samples and 3 (3.2%) of 92 unnatural samples. The fact that there was a statistically significant correlation between VZV and teratospermia shows a need for more research into its effects on sperm morphology, and such results should be carefully evaluated. In the study performed by Bezold et al., the prevalence of chicken pox virus, HHV-6, and HHV-7 was reported in sperm samples from patients. The prevalence of HHV-6 was 4%, and HHV-7 was 0.4%. The result for VZV and HHV-8 was negative [19].
There were some limitations in our study. First, the sample size was small. A larger sample size might give better information about the role of the mentioned viruses. Second, all samples were obtained from the infertility centers in Tehran. Sampling from infertility centers and hospitals in different geographical regions of Iran can be useful for a more accurate diagnosis of viruses. Further studies with larger sample sizes and sampling from various regions within the country are recommended.
Conclusion
Our findings revealed that the prevalence of HHV-6 and VZV were 7.3% and 4.9%, respectively. Also, HHV-7 was not detected in sperm samples. Although the prevalence of herpesviruses seems low, further studies with larger sample sizes and sampling from various regions within the country are required to obtain better results.
Conflict of Interest
The authors have no conflict of interest.
Acknowledgments
The Iran University of Medical Sciences financially supported this study, Grants Number: 98-2-4-14394
References
[1]. Wortley PM, Hammett TA, Fleming PL. Donor insemination and human immunodeficiency virus transmission. Obstet Gynecol. 1998;91(4): 515-18.
[2]. Berry WR, Gottesfeld RL, Alter HJ, Vierling JM. Transmission of hepatitis B virus by artificial insemination. JAMA. 1987;257(8): 1079-1081.
[3]. Kapranos N, Petrakou E, Anastasiadou C, Kotronias D. Detection of herpes simplex virus, cytomegalovirus, and Epstein-Barr virus in the semen of men attending an infertility clinic. Fertil Steril. 2003;79(Suppl 3): 1566-570.
[4]. Neofytou E, Sourvinos G, Asmarianaki M, Spandidos DA, Makrigiannakis A. Prevalence of human herpes virus types 1-7 in the semen of men attending an infertility clinic and correlation with semen parameters. Fertil Steril. 2009;91(6): 2487-494.
[5]. Naumenko V, Tyulenev Y, Kurilo L, Shileiko L, Sorokina T, Evdokimov V, et al. Detection and quantification of human herpes viruses types 4-6 in sperm samples of patients with fertility disorders and chronic inflammatory urogenital tract diseases. Andrology 2014;2(5): 687-94.
[6]. Chen M, Cai LY, Kanno N, Kato T, Lu J, Jin F, et al. Detection of human herpesviruses (HHVs) in semen of human male infertile patients. J Reprod Dev. 2013;59(5): 457-62.
[7]. World Health Organisation. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th ed, UK: Cambridge university press;1999.
[8]. Skakkebæk NE, Giwercman A, de Kretser D. Pathogenesis and management of male infertility. Lancet. 1994;343(8911):1473-479.
[9]. Garolla A, Pizzol D, Bertoldo A, Menegazzo M, Barzon L, Foresta C. Sperm viral infection and male infertility: Focus on HBV, HCV, HIV, HPV, HSV, HCMV, and AAV. J Reprod Immunol. 2013;100(1): 20-29.
[10]. Oliva A, Spira A, Multigner L. Contribution of environmental factors to the risk of male infertility. Hum Reprod. 2001;16(8): 1768-776.
[11]. Schlehofer JR, Boeke C, Reuland M, Eggert-Kruse W. Presence of DNA of adeno-associated virus in subfertile couples, but no association with fertility factors. Hum Reprod. 2012; 27(3):770-78.
[12]. Bezold G, Politch JA, Kiviat NB, Kuypers JM, Wolff H, Anderson DJ. Prevalence of sexually transmissible pathogens in semen from asymptomatic male infertility patients with and without leukocytospermia. Fertil Steril. 2007;87(5): 1087-1097.
[13]. Kaspersen MD, Höllsberg P. Seminal shedding of human herpesviruses. Virol J. 2013;10(1): 1-8.
[14]. Kaspersen MD, Larsen PB, Kofod-Olsen E, Fedder J, Bonde J, Höllsberg P. Human Herpesvirus-6A/B binds to spermatozoa acrosome and is the most prevalent herpesvirus in semen from sperm donors. PLoS One. 2012;7(11): 48810.
[15]. Wyatt LS, Rodriguez WJ, Balachandran N, Frenkel N. Human herpesvirus 7: antigenic properties and prevalence in children and adults. J Virol. 1991;65(11): 6260-265.
[16]. Caserta MT, Hall CB, Schnabel K, Lofthus G, McDermott MP. Human herpesvirus (HHV)-6 and HHV-7 infections in pregnant women. J Infect Dis. 2007;196(9): 1296-303.
[17]. Pallier C, Tebourbi L, Chopineau-Proust S, Schoevaert D, Nordmann P, Testart J, et al. Herpesvirus, cytomegalovirus, human sperm and assisted fertilization. Hum Reprod. 2002;17(5): 1281-287.
[18]. Volpi A. Severe complications of herpes zoster. Herpes. 2007;14 (Suppl 2): 35-9.
[19]. Bezold G, Schuster-Grusser A, Lange M, Gall H, Wolff H, Peter RU. Prevalence of human herpesvirus types 1-8 in the semen of infertility patients and correlation with semen parameters. Fertil Steril. 2001;76(2): 416-18.
Type of Study: Research |
Subject:
Virology
Received: 2023/04/15 | Accepted: 2023/09/9 | Published: 2023/10/2
Received: 2023/04/15 | Accepted: 2023/09/9 | Published: 2023/10/2
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




