There are over a hundred types of organophosphate-containing compounds on a large commercial scale worldwide, with different formulations used as insecticides in agriculture, animal husbandry, and home use (such as rat death). These compounds are usually composed of phosphate esters, which include a central phosphate atom and three sub-organic chains, two of which are ethyl or methyl, and the other, which is more specific, is used to kill
insects [1]. The toxicity and side effects of organophosphorus toxins on the human body are evident to everyone. Cholinesterase is one
of the most important enzymes that function properly in the nervous system [2]. Some chemical compounds in pesticides, such as organophosphates, interfere with or inhibit cholinesterase activity. Breathing, eating, and absorbing through the skin and eyes are ways that cholinesterase inhibitors can infect humans. Although the symptoms of cholinesterase inhibition by carbamates are similar to those of organophosphate, blood cholinesterase levels return to normal faster than those of organophosphates after carbamate intoxication. This time varies from a few hours to a few days for carbamate and several days to several weeks for organophosphates, depending on the amount of toxin and the length of time the person has been exposed to it [3]. A poisoned person’s cholinesterase returns to normal after 82 days if not exposed to toxins. It is best to measure each person’s cholinesterase before starting work in a pesticide factory and to consider its value as the baseline for the same person because the natural range of cholinesterase is extensive.
For this reason, changes in cholinesterase levels in a person may be very significant but within the normal range. If the baseline is unavailable from this person, changing the results will be very difficult. A reduction of 25 to 35% often means moderate contact, and a reduction of 35 to 50% indicates severe poisoning [4]. Because muscle cholinesterase is not available for direct measurement, blood cholinesterase is a reliable alternative. The level of this enzyme is an important biochemical indicator and a sensitive parameter to exposure to toxins or the presence of toxic substances in the body [5]. Since workers are exposed to these toxins in organophosphate pesticide production plants, these toxins, according to what was mentioned, reduce the level of acetylcholinesterase, followed by mild to severe poisoning. We decided to check the level of this enzyme in these people so that we can monitor the performance of this enzyme in these workers to keep the level of cholinesterase enzyme in these people at normal levels.
Materials and Methods
Our experiment was performed on the workers of Elixir pesticide factory in Mehriz, Yazd. We have two sample groups (exposure group of 52 people and control group of 24 people), a group of workers who are supposed to work daily in the field of organophosphorus toxins (exposure group), and another group of workers who work in the administrative part of the factory. They have no contact with organophosphate toxins and are similar in age and sex to the first group (non-exposure group). Each person completed a questionnaire about their health status, which included questions about their medication history, work-related illness, and smoking. Also, written consent was obtained from them to participate in this study, and those who did not meet the required conditions were excluded from the study. To do this, one milliliter of blood was taken from the workers before measuring acetylcholinesterase activity. After 3 months after the start of work, the activity of acetylcholine was measured again in the same workers, and the results were recorded. Then, the mean of acetylcholinesterase activity in the 2 groups after starting work was compared to before. The mean of acetylcholinesterase was also compared between the two groups. An incubation solution was first prepared to measure plasma acetylcholinesterase activity. This solution is a mixture of phosphate buffer and 5,5′-dithiobis-(2-nitrobenzoic acid (DTNB) or Ellman’s reagent). Pour 3 ml of incubation solution and 10 μl of plasma into each tube and place the tube at 37 °C to establish temperature balance. Then, we transferred the contents of the test tube and the blank tube to another tube. Add 5 microliters of distilled water to the blank tube and
5 microliters of the substrate to the test tube, and finally, add acetylthiocholine iodide. We immediately read the absorption changes kinetically with a spectrophotometer at 412 nm. Acetylthiocholine iodide with a concentration of 3 mM was prepared as a substrate before the test. Water was distilled to prepare 250 ml of phosphate buffer with a concentration of 75 mM and 7.9 PH; we weighed 0.425 g of monobasic potassium phosphate and 2.72 g of diphasic phosphate to a volume of 230-220 ml. Then, we adjusted the pH to 7.9 degrees at 37 degrees Celsius and then increased its volume to 250 ml. This solution is considered as incubation. All ingredients were stored in the refrigerator after preparation.
Statistical analysis
Kolmogorov-Smirnov test was used to evaluate the normal distribution of data, which was not normal for acetylcholinesterase activity in zero minutes of distribution (p < 0.05). Therefore, the non-parametric Mann-Whitney test was used for intergroup comparison.
Results
The mean activity of acetylcholinesterase was 12.78. ±10.70 in the control group and 10.77± 8.69 in the exposure group. The results of the Mann-Whitney test did not show a significant difference between the two groups (p > 0.05). To measure the activity of acetylcholinesterase enzyme at 10 minutes, data distribution was not normal (p <0.05). The mean activity of acetylcholinesterase was 14.24±10.49 ku / l in the control group and 10.40 ± 8.64 ku / l in the exposure group. The results of Mann-Whitney test showed a significant difference between the two groups (p < 0.05). Therefore, the mean activity of acetylcholinesterase (10 min) in the exposure group was significantly lower than the
control group. Data distribution was not normal to measure the activity of acetylcholinesterase in 20 minutes (p < 0.05). The mean activity of acetylcholinesterase was 15.45±10.17 ku / l in the control group and 10.36±8.35 ku / l in the exposure group. The results of the Mann-Whitney test showed a significant difference between the two groups (p < 0.05) so that the activity of acetylcholinesterase enzyme in the exposed group was significantly higher than the control group.
Discussion
In the present study, we measured the activity of acetylcholinesterase in the blood of workers of Mehriz organophosphate toxin factory. Blood samples were taken from workers exposed to the toxin, and blood samples were taken from factory workers who were not exposed to it, and their acetylcholinesterase activity was measured. The results of this study showed a significant difference in the level of steel cholinesterase between the two groups of workers, the first group of which was exposed to the poison (as the experimental group) and the second group of employees who worked in the administrative department (As a control group), shows us. After three months of working in the factory, workers exposed to the toxin had lower average levels of acetylcholinesterase than workers in the administrative sector. This difference is so great that as we move from the group in contact with the toxin to the control group, the acetylcholinesterase level increases by 1.4 times. The study by Dhananjayan et al. (2012), which aimed to evaluate the activity of acetylcholinesterase and butyrylcholinesterase in the plasma of agricultural workers, showed that acetylcholinesterase activity in the exposure group ranged from 1.65 to 3.54 μmol / ml/min and in the control group [6]. It ranged from 2.22 to 3.51 μmol/ ml/ min.
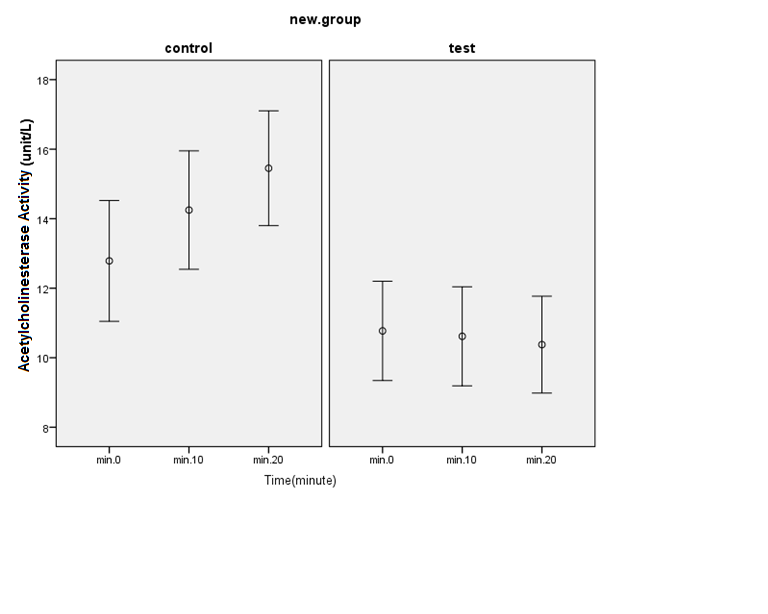
Fig. 1. Comparison of mean acetylcholinesterase activity between the two groups at 0, 10, and 20 minutes. The difference between acetylcholinesterase activity in the control and exposure groups increases over time, so acetylcholinesterase activity in the control group rapidly increased. (p < 0.05)
In this study, Chambers determined the level of acetylcholinesterase activity according to the modified Elman method, which is slightly different from our research methodology. However, the results showed a higher level of acetylcholinesterase activity in the control group, which is consistent with the present study. These findings were previously reported in the Gomes et al. (1997) study of field farmers, where acetylcholinesterase activity was 3.89±0.64 and 4.15±0.29 Ul/ml in controls [7]. The activity of acetylcholinesterase activity has also been investigated in more recent studies. The study by Silvério et al. was performed on 94 individuals exposed to organophosphate-free pesticides and 94 individuals exposed to organophosphate-containing pesticides [8]. Acetylcholinesterase activity was 63.8% lower in the exposed group. Quandt et al. also measured acetylcholinesterase activity in farmers using pesticides and compared it with other workers [9]. The results showed a significant difference between the activity levels of this enzyme in the two groups. In a study of the last two years, a study in Iran by Salari et al. (2019) on workers in poison-producing industries showed that the mean level of serum acetylcholinesterase in the control group was significantly higher than the exposure group [10].
Ethical Considerations
Ethics code received from the Research Council of Shahid Sadoughi University of Medical Sciences, Yazd: IR.SSU.MEDICINE.REC.1397.138
Funding
This study funded by Shahid Sadoughi University of Medical Sciences in Yazd.
Conflict of Interest
The authors have no conflict of interest to disclose.
Acknowledgment
The authors gratefully acknowledge all colleagues who assisted in this research.
Authors’ Contributions
HR.J conceived and planned conceptualization, experimental set-up, data interpretation, manuscript writing, and figure preparation. L.B conducted the experimental work, data analysis, interpretation manuscript, and figure formatting. All authors reviewed and approved the final version of the manuscript.
References
- Sánchez-Santed F, Colomina MT, Hernández EH. Organophosphate pesticide exposure and neurodegeneration. Cortex 2016; 74(1): 417-26.
- Pepeu G, Giovannini MG, Bracco L. Effect of cholinesterase inhibitors on attention. Chemico-Biological Interactions 2013; 203(1): 361-64.
- King AM, Aaron CK. Organophosphate and carbamate poisoning. Emergency Medicine Clinics 2015; 33(1): 133-51.
- Ballantyne B, Marrs TC. Clinical and experimental toxicology of organophosphates and carbamates: Elsevier; 2017.
- Fareed M, Pathak MK, Bihari V, Kamal R, Srivastava AK, Kesavachandran CN. Correction: adverse respiratory health and hematological alterations among agricultural workers occupationally exposed to organophosphate pesticides: A cross-sectional study in north India. PLOS ONE 2013; 8(8): 69755.
- Dhananjayan V, Ravichandran B, Anitha N, Rajmohan H. Assessment of acetylcholinesterase and butyrylcholinesterase activities in blood plasma of agriculture workers. Indian Journal of Occupational and Environmental Medicine 2012; 16(3): 127-35.
- Gomes J, Lloyd O, Revitt D, Norman J. Erythrocyte cholinesterase activity levels in desert farm workers. Occupational Medicine 1997; 47(2): 90-4.
- Silvério ACP, Machado SC, Azevedo L, Nogueira DA, de Castro Graciano MM, Simões JS, et al. Assessment of exposure to pesticides in rural workers in southern of Minas Gerais, Brazil. Environmental Toxicology and Pharmacology 2017; 55(1): 99-106.
- Quandt SA, Pope CN, Chen H, Summers P, Arcury TA. Longitudinal assessment of blood cholinesterase activities over two consecutive years among Latino non-farmworkers and pesticide-exposed farmworkers in North Carolina. Journal of Occupational and Environmental Medicine 2015; 57(8): 851.
- Salari M, Rahimi J, Moradnia M, Tarin Z, Darvishmotevalli M, Eslami F, et al. Evaluation of the relation of acetylcholinesterase enzyme level of the worker of a poison-producing industry with the application of personal protective equipment and the amount of poison production within 2012–2015. International Journal of Environmental Health Engineering 2019; 8(1): 3-8.









































