Sat, Feb 7, 2026
[Archive]
Volume 10, Issue 4 (November 2023)
IJML 2023, 10(4): 314-321 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Eslami N, Anzabi Y, NourAzar M A. Comparison of the Effect of Temperature and Different Culture Media on the Possibility of Growth of Salmonella Typhimurium (ATCC: 14028). IJML 2023; 10 (4) :314-321
URL: http://ijml.ssu.ac.ir/article-1-485-en.html
URL: http://ijml.ssu.ac.ir/article-1-485-en.html
Department of Pathobiology, Faculty of Veterinary Medicine, Tabriz Medical Sciences, Tabriz Islamic Azad University, Tabriz, Iran.
Full-Text [PDF 318 kb]
(889 Downloads)
| Abstract (HTML) (845 Views)
Introduction
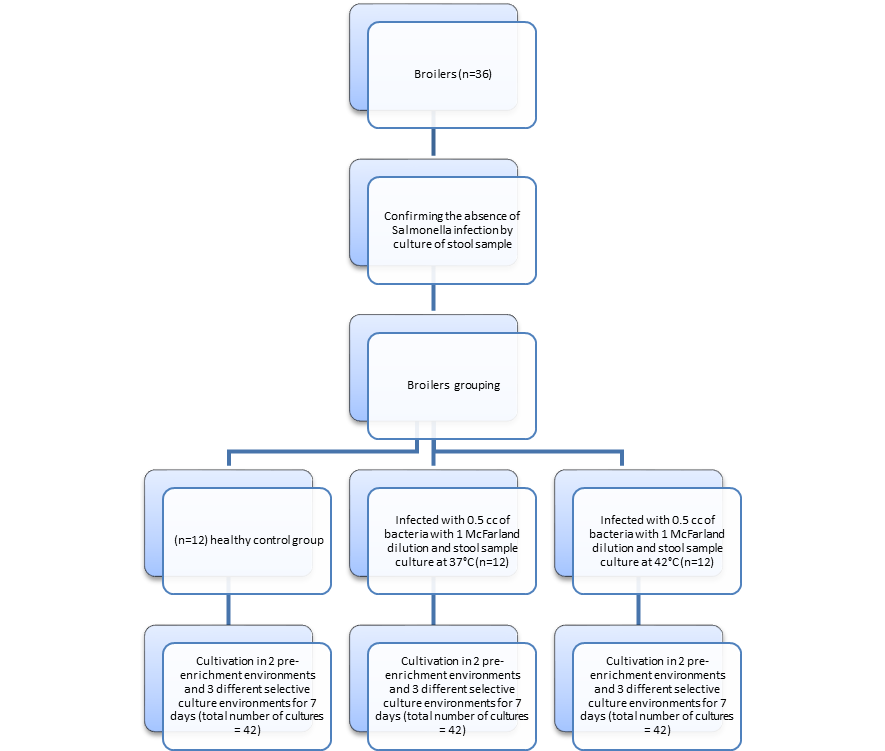
Table 2. The development rate of S. Typhimurium in Tetrathionate enrichment medium at two temperatures of 37°C and 42°C
Table 3. The development rate of S. Typhimurium in Brilliant Green culture medium at two temperatures of 37 °C and 42 °C
Table 4. The development rate of S. Typhimurium in McConkey agar culture medium at two temperatures of 37 °C and 42 °C
Table 5. The development rate of S. Typhimurium in Xylose Lysine Deoxycholate culture medium at two temperatures of 37°C and 42°C
Table 6. Results of imvic and urease differential tests for isolating suspected Proteus colonies from Salmonella Typhimurium colonies.
Full-Text: (583 Views)
Introduction
Foodborne salmonellosis remains an important public health problem in developed and developing countries [1, 2]. Salmonella Typhimurium (S. Typhimurium) gastroenteritis is a disease caused by this infection, and the use of stool samples or rectal swabs is an important diagnostic method in laboratories. However, in stool samples, saprophytic flora, especially other coliforms, complicates Salmonella’s isolation. Therefore, there is no best way to detect Salmonella, and improving media selection is important for diagnostic and therapeutic purposes. Conventional media-based identification relies on producing H2S and pH indicators to detect lactose fermentation, while bile salt and dyes inhibit saprophytic flora. However, this type of media is not sufficient to distinguish Salmonella spp. Other members of the Enterobacteriaceae family, such as Proteus spp. and Citrobacter spp. [3].
Enrichment culture is a competition among microbiota for available nutrients and against growth inhibitors. While enrichment media are designed to favor a target organism, the conditions may not equally favor every strain or sub-group (e.g., serotype, serogroup) of that species. This is of particular concern when the organism being sought is a pathogen from a complex matrix, such as a foodborne pathogen. The issue of cultural bias and culture fitness between strains of the same species or subgroup has been described for Listeria monocytogenes [4] and Salmonella [5]. Natural variants present in Escherichia Coli (O157:H7) populations show major differences in stress resistance affecting isolated phenotypes [6].
Therefore, this study aimed to compare the temperature, enrichment, and selective culture media for the better growth of S. Typhimurium from the fecal samples of diseased broiler chickens.
Materials and Methods
This study was conducted within the frame of a Preclinical Trial and Experimental sort in the winter of 2022 at Tabriz Medical Sciences, Islamic Azad University.
Preparation of bacterial strain
The examined microorganism was S. Typhimurium (ATCC: 14028), obtained from the Scientific and Industrial Research Center of Iran. To resuscitate the bacteria, to begin with, a pellet dipped in the desired bacteria solution was removed from the cryobank and placed in 3 ccs of Brain Heart Infusion (BHI) broth medium. At that point, this culture medium was incubated for 24 hours at 37 °C for the bacteria to multiply again. After the revival study, an isolated culture was done on the BHI agar medium to obtain pure colonies from the BHI broth culture medium containing pathogen bacteria. Then, after 24 hours (in this case, it is in the logarithmic phase of growth), a suspension was prepared from the colonies obtained from the bacterial culture in Hinton broth medium and standardized to 0.5 McFarland by visual perception within the light source [7].
Study method
This study divided 36 14-day-old Ross chickens into 3 groups of 12 each. The study took stool samples from all chickens that used sterile chemicals before infection. It is placed in the box and sent to the laboratory to confirm that it does not contain Salmonella. All samples were processed on the day of collection. The samples were first dissolved in Peptone buffer and incubated in Tetrathionate at 37 °C for 18-24 hours. After incubation, centrifuge at 360 rpm for 1 minute, discard the supernatant, make a culture for Xylose Lysine Deoxycholate (XLD) medium from the remaining pellet, and incubate at 37 °C for 24 hours. After verifying that there was no contamination, grouping was done. The first group was used as a healthy control group, and the second group of broilers was tube-infected with 0.5 cc bacteria with 1 McFarland turbidity. The chickens of the third group were also infected with 0.5 cc of bacteria with 1 McFarland dilution, and stool samples of this group were also taken and incubated at 42 °C [5]. The working method is roughly shown in Figure 1. For Salmonella isolation, stool samples were collected from each group for 7 days and taken to selected media.
Enrichment culture is a competition among microbiota for available nutrients and against growth inhibitors. While enrichment media are designed to favor a target organism, the conditions may not equally favor every strain or sub-group (e.g., serotype, serogroup) of that species. This is of particular concern when the organism being sought is a pathogen from a complex matrix, such as a foodborne pathogen. The issue of cultural bias and culture fitness between strains of the same species or subgroup has been described for Listeria monocytogenes [4] and Salmonella [5]. Natural variants present in Escherichia Coli (O157:H7) populations show major differences in stress resistance affecting isolated phenotypes [6].
Therefore, this study aimed to compare the temperature, enrichment, and selective culture media for the better growth of S. Typhimurium from the fecal samples of diseased broiler chickens.
Materials and Methods
This study was conducted within the frame of a Preclinical Trial and Experimental sort in the winter of 2022 at Tabriz Medical Sciences, Islamic Azad University.
Preparation of bacterial strain
The examined microorganism was S. Typhimurium (ATCC: 14028), obtained from the Scientific and Industrial Research Center of Iran. To resuscitate the bacteria, to begin with, a pellet dipped in the desired bacteria solution was removed from the cryobank and placed in 3 ccs of Brain Heart Infusion (BHI) broth medium. At that point, this culture medium was incubated for 24 hours at 37 °C for the bacteria to multiply again. After the revival study, an isolated culture was done on the BHI agar medium to obtain pure colonies from the BHI broth culture medium containing pathogen bacteria. Then, after 24 hours (in this case, it is in the logarithmic phase of growth), a suspension was prepared from the colonies obtained from the bacterial culture in Hinton broth medium and standardized to 0.5 McFarland by visual perception within the light source [7].
Study method
This study divided 36 14-day-old Ross chickens into 3 groups of 12 each. The study took stool samples from all chickens that used sterile chemicals before infection. It is placed in the box and sent to the laboratory to confirm that it does not contain Salmonella. All samples were processed on the day of collection. The samples were first dissolved in Peptone buffer and incubated in Tetrathionate at 37 °C for 18-24 hours. After incubation, centrifuge at 360 rpm for 1 minute, discard the supernatant, make a culture for Xylose Lysine Deoxycholate (XLD) medium from the remaining pellet, and incubate at 37 °C for 24 hours. After verifying that there was no contamination, grouping was done. The first group was used as a healthy control group, and the second group of broilers was tube-infected with 0.5 cc bacteria with 1 McFarland turbidity. The chickens of the third group were also infected with 0.5 cc of bacteria with 1 McFarland dilution, and stool samples of this group were also taken and incubated at 42 °C [5]. The working method is roughly shown in Figure 1. For Salmonella isolation, stool samples were collected from each group for 7 days and taken to selected media.

Fig. 1. The overview of the working method is presented in this flowchart. After contamination, stool samples were collected from all three groups for seven days, and after enrichment in Selenite Cysteine and Tetrathionate cultures, they were cultured in three BGA, XLD agar, and McC agar culture media to compare the growth rate. 126 samples were cultured for 3 cultures with two pre-enrichment environments.
Enrichment
Two grams of stool samples were collected from each group and diluted with Peptone buffer at a ratio of 1:9. Approximately 1 ml of each stool suspension was incubated into Tetrathionate and Selenite Cysteine enrichment broths and incubated at 37 °C for 18-24 hours. Tetrathionate broth provides good control for Proteus and Psuedomonas [8].
Culture media
MacConkey agar (Merck, Germany), Brilliant Green agar (Merck, Germany), and XLD (Merck, Germany) cultures were used to compare the growth of Salmonella bacteria in stool samples. A total of 21 stool samples were collected from the groups. Streak 10 μl loops filled with Tetrathionate or Selenite Cystine broth were inoculated, incubated on XLD, Brilliant Green agar (BGA), and McConkey agar (MCA), and incubated for 24 hours at 37 °C and 42 °C. Then suspicious plate colonies from XLD, MCA, and BGA on a non-selective medium such as nutrient agar for biochemical confirmation were cultured. Growth on XLD plates was examined for a slightly translucent red halo and a black center, BGA plates for red/pink color, and MCA plates for red color with colorless colonies [8].
Biochemical identification
For diagnosis and final conformation, in addition to colony appearance, IMViC, and Lysin-urea-glutamate differential tests were performed for cultures. Typically, for all Enterobacteriaceae, Salmonella is gram-negative and oxidase-negative. Also, facultative anaerobes, negative Voges-Proskauer, Methyl Red positive, and Nitrate to Nitrite no gas production, are usually Indole and urease negative, although rare, indole and urease are positive strains may be encountered [8].
Statistical analysis
Cohen’s Kappa test was used to investigate the correlation between selective culture medium, enrichment culture medium, and different temperatures to detect S. Typhimurium. The kappa coefficient was calculated to test the agreement among different methods in classifying the samples as positive and negative. Based on the kappa coefficient, the results were interpreted as having slight agreement (0.01 to 0.20), fair (0.21–0.40), moderate (0.41–0.60), substantial (0.61–0.80) and almost perfect (0.81–1.0) between the raters [9].
Results
Stool tests were tried for S. Typhimurium using different selective culture mediums and enrichment culture mediums at several temperatures. They think about demonstrated that out of 126 tests of broiler’s stool, 45 (35.71%) were positive. The development rate of salmonella in totally different culture mediums appears independently in Tables 1 to 5. The results of Urease and IMViC tests for differentiating colonies suspected of Proteus from Salmonella are shown in Table 6.
Two grams of stool samples were collected from each group and diluted with Peptone buffer at a ratio of 1:9. Approximately 1 ml of each stool suspension was incubated into Tetrathionate and Selenite Cysteine enrichment broths and incubated at 37 °C for 18-24 hours. Tetrathionate broth provides good control for Proteus and Psuedomonas [8].
Culture media
MacConkey agar (Merck, Germany), Brilliant Green agar (Merck, Germany), and XLD (Merck, Germany) cultures were used to compare the growth of Salmonella bacteria in stool samples. A total of 21 stool samples were collected from the groups. Streak 10 μl loops filled with Tetrathionate or Selenite Cystine broth were inoculated, incubated on XLD, Brilliant Green agar (BGA), and McConkey agar (MCA), and incubated for 24 hours at 37 °C and 42 °C. Then suspicious plate colonies from XLD, MCA, and BGA on a non-selective medium such as nutrient agar for biochemical confirmation were cultured. Growth on XLD plates was examined for a slightly translucent red halo and a black center, BGA plates for red/pink color, and MCA plates for red color with colorless colonies [8].
Biochemical identification
For diagnosis and final conformation, in addition to colony appearance, IMViC, and Lysin-urea-glutamate differential tests were performed for cultures. Typically, for all Enterobacteriaceae, Salmonella is gram-negative and oxidase-negative. Also, facultative anaerobes, negative Voges-Proskauer, Methyl Red positive, and Nitrate to Nitrite no gas production, are usually Indole and urease negative, although rare, indole and urease are positive strains may be encountered [8].
Statistical analysis
Cohen’s Kappa test was used to investigate the correlation between selective culture medium, enrichment culture medium, and different temperatures to detect S. Typhimurium. The kappa coefficient was calculated to test the agreement among different methods in classifying the samples as positive and negative. Based on the kappa coefficient, the results were interpreted as having slight agreement (0.01 to 0.20), fair (0.21–0.40), moderate (0.41–0.60), substantial (0.61–0.80) and almost perfect (0.81–1.0) between the raters [9].
Results
Stool tests were tried for S. Typhimurium using different selective culture mediums and enrichment culture mediums at several temperatures. They think about demonstrated that out of 126 tests of broiler’s stool, 45 (35.71%) were positive. The development rate of salmonella in totally different culture mediums appears independently in Tables 1 to 5. The results of Urease and IMViC tests for differentiating colonies suspected of Proteus from Salmonella are shown in Table 6.
Table 1. The development rate of S. Typhimurium in Selenite Cysteine enrichment medium at two temperatures of 37°C and 42°C
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | |
| Control group | - | - | - | - | - | - | - |
| Incubated at 37 °C | + | + | + | + | + | + | + |
| Incubated at 42 °C | + | + | + | + | + | + | + |
Table 2. The development rate of S. Typhimurium in Tetrathionate enrichment medium at two temperatures of 37°C and 42°C
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | |
| Control group | - | - | - | - | - | - | - |
| Incubated at 37°C | + | + | + | + | + | + | + |
| Incubated at 42°C | + | + | + | + | + | + | + |
Table 3. The development rate of S. Typhimurium in Brilliant Green culture medium at two temperatures of 37 °C and 42 °C
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | |
| Control group | - | - | - | - | - | - | - |
| - | - | - | - | - | - | - | |
| Incubated at 37 °C | - | - | - | - | - | - | - |
| - | - | - | - | - | - | - | |
| Incubated at 42 °C | + | - | - | - | - | + | - |
| + | - | - | - | + | + | - |
Table 4. The development rate of S. Typhimurium in McConkey agar culture medium at two temperatures of 37 °C and 42 °C
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | |
| Control group | - | - | - | - | - | - | - |
| - | - | - | - | - | - | - | |
| Incubated at 37 °C | - | - | - | - | - | - | - |
| - | - | - | - | - | - | - | |
| Incubated at 42 °C | - | - | + | + | - | - | - |
| - | - | + | + | - | + | - |
Table 5. The development rate of S. Typhimurium in Xylose Lysine Deoxycholate culture medium at two temperatures of 37°C and 42°C
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | ||
| Control group | - | - | - | - | - | - | - | |
| - | - | - | - | - | - | - | ||
| Incubated at 37 °C | - | - | - | - | - | - | - | |
| - | - | - | - | - | - | - | ||
| Incubated at 42 °C | + | + | + | - | - | + | - | |
| + | + | + | - | - | + | + | ||
Table 6. Results of imvic and urease differential tests for isolating suspected Proteus colonies from Salmonella Typhimurium colonies.
| Urease | Citrate Utilization | Indol | Voges-Proskauer | Methyl Red | Test |
| - | + | - | - | + | Result |
Agreeing with the Kappa test, the agreement between Tetrathionate and Selenite Cysteine was almost perfect, with a numerical value of 0.81. Slight agreement was observed between incubation at 37 °C and 42 °C in Tetrathionate (0.01) and incubation at 37 °C and 42 °C in Selenite Cysteine (0.01). Almost perfect agreement between MCA and BGA was obtained with the rate of 0.82. The moderate agreement between BGA and XLD was 0.57, and the agreement between MCA and XLD environments was fair, with a numerical value of 0.33.
Discussion
In the current study, the growth rate of S. Typhimurium in face samples from broiler chickens infected with this bacterium on three selective culture mediums, including XLD, BGA, and MCA, as well as two enrichment mediums containing Selenite Cystine and Tetrathionate, were examined at two temperatures, 37 °C, and 42 °C. According to the results obtained from the Kappa test, the agreement between the Tetrathionate and Selenite Cystine enrichment medium is almost perfect (0.81). BGA and MCA are in perfect agreement (0.8), so both can be used to isolate S. Typhimurium in connection with the selective culture medium. According to the Kappa test, the agreement between the selected culture medium BGA and XLD is a medium agreement (0.57), and the agreement between XLD and MCA is fair (0.33), therefore, XLD culture medium can be used instead of the other two media for better isolation of S. Typhimurium used stool samples. In this study, it was found that at 37 °C, bacteria could not grow in any of the chosen culture mediums; however, 42 °C was found to be the ideal temperature for bacterial growth in all culture mediums (k<0.1).
In the 2022 study by Thames et al. Tetrathionate broth was used for enrichment, and the XLD culture medium was used for the culture of Salmonella-infected samples. According to their report, the bacteria have grown well, and their results are consistent with the results of this study [10]. In 2022, Cull et al. reported the growth of Salmonella from stool specimens in the Tetrathionate-enriched xylose lysine agar Tergitol 4 culture medium [11]. Tiwari et al. 2022 used two Tetrathionate and Rappaport Vasiliadis broths to enrich and two selective XLD and BGA culture media at 37 °C and 42 °C for Salmonella culture from chicken fecal matter. In their study, Rappaport Vasiliadis bouillon improved Salmonella growth. According to their report, the growth rate of salmonella in Tetrathionate is low, which is not consistent with the results of this research. They also noted that Salmonella had grown well in both XLD and BGA environments at both temperatures; meanwhile, our research observed growth only at 42 °C in both cultures [12].
According to the report of Yue et al., the Tetrathionate enrichment medium had a greater effect on the isolation rate of salmonella than Selenite Cystine and Rappaport Vasiliadis mediums. However, in this study, Salmonella increased at the same rate in both Tetrathionate and Selenite Cystine broths. Therefore, the results of their research are contrary to this research [13]. Kumar et al. reported that Salmonella growth was higher in XLD than in BGA, hektoen enteric, and bismuth sulphite. Results are similar to ours [14]. El Shamy et al. reported that the recovery rate of salmonella with enrichment in Rappaport Vasiliadis and Tetrathionate media was higher in CHROmagar Salmonella (CAS) media than XLD; in that media, it was more than BGA. The fact that the growth rate of salmonella in XLD is higher than in BGA is consistent with the present research [15]. Hammack et al., reported that selenite cystine and Tetrathionate broths were significantly more effective than Rappaport Vasiliadis culture medium, but no significant difference was observed between Tetrathionate and Selenite Cystine for the isolation of Salmonella. The reported result is completely consistent with the result of the present research [16]. In a study by Valentin-Bon et al., Salmonella samples were pre-enriched in Rappaport Vasiliadis and Tetrathionate broths and then cultured in BGA, brilliant green with novobiocin, selenite broth, XLD, and Tetrathionate-enriched xylose lysine agar Tergitol 4 media. According to their report, Salmonella was isolated from all environments, and there was no significant difference in the growth rate in different environments [17]. While in this study, the growth rate of salmonella in XLD is higher than in BGA. The difference in the results obtained can be related to the Salmonella strain used in the experiments of different laboratory conditions [18].
Conclusion
According to the results obtained in this study, S. Typhimurium can grow better when cultured in XLD selection medium and at 42 °C. Therefore, this method is recommended for isolating S. Typhimurium from broiler stool samples.
Ethical Considerations
The Tabriz Medical Science Research Ethics Committee, Islamic Azad University, Tabriz, Iran, approved the ethical review. This project was evaluated following the cultural and national standards and standards for medical research in Iran (IR.IAU.TABRIZ.REC.1401.133).
Funding
This research was the result of a student project and all financial resources were provided by the student.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgments
We would like to thank and thank the officials and experts of Islamic Azad University Tabriz Medical Faculty and Islamic Azad University Tabriz Branch, as the information in this article was taken from student’s master’s thesis in microbiology. It should be noted that the financial resources required to implement the project were the student’s responsibility.
Authors’ Contributions
Y.A conceived and supervised the study, N.E collected the data drafted the first manuscript; Y.A contributed to the manuscript revision and interpretation, and N.E analyzed the data. MA.N and N.A critically revised the manuscript for important intellectual contents; all the authors read and approved the final manuscript.
Discussion
In the current study, the growth rate of S. Typhimurium in face samples from broiler chickens infected with this bacterium on three selective culture mediums, including XLD, BGA, and MCA, as well as two enrichment mediums containing Selenite Cystine and Tetrathionate, were examined at two temperatures, 37 °C, and 42 °C. According to the results obtained from the Kappa test, the agreement between the Tetrathionate and Selenite Cystine enrichment medium is almost perfect (0.81). BGA and MCA are in perfect agreement (0.8), so both can be used to isolate S. Typhimurium in connection with the selective culture medium. According to the Kappa test, the agreement between the selected culture medium BGA and XLD is a medium agreement (0.57), and the agreement between XLD and MCA is fair (0.33), therefore, XLD culture medium can be used instead of the other two media for better isolation of S. Typhimurium used stool samples. In this study, it was found that at 37 °C, bacteria could not grow in any of the chosen culture mediums; however, 42 °C was found to be the ideal temperature for bacterial growth in all culture mediums (k<0.1).
In the 2022 study by Thames et al. Tetrathionate broth was used for enrichment, and the XLD culture medium was used for the culture of Salmonella-infected samples. According to their report, the bacteria have grown well, and their results are consistent with the results of this study [10]. In 2022, Cull et al. reported the growth of Salmonella from stool specimens in the Tetrathionate-enriched xylose lysine agar Tergitol 4 culture medium [11]. Tiwari et al. 2022 used two Tetrathionate and Rappaport Vasiliadis broths to enrich and two selective XLD and BGA culture media at 37 °C and 42 °C for Salmonella culture from chicken fecal matter. In their study, Rappaport Vasiliadis bouillon improved Salmonella growth. According to their report, the growth rate of salmonella in Tetrathionate is low, which is not consistent with the results of this research. They also noted that Salmonella had grown well in both XLD and BGA environments at both temperatures; meanwhile, our research observed growth only at 42 °C in both cultures [12].
According to the report of Yue et al., the Tetrathionate enrichment medium had a greater effect on the isolation rate of salmonella than Selenite Cystine and Rappaport Vasiliadis mediums. However, in this study, Salmonella increased at the same rate in both Tetrathionate and Selenite Cystine broths. Therefore, the results of their research are contrary to this research [13]. Kumar et al. reported that Salmonella growth was higher in XLD than in BGA, hektoen enteric, and bismuth sulphite. Results are similar to ours [14]. El Shamy et al. reported that the recovery rate of salmonella with enrichment in Rappaport Vasiliadis and Tetrathionate media was higher in CHROmagar Salmonella (CAS) media than XLD; in that media, it was more than BGA. The fact that the growth rate of salmonella in XLD is higher than in BGA is consistent with the present research [15]. Hammack et al., reported that selenite cystine and Tetrathionate broths were significantly more effective than Rappaport Vasiliadis culture medium, but no significant difference was observed between Tetrathionate and Selenite Cystine for the isolation of Salmonella. The reported result is completely consistent with the result of the present research [16]. In a study by Valentin-Bon et al., Salmonella samples were pre-enriched in Rappaport Vasiliadis and Tetrathionate broths and then cultured in BGA, brilliant green with novobiocin, selenite broth, XLD, and Tetrathionate-enriched xylose lysine agar Tergitol 4 media. According to their report, Salmonella was isolated from all environments, and there was no significant difference in the growth rate in different environments [17]. While in this study, the growth rate of salmonella in XLD is higher than in BGA. The difference in the results obtained can be related to the Salmonella strain used in the experiments of different laboratory conditions [18].
Conclusion
According to the results obtained in this study, S. Typhimurium can grow better when cultured in XLD selection medium and at 42 °C. Therefore, this method is recommended for isolating S. Typhimurium from broiler stool samples.
Ethical Considerations
The Tabriz Medical Science Research Ethics Committee, Islamic Azad University, Tabriz, Iran, approved the ethical review. This project was evaluated following the cultural and national standards and standards for medical research in Iran (IR.IAU.TABRIZ.REC.1401.133).
Funding
This research was the result of a student project and all financial resources were provided by the student.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgments
We would like to thank and thank the officials and experts of Islamic Azad University Tabriz Medical Faculty and Islamic Azad University Tabriz Branch, as the information in this article was taken from student’s master’s thesis in microbiology. It should be noted that the financial resources required to implement the project were the student’s responsibility.
Authors’ Contributions
Y.A conceived and supervised the study, N.E collected the data drafted the first manuscript; Y.A contributed to the manuscript revision and interpretation, and N.E analyzed the data. MA.N and N.A critically revised the manuscript for important intellectual contents; all the authors read and approved the final manuscript.
References
- Bodhidatta L, Vithayasai N, Eimpokalarp B, Pitarangsi C, Serichantalergs O, Isenbarger DW. Bacterial enteric pathogens in children with acute dysentery in Thailand: increasing importance of quinolone-resistant Campylobacter. The Southeast Asian Journal of Tropical Medicine and Public Health 2002; 33(4): 752-57.
- Voetsch AC, Van Gilder TJ, Angulo FJ, Farley MM, Shallow S, Marcus R, et al. FoodNet estimate of the burden of illness caused by nontyphoidal Salmonella infections in the United States. Clinical infectious diseases: An official publication of the Infectious Diseases Society of America 2004; 38 (S3): 127-34.
- Kuijpers LMF, Post AS, Jacobs J. Chromogenic media for the detection of Salmonella enterica serovar Paratyphi A in human stool samples: evaluation in a reference setting. European Journal of Clinical Microbiology & Infectious Diseases 2018; 37(11): 2181-190.
- Gorski L, Flaherty D, Mandrell RE. Competitive fitness of Listeria monocytogenes serotype 1/2a and 4b strains in mixed cultures with and without food in the U.S. Food and Drug Administration enrichment protocol. Applied and Environmental Microbiology 2006; 72(1): 776-83.
- Singer RS, Mayer AE, Hanson TE, Isaacson RE. Do microbial interactions and cultivation media decrease the accuracy of Salmonella surveillance systems and outbreak investigations? Journal of Food Protection 2009; 72(4): 707-13.
- Carter MQ, Brandl MT, Louie JW, Kyle JL, Carychao DK, Cooley MB, et al. Distinct acid resistance and survival fitness displayed by Curli variants of enterohemorrhagic Escherichia coli O157:H7. Applied and Environmental Microbiology 2011; 77(11): 3685-395.
- Li S, Kundu D, Holley RA. Use of lactic acid with electron beam irradiation for control of Escherichia coli O157:H7, non-O157 VTEC E. coli, and Salmonella serovars on fresh and frozen beef. Food Microbiology 2015; 46(1): 34-9.
- Ramatla TA, Mphuthi N, Ramaili T, Taioe MO, Thekisoe OMM, Syakalima M. Molecular detection of virulence genes in Salmonella spp. isolated from chicken faeces in Mafikeng, South Africa. Journal of the South African Veterinary Association 2020; 91(1): 1-7.
- Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Family Medicine 2005; 37(5): 360-63.
- Thames HT, Fancher CA, Colvin MG, McAnally M, Tucker E, Zhang L, et al. The prevalence of Salmonella and Campylobacter on broiler meat at different stages of commercial poultry processing. Animals 2022; 12(18): 2460.
- Cull C, Singu VK, Cull BJ, Lechtenberg KF, Amachawadi RG, Schutz JS, et al. Efficacy of Lactobacillus animalis and Propionibacterium freudenreichii-based feed additives in reducing salmonella-associated health and performance effects in commercial beef calves. Antibiotics 2022; 11(10): 1328.
- Tiwari A, Swamy M, Mishra P, Verma Y, Dubey A, Srivastav N. Molecular detection of Salmonella isolated from commercial chicken. Iranian Journal of Veterinary Research 2022; 23(1): 39-45.
- Yue H, Zhang B, Zhu X, Zhang H, Tang C. Comparison of culture methods for isolation of salmonella in yak fecal samples. Indian Journal of Microbiology 2014; 54(2): 223-26.
- Kumar R, Surendran PK, Thampuran N. Evaluation of culture media for selective enrichment and isolation of Salmonella in seafood. Journal of AOAC International 2010; 93(5): 1468-471.
- El Shamy HA, Bakr WM, Gomaa NF, Barheem OH. Evaluation of two enrichment broths, three plating media and ELISA technique for the isolation of salmonella from dairy products. The Journal of the Egyptian Public Health Association 2008; 83(1-2): 133-37.
- Hammack TS, Jacobson AP, Andrews WH. The effect of preenrichment and selective enrichment media on recovery of Salmonella Typhi from the tropical fruit mamey. Journal of AOAC International 2008; 91(1): 83-91.
- Valentín-Bon IE, Brackett RE, Seo KH, Hammack TS, Andrews WH. Preenrichment versus direct selective agar plating for the detection of Salmonella enteritidis in shell eggs. Journal of Food Protection 2003; 66(9): 1670-674.
- Nah EH, Cho S, Kim S, Cho HI, Stingu CS, Eschrich K, et al. International Organization for Standardization (ISO) 15189. Annals of laboratory Medicine 2017; 37(5): 365-70.
Type of Study: Research |
Subject:
Bactriology
Received: 2023/05/2 | Accepted: 2023/11/29 | Published: 2023/12/1
Received: 2023/05/2 | Accepted: 2023/11/29 | Published: 2023/12/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







