Introduction
Toxoplasma gondii (T. gondii), an obligate intracellular parasite, causes toxoplasmosis. It is one of the most widespread diseases in the world and infects approximately one-third of the world’s population [1]. Although felines are definitive hosts, warm-blooded animals are intermediate hosts of this parasite [2]. T. gondii is transmitted by eating raw or half-cooked meat of infected animals, eating sporulated oocytes through consuming contaminated food or drink, and the placenta in seronegative pregnant women and organ transplantation [3, 4]. It has been confirmed that in a person with acute toxoplasmosis, tachyzoites are present in milk, saliva, sperm, urine, feces, and all body fluids. In the acute stage, Tachyzoites are usually found in various organs [5-7]. It has some adverse effects on reproduction, which disrupts the reproduction of some intermediate hosts [8, 9], which is very important from the viewpoint of public health and medicine.
In general, various causes and factors are effective in couples’ infertility, among which protozoan infections, such as T. gondii, the reproductive system, can damage the males’ reproductive system, such as sexual dysfunction and infertility. Other mechanisms in males’ and females’ reproductive systems include changes in sperm factors, reduction in the quality, concentration, and mobility of sperm in men or eggs in women, and these people, despite not having genetic factors, suffer from fertility problems [10]. It has even been shown that toxoplasmosis is more common in infertile couples than in fertile couples. Also, in some studies, anti-sperm antibodies in infertile couples with this infection were significantly higher than in uninfected fertile couples, and this may be related to Toxoplasma antibodies as anti-sperm agents [11].
In addition, there have been many reports of male genital disorders with specific characteristics of testicular toxoplasmosis [12], toxoplasma-related orchitis [13, 14], and hypogonadotropic hypogonadism caused by congenital toxoplasmosis [15]. Recent studies on disease transmission through sexual contact from husband to wife and vice versa have shown that T. gondii can also be transmitted to female animals with semen [16, 17]. Considering that one-third of the human population is infected with toxoplasmosis and the increasing number of couples with idiopathic infertility, there is few research on the epidemiology of toxoplasma in infertile men and its effect on reproductive parameters; however, the relationship between toxoplasma infection and infertility in men has not been fully proven to date. Therefore, we aimed to evaluate the frequency of toxoplasmosis in infertile and fertile men using serological and molecular methods.
Materials and Methods
Population study and samples
In this case-control study, in the year 2022, 129 men referred to the infertility center of Chaharmahal and Bakhtiari province participated, including 67 infertile men with fertility problems in their first or second child Infertility can be primary or secondary. Primary infertility is when a pregnancy has never been achieved by a person, and secondary infertility is when at least one prior pregnancy has been achieved) and 62 fertile men as the control group. Seminal fluid samples were taken from them. Immediately after receiving the samples, a portion was used for cell analysis, and another portion was kept for polymerase chain reaction (PCR) at -20 °C. Seminal cell analyses, including sperm count, morphology, motility, and viscosity, were performed. Two ml of blood samples were taken from each participant for serological method, and their sera were separated and stored at -20 °C until used. A questionnaire, including age, number of children, duration of marriage, age at marriage, smoking, alcohol consumption, etc., was completed by all the studied subjects. Smoking and alcohol consumption were considered the exclusion criteria because these two factors have a great impact on infertility. All of the cases were seronegative from human immunodeficiency viruses (HIV), Hepatitis B (HBV) and C (HCV), and Human papillomavirus (HPV).
Enzyme-linked immunosorbent assay (ELISA)
The frozen sera were thawed at room temperature (25 °C) and evaluated for anti-T. gondii antibodies, including IgG for the chronic stage and IgM for the acute stage of toxoplasmosis, using a commercial quantity ELISA kit (Vircell, Toxoplasma IgG and IgM, Germany).
Primer design
A REP-529 with accession No. AF146527 was used because it was identified as having 200-300 copies in the genome, making it a more suitable target for PCR. Other repetitive sequences, such as mobile genetic elements with 100 to 500 copies, are used to diagnose toxoplasmosis [18]. (F) 5'-TGTGCTTGGAGCCACAGAAG-3' and (R) 5'-GCAGCCAAGCCGGAAACAT-3'.
DNA extraction and PCR
The DNA was extracted from Tehran strain tachyzoites as a positive control and seminal fluids using an Addpreb Genomic DNA extraction Kit, Korea, according to the manufacturer’s protocol. The DNA concentration and purity were measured using a NanoDrop spectrophotometer (Thermofisher Scientific, USA); however, the integrity of the extracted DNA was confirmed using a Safestained agarose gel. DNA was stored at -20 °C until use. The PCR reaction was performed in a 25 µl final reaction mixture, containing 10 pM of each primer, 0.2 µM dNTP (Pharmacia Biotech), 30 mM Tris±HCl (pH 9.0), 7 mM (NH4)2SO4, 1.5 mM MgCl2, 2.5 U Taq polymerase. Amplification was performed on an ASTEC thermal cycler in Japan by 7 min incubation at 94 °C, followed by 30 cycles of 45 sec at 94 °C, 45 sec at 57 °C, 45 sec at 728 °C, and a final 10-min incubation at 72 °C. The PCR products were analyzed on a 1% agarose gel against positive (Tehran strain) and negative controls (distill water) and DNA markers.
Statistical analysis
All data were analyzed with Chi-square, logistic regression, and student T-test using SPSS version 21. A 95% confidence level and a P-value of less than 0.05 were considered statistically significant.
Results
Sperm analysis results in infertile and fertile groups
129 men in the two infertile (67 people) and fertile (62 people) groups participated in this study. Analysis of their sperms showed that 11 of the 67 men in the infertile group had low sperm count (<15 M/ml), and the other cases (118 males) in the two groups had normal sperm count. In the study of sperm motility, 48 of 67 men in the infertile group had slow sperm movement (<40%). In terms of morphology, 62 men in the fertile group had normal sperm, and 67 men in the infertile group had abnormal sperm. Most sperm shape abnormalities in the head area are conical (76.9%), pear-shaped (63.1%), round (52.3%), needle-shaped (23.1%), and double-headed (3.1%). Problems in the sperm neck area included sperm with bent neck (56.1%), thick neck (12.1%), cytoplasmic drops (39.4%), and two people (1.2%) with sperm tail problems.
Frequency of quantitative variables in infertile and control groups
Quantitative variables of the study included age, weight, height, body mass index (BMI), age of marriage, and duration of marriage in the infertile and the control groups. An independent T-test showed no significant difference between these variables in both groups (p > 0.05) (Table 1).
The frequency of T. gondii infection in infertile and fertile groups by serological and molecular methods
Regarding the frequency of T. gondii in the serum of men in the two groups by serological method, only two subjects (3%) in the infertile group had IgM antibodies. According to the Chi-square statistical test, no significant difference was found in the presence of T. gondii infection in two fertile and infertile groups (p >0.05). Based on IgG antibodies in the people’s sera, a significant difference was observed in T. gondii infection in fertile and infertile groups. Using the ELISA method, ten subjects (16.1%) in the fertile group and 27 subjects (40.3%) in the infertile group had a high titer of IgG antibodies (p<0.05). PCR results also did not show a significant relationship between fertile and infertile groups in T. gondii infection. The PCR results showed that 7 subjects (10.4%) in the infertile group and 2 subjects (3.2%) in the control group had a positive result using the molecular method (p >0.05) (Table 2).
The results of IgM frequency indicated T. gondii infection showed no relationship with any of the variables of age, duration of marriage, marriage age, sperm count, sperm motility and morphology, and contact with pets in both infertile and fertile groups (p > 0.05). Also, the results showed that no relationship was found between toxoplasmosis and primary infertility or secondary infertility (p >0.05) (Data not shown).
Prevalence of IgG antibody against Toxoplasma gondii in infertile and fertile men according to study variables by serological method
According to the results of IgG antibody frequency in table 3, Toxoplasma infection did not show any relationship with any of the variables of age, duration of marriage, age of marriage, sperm motility, type of infertility, and contact with pets in the infertile and fertile groups (p >0.05). However, using the the Chi-square test, we found a significant relationship between Toxoplasma infection with sperm count and sperm morphology, so its frequency was 72.7% in people with low sperm count and 24.6% in the group with high sperm (p < 0.05). Also, the frequency of anti-toxoplasma IgG antibody was 16.1% in the group of people with normal sperm shape and 40.3% in the group with abnormal morphology (p < 0.05). The highest frequency of anti-toxoplasma IgG antibody in men with abnormal sperm is in the infertile group, which shows a significant difference compared to the fertile group (Table 3).
Frequency distribution of Toxoplasma gondii in infertile and fertile men according to study variables by PCR
Based on the PCR results in Table 4, Toxoplasma infection was unrelated to age, duration of marriage, age of marriage, sperm count, sperm motility and morphology, and contact with pets in both case and control groups (p > 0.05). The results of PCR only showed a relationship with the marriage duration, which was not a directional relationship by examining Fisher’s exact test (p = 0.04).
The results of the PCR
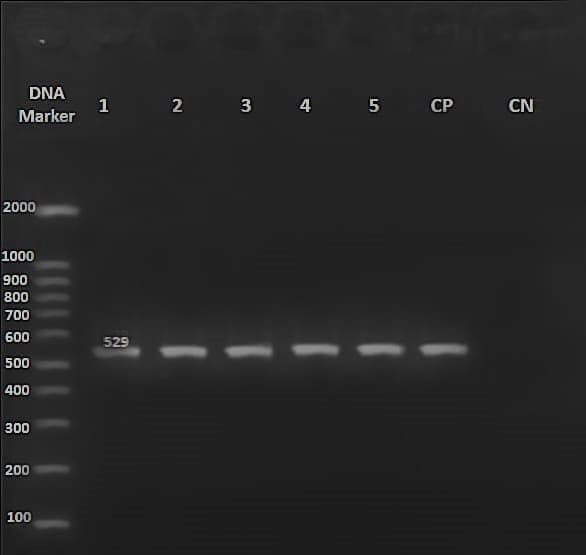
Out of 129 sperm samples whose DNA was extracted, nine samples contained T. gondii genes. So, upon loading their PCR products with specific primers, a fragment with 529 bp was observed on the agarose gel compared to the DNA marker, indicating T. gondii infection (Fig. 1).
Discussion
Infertility in men is a multifactorial syndrome that includes a wide range of disorders. The cause of their infertility is unknown (idiopathic) in more than 50% of infertile men and can be either congenital or acquired. However, several factors are involved, including the parasitic factors, including T. gondii, whose effect on semen quality has been examined [10]. The present study shows that in the serum of 40.3% of people from the infertile and 16.1% of people from the fertile groups, there is an anti-Toxoplasma IgG antibody, which is significantly different between the two groups (p < 0.05). In contrast to IgG, the results of IgM show no significant difference between them.
Table 1. Quantitative variables in the two groups of infertile and fertile groups
| P-value |
Infertile group (67 subjects) |
Fertile group (62 subjects) |
Variable |
| 0.203 |
34.1± 5.43 |
35.4 ± 6.6 |
Age (years) |
| 0.118 |
25.8± 3.7 |
24.9 ± 2.6 |
Body Mass Index |
| 0.79 |
26.5 ± 4.1 |
26.3± 3.6 |
Age of marriage (years) |
| 0.12 |
7.5± 5.2 |
9.1± 5.9 |
Duration of marriage (years) |
Data are presented as Mean ± SD.
Table 2. Frequency of T. gondii in the infertile and control group by serological and molecular methods
| P-value |
Infertile N (%) |
Fertile N (%) |
Group |
Test |
| 0.49 |
2 (3) |
0 |
Positive |
IgM |
| 65 (97) |
62 (100) |
Negative |
| 67 (100) |
62 (100) |
- |
Total |
| 0.002* |
27 (40.3) |
10 (16.1) |
Positive |
IgG |
| 40 (59.7) |
52 (83.0) |
Negative |
| 67 (100) |
62 (100) |
- |
Total |
| 0.16 |
7 (10.4) |
2 (3.2) |
Positive |
| 60 (89.6) |
63 (96.8) |
Negative |
PCR
Total |
| 67 (100) |
65 |
- |
* p < 0.05 is considered as significant.
Table 3. Prevalence of IgG antibody against T. gondii in infertile and fertile men according to study variables by serological method.
| P-value |
Fertile group/
IgG Positive N (%) |
Infertile group/
IgG Positive N (%) |
|
Variable |
| 0.27 |
3 (20) |
7 (35) |
20-30 |
Age group |
| 4 (11.8) |
14 (35.9) |
31-40 |
| 3 (23.1) |
6 (75) |
41 ≥ |
| 0.5 |
2 (20) |
4 (30.8) |
≤ 22 |
Age at the time of marriage |
| 3 (15) |
5 (27.8) |
23-26 |
| 5 (19.2) |
14 (48.3) |
27-30 |
| 0 |
4 (57.1) |
31 ≥ |
| 0.54 |
4 (22.2) |
11 (40.7) |
1-4 |
Duration of marriage |
| 2 (9.1) |
8 (36.4) |
5-10 |
| 4 (18.2) |
8 (44.4) |
10 ≥ |
| 1 |
0 |
21 (39.6) |
Primary |
Type of infertility |
| 0 |
6 (42.9) |
Secondary |
| 0.002* |
0 |
8 (72.7) |
Low |
Sperm count |
| 10 (16.1) |
19 (33.9) |
Normal |
| 0.29 |
0 |
17 (36.2) |
Slow |
Sperm movement |
| 10 (16.4) |
10 (50) |
Normal |
| 0.003* |
0 |
27 (40.3) |
Abnormal |
Sperm shape |
| 10 (16.1) |
0 |
Normal |
| 0.62 |
3 (33.3) |
5 (33.3) |
Yes |
Pet |
| 7 (13.2) |
22 (42.3) |
No |
* P-value < 0.05 is considered as significant.
Table 4. Frequency distribution of Toxoplasma gondii in infertile and fertile men according to study variables by PCR
| P-value |
Fertile group
Positive N (%) |
Infertile group
Positive N (%) |
|
Variable |
| 0.8 |
0 (0) |
2 (10) |
20-30 |
Age group |
| 1 (2.9) |
5 (12.8) |
31-40 |
| 1 (7.7) |
0 (0) |
41 ≥ |
| 0.12 |
2 (2) |
2 (15.4) |
≤ 22 |
Age at the time of marriage |
| 0 |
3 (16.7) |
23-26 |
| 0 |
2 (6.9) |
27-30 |
| 0 |
0 (0) |
31 ≥ |
| 0.04* |
0 |
4(14.8) |
1-4 |
Duration of marriage |
| 0 |
0 (0) |
5-10 |
| 2 (9.1) |
3 (16.7) |
10 ≥ |
| 1 |
0 |
6 (11.3) |
Primary |
Type of infertility |
| 0 |
1 (7.1) |
Secondary |
| 0.17 |
0 |
2 (18.2) |
Low |
Sperm count |
| 2 (3.2) |
5 (8.9) |
Normal |
| 0.07 |
0 |
6(12.8) |
Slow |
Sperm movement |
| 2 (3.3) |
1 (5) |
Normal |
| 0.16 |
0 |
7 (10.4) |
Abnormal |
Sperm shape |
| 2 (3.2) |
0 (0) |
Normal |
| 0.22 |
1 (11.1) |
2 (13.3) |
Yes |
Pet |
| 1 (1.9) |
5 (9.6) |
No |
* P-value < 0.05 is considered as significant.
Fig. 1. Gel electrophoresis of PCR products
Agarose gel electrophoresis of PCR products amplified using Ref-529 primers and DNA of T. gondii Tehran strain (positive control). Marker 100 bp, Lane 1-5: PCR products of T. gondii-infected semen samples, CP= positive control (Tehran T. gondii strain), CN= negative control.
In most of the studies conducted by serological methods, there is a significant relationship between infertility and IgG antibodies in their sera. From 2 patients with IgM positive, their PCR was also positive, which means that the disease is acute and the parasite is present in body fluids. It may be necessary to test more samples in order to obtain a meaningful relationship.
Ahmed et al. in 2013 in Baghdad showed that 41.81% of men had IgG against T. gondii, and 58.18% of men were negative serologically. This study showed that the presence of toxoplasmosis was not rare in infertile men [26]. In 2005 in China, in infertile men, 36% had IgG antibodies against T. gondii, while 11% were positive in fertile men [20]. Zhou et al. in 2002 revealed anti-toxoplasmosis antibodies in infertile couples (34.83%), which is significantly higher than fertile couples (12.11%) [11]. Al-Ezzy et al. in 2016 in Iraq showed that anti-T. gondii IgM was 23.23% in infertile men [19]. In all these studies, there is a significant relationship between T. gondii and infertility in serology reports, and our study was in line with these studies. It shows that T. gondii can be a biological factor in infertility playing a role, and probably chronic toxoplasmosis is one of the factors that can be involved in fertility disorders.
In the present study, no significant difference was found in toxoplasmosis in the fertile and infertile groups after examining the PCR results. While the presence of T. gondii in the semen of seven people (10.4%) from the infertile group and two people (3.2%) from the fertile group was confirmed using the PCR method. The first report of isolation of T. gondii in the semen of 125 men was mentioned by Disco et al. in 1971. Toxoplasma parasite was detected in 3 out of 125 semen samples [21]. Most molecular research has been conducted on the semen of experimentally infected male animals. For example, in the study by Moura et al. in 2007, which was conducted on adult pigs infected with T. gondii, [22], Scarpelli et al. in 2009, carried out on young deer infected to T. gondii [23], Arantes and his colleagues were conducted on dog infected to T. gondii, [24] the PCR method indicated the presence of Toxoplasma in the semen samples of animals infected with oocysts or tachyzoites. Nevertheless, our study was not in line with these experimental studies because the parasite has been directly inoculated into the animals in experimental studies, and the source of infection is known. At the same time, transmission occurs randomly from an unknown source in humans.
In this study, age, age at marriage, and duration did not show a significant relationship with the anti-T. gondii antibodies and PCR in both infertile and fertile groups. Only PCR had a relationship with the marriage duration, which was not a directional relationship either. Also, the highest frequency of T. gondii infection by serology and molecular methods was in the age group of 31-40 years, consistent with Al-ezzy et al.’s study in Iraq. Also, no correlation was seen between IgM antibody and age groups, but the highest prevalence of T. gondii was 41-48 years old [19]. Our study was in line with Abdulla et al.’s 2015 study. They found the highest prevalence of toxoplasma was in the age group of 23-30 years [25]. Also, in a cross-sectional study by Ahmed et al., the results showed that the frequency of toxoplasmosis was in the age group of 35-39 years, which is almost in line with this study [26]. The serology results of Al-Bajalan et al. 2015 also showed that the frequency of toxoplasmosis is higher in the age group of 31-35 years [27]. This difference in age groups can also be seen in the study of Mahmood et al. in 2013 in Baghdad, where the highest prevalence of IgG antibody in the age group (18-25) and (26-33) years and IgM antibody in the age group 18-25 the years old [28]. On the other hand, studies by Muluye et al. in 2013 from people living in northwestern Ethiopia [29] and Chiang et al. in 2014 in Taiwan [30] showed no significant difference between age and infection with T. gondii. This difference in the age groups of patients with toxoplasmosis may be due to the difference in the age groups of marriage, the unequal number of total samples in these studies, and factors such as food habits, regional climate, and cultural characteristics.
In relation to T. gondii with the age of marriage and the duration of marriage, no study has been done in the past that can be matched with the present study. However, according to the present study, it seems to be the highest frequency of anti-T. gondii antibodies in both fertile and infertile groups at the age of marriage 27-30. Further, there is no significant relationship between toxoplasma infection and age of marriage in the two infertile and fertile groups. Although there is a relationship between toxoplasmosis and duration of marriage only in the molecular method, there is no directional relationship, and the highest frequency of toxoplasmosis in both serological and molecular methods is in the duration of marriage 1-4 years.
In this study, the results of serology and PCR showed no significant relationship with the type of infertility (primary and secondary) in the infertile group. In this study, the frequency of toxoplasmosis both serologically and molecularly in the infertile group shows that the primary infertility group has a higher frequency than the second group. There are few studies on the frequency of toxoplasmosis among infertile groups (primary and secondary). In the study of Abdulla et al. in Baghdad, the relationship between the anti-T. gondii IgG and the type of infertility (primary and secondary) were revealed, but they were not significantly related to the result of the present study [25]. However, in a study conducted in Iraq by Al-Ezzy et al., contrary to this study, the result of serology (IgM antibody) showed a significant relationship with the type of infertility [19]. The serological results of Al-Bajalan et al. in 2015 showed a relationship between toxoplasmosis and secondary infertility in infertile men, unlike our study [27]. These differences in the results may be related to the duration of toxoplasmosis, the number of samples, and the time of toxoplasmosis treatment before and after infertility investigation.
In this study, the result of PCR and IgM anti-toxoplasma showed no significant relationship with any of the variables of sperm number, motility, and morphology in both fertile and infertile groups. However, IgG results found a significant relationship between T. gondii infection and sperm count and sperm morphology. Out of 67 infertile men with IgG antibodies, eight (72.7%) had low sperm count, and 27 (40.3%) had abnormal sperm morphology. In Ahmed et al.’s study in Baghdad, out of 46 infertile men with anti-Toxoplasma antibodies, 56.52% of men had abnormal sperm morphology, 67.39% of men had low sperm count, and 73.91% of men had Abnormal sperm movement observed. According to statistical analysis, there was a significant relationship between toxoplasma-positive infertile men with sperm count and movement, but no significant relationship with sperm morphology [26]. Our results are consistent with this study regarding the relationship between Toxoplasma and the sperm count parameter in the semen of infertile men in the serological method (IgG antibody).
This study is also consistent with the study by Hlaváčová et al. in 2021, in which at the Infertility Center in Prague, fertility problems in 163 men infected with Toxoplasma by serology were significantly higher than in men without Toxoplasma, and there was a significant relationship between sperm parameters (sperm count, sperm motility and morphology) with T. gondii in infertile men [31]. Many studies have been conducted on animals, which mostly show the effect of T. gondii on sperm parameters and fertility [32-34]. It has been demonstrated that the high amount of some substances, such as nitric oxide reactive oxygen species, with the action of peroxidation of unsaturated fatty acids in the sperm plasma membrane, negatively affects motility, abnormalities, and sperm-oocyte fertilization. These substances are produced in large quantities in infection with T. gondii and the host’s immune response pathway stimulation. Therefore, this may be a reason for expressing a significant relationship between Toxoplasma infection and sperm abnormalities [35].
Insufficient results have been found or published regarding the relationship between Toxoplasma infection in infertile men and contact with animals. In our study, having a cat or a pet did not show any relationship with the anti-toxoplasma antibodies in the serological and molecular methods in both fertile and infertile groups. Nevertheless, Al-Bajalan et al. conducted a study among male military personnel of the Czech Republic in 2007. They showed a relationship between toxoplasma infection and contact with animals (cats) [27].
Felines are the only definite host of T. gondii parasites and expel the infectious oocyst into the environment. This parasite can be transmitted from cat to human, including eating raw or half-cooked meat with tissue cysts, drinking water, or food contaminated with oocysts or oocysts in the soil, etc. Therefore, humans may be infected without direct contact with felines occur. For this reason, the attempt to show the relationship between T. gondii infection and previous contact with cats provides contradictory results. However, regardless of the groups of infertile and fertile men, studies on the relationship between Toxoplasma and contact with animals have been conducted in different groups. They have shown different results, and only half have confirmed this relationship. Studies by Al-Bajalan et al. [27]., Senthamarai et al. [36]., showed no relationship between contact with cats and Toxoplasma infection.
Conclusion
The effect of toxoplasmosis on specific parameters of male fertility has been broadly studied in detail in animals, apart from a few preliminary studies conducted on a small sample of men. According to the studies conducted and the recent study that shows the effect of latent or chronic toxoplasmosis on the number and morphology of sperm in men, a theoretical framework can be provided for the relationship between Toxoplasma infection and male infertility parameters. Measuring the antibody level against T. gondii in the serum of people with infertility problems can be an important step in treating infertility. According to the results of studies, T. gondii may directly or indirectly affect the quality and quantity of sperm, which is the leading cause of infertility in men. Clarifying these factors is an essential goal in understanding the general role of Toxoplasma infection in male infertility and an important step in the final treatment of the problem of infertility caused by T. gondii.
The limitations of the study
It was difficult to take samples from healthy groups at the infertility center, so samples were taken from private laboratories from people who came for a check-up after obtaining consent.
Ethical Consideration
This study was approved by the Human Research Ethics Committees of Shahrekord University of Medical Sciences (SKUMS), Iran, with Ethics No. IR.SKUMS.REC.1398.197. Also, written informed consent was taken from all participants before the study.
Funding
This research received no specific grant, funding, equipment, or supplies from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare no competing interests.
Acknowledgments
The work described in this publication was supported by the Human Research Ethics Committees, Medical University of Shahrekord (SKUMS), Iran No IR.SKUMS.REC.1398.197 and project No. 389 grants.
Authors’ Contributions
K.M conceived and supervised the study, F.ES collected the data, and drafted the first manuscript; E.S contributed to the manuscript revision and interpretation, and S.KH analyzed the data. F.S critically revised the manuscript for important intellectual contents; all the authors read and approved the final manuscript.
References
- Dubey JP, Beattie CP. Toxoplasmosis of animals and man. CRC Press, Inc. 1988.
- Ahmadpour E, Daryani A, Sharif M, Sarvi S, Aarabi M, Mizani A, et al. Toxoplasmosis in immunocompromised patients in Iran: A systematic review and meta-analysis. JIDC. 2014; 8(12): 10-15.
- Prandovszky E, Gaskell E, Martin H, Dubey J, Webster JP, McConkey GA. The neurotropic parasite Toxoplasma gondii increases dopamine metabolism. PloS One 2016; 6(9): 23866.
- Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet 2004; 363: 1965-976.
- Aguirre AA, Longcore T, Barbieri M, Dabritz H, Hill D, Klein PN, et al. The one health approach to toxoplasmosis: Epidemiology, control, and prevention strategies. EcoHealth. 2019; 16(2): 378-90.
- Weiss LM, Dubey JP. Toxoplasmosis: A history of clinical observations. Int. J. Parasitol. 2009; 39(8): 895-901.
- Zare F, Dalimi Asl A, Ghaffarifar F. Detection of active Toxoplasma gondii (RH strain) in the different body tissues of experimentally infected rats. Pathobiology Research. 2010; 9: 19-23.
- Suresh Babu PS, Nagendra K, Sarfaraz Navaz R, Ravindranath HM. Congenital toxoplasmosis presenting as hypogonadotropic hypogonadism. IJP, 2007; 74(6): 577-79.
- Komijani M, Shaykh-Baygloo N, Ghasemi SM, Azad F. A systematic review on the role of infectious agents in female and male infertility. J Res Med Sci. 2018; 29(4): 295-304.
- Martinez-Garcia F, Regadera J, Mayer R, Sanchez S, Nistal M. Protozoan infections in the male genital tract. J Urol. 1996; 156(2): 340-49.
- Zhou YH, Lu YJ, Wang RB, Song LM, Shi F, Gao QF, et al. Survey of infection of Toxoplasma gondii in infertile couples in Suzhou countryside. Natl J androl. 2002; 8(5): 350-52.
- Sharma RK, Agarwal A. Role of reactive oxygen species in male infertility. Urology 1996; 48(6): 835-50.
- De Lamirande E, Jiang H, Zini A, Kodama H, Gagnon C. Reactive oxygen species and sperm physiology. Reviews of Reproduction 1997; 2: 48-54.
- Sanocka D, Kurpisz M. Reactive oxygen species and sperm cells. Reprod Biol Endocrinol. 2004; 2(1): 1-7.
- Pellati D, Mylonakis I, Bertoloni G, Fiore C, Andrisani A, Ambrosini G, Armanini D. Genital tract infections and infertility. Eur J Obstet Gynecol. 2008; 140(1): 3-11.
- Crider SR, Horstman WG, Massey GS. Toxoplasma orchitis: Report of a case and a review of the literature. Am J Med. 1988; 85(3): 421-24.
- Haskell L, Fusco MJ, Ares L, Sublay B. Case report: Disseminated toxoplasmosis presenting as symptomatic orchitis and nephrotic syndrome. Am J Med Sci. 1989; 298(3): 185-90.
- Montoya JG. Laboratory diagnosis of Toxoplasma gondii infection and toxoplasmosis. J Infect Dis. 2002; 185(S1): 73-82.
- Al-Ezzy AI. Effect of TORCH agents and Chlamydia trachomatis on reproductive parameters and fertility hormones of Iraqi infertile males. Asian J Pharm Clin Res. 2016; 9(3): 47-56.
- Qi R, Su XP, Gao XL, Liang XL. Toxoplasma infection in males with sterility in Shenyang, China. Natl J Androl. 2005; 11(7): 503-504.
- Disko R, Braveny I, Vogel P. Examination of human seminal fluid for Toxoplasma gondii. Z. Tropenmed. Parasitol. 1971; 22(4): 391-96.
- Moura AB, Costa AJ, Jordão Filho S, Paim BB, Pinto FR, Di Mauro DC. Toxoplasma gondii in semen of experimentally infected swine. Pesqui Vet Bras. 2007; 27: 430-34.
- Scarpelli L, Lopes WD, Migani M, Bresciani KD, Costa AJ. Toxoplasma gondii in experimentally infected Bos taurus and Bos indicus semen and tissues. Pesqui Vet Bras. 2009; 29(1): 59-64.
- Arantes TP, Lopes WD, Ferreira RM, Pieroni JS, Pinto VM, Sakamoto CA, et al. Toxoplasma gondii: Evidence for the transmission by semen in dogs. Exp. Parasitol. 2009; 123(2): 190-94.
- Abdulla HE, Al-bashier NM, Al-kawaz U, Al-Shuwaikh AM, Abood AS. Cross-sectional study of infertile males with toxoplasmosis in Baghdad province. Int J Sci Eng. 2015; 6(1): 254-59.
- Ahmed AM, Mohammed JQ, Nuhal Noel J. Affliction with toxoplasmosis among infertile men In Kamal Al-Samarae Hospital -Baghdad City. AL Taqani 2013; 26(3): 56-62.
- Al-Bajalan RR, Al-Nasiri FS, Mahmood SM. Detection Toxoplasma gondii by latex and ELISA test in infertile and fertile men in Kalar City, Kurdistan region, Iraq. Int J Curr Microbiol App Sci. 2015; 4(10): 570-85.
- Mahmood SH, AL-Qadhi BN, Zghair KH. Prevalence of toxoplasmosis of males blood donors in Baghdad. Iraqi J Sci. 2013; 54(4): 832-41.
- Muluye D, Wondimeneh Y, Belyhun Y, Moges F, Endris M, Ferede G, et al. Prevalence of Toxoplasma gondii and associated risk factors among people living with HIV at Gondar University Hospital, Northwest Ethiopia. Int Sch Res Notices. 2013; 2013(12): 1-6.
- Chiang TY, Kuo MC, Chen CH, Yang JY, Kao CF, Ji DD, et al. Risk factors for acute Toxoplasma gondii diseases in Taiwan: A population-based case-control study. PLoS One 2014; 9(3): 90880.
- Hlaváčová J, Flegr J, Řežábek K, Calda P, Kaňková S. Association between latent toxoplasmosis and fertility parameters of men. Andrology 2021; 9(3): 854-62.
- Terpsidis KI, Papazahariadou MG, Taitzoglou IA, Papaioannou NG, Georgiadis MP, Theodoridis IT. Toxoplasma gondii: reproductive parameters in experimentally infected male rats. Exp Parasitol. 2009; 121(3): 238-41.
- Taherimoghaddam M, Bahmanzadeh M, Tapak L, Maghsood AH, Fallah M, Foroughi-Parvar F. Effects of Toxoplasma gondii on sperm parameters and histomorphometry of testis in experimentally infected rats. Arch Clin Infect Dis. 2021; 16(2): 99855.
- Sun LH, Fan F, Wang JJ, Gong J. Acute Toxoplasma gondii infection affects the reproductive function of male mice. National Journal of Andrology 2008; 14(1): 55-7.
- Abdoli A, Dalimi A, Movahedin M. Impaired reproductive function of male rats infected with Toxoplasma gondii. Andrologia 2012; 44: 679-87.
36. Senthamarai S., Sivasankari S, Apurba SS, Sandhya BK, Kumudavathi MS, Anitha C, et al. Seroprevalence of toxoplasmosis in pregnant women with bad obstetricstory in a tertiary care hospital, Kanchipuram-apilotstudy. 2013; Disease 3(9): 2932.