Mon, Feb 2, 2026
[Archive]
Volume 11, Issue 1 (February 2024)
IJML 2024, 11(1): 72-85 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Azadbakht M, Bagheri P, Akbarzadeh M, Foruozandeh H, Khaleghi A, Gholami M S, et al . Frequency of Blood Components Wastages and Related Factors in Fars Blood Transfusion Centers, Iran. IJML 2024; 11 (1) :72-85
URL: http://ijml.ssu.ac.ir/article-1-494-en.html
URL: http://ijml.ssu.ac.ir/article-1-494-en.html
Mojtaba Azadbakht 
 , Parisa Bagheri
, Parisa Bagheri 
 , Majid Akbarzadeh
, Majid Akbarzadeh 
 , Hossein Foruozandeh *
, Hossein Foruozandeh * 
 , Aliasghar Khaleghi
, Aliasghar Khaleghi 
 , Mohammad Saeed Gholami
, Mohammad Saeed Gholami 
 , Davood Zarei
, Davood Zarei 
 , Zahra Nasiri
, Zahra Nasiri 
 , Amir Abbas Asadi
, Amir Abbas Asadi 


 , Parisa Bagheri
, Parisa Bagheri 
 , Majid Akbarzadeh
, Majid Akbarzadeh 
 , Hossein Foruozandeh *
, Hossein Foruozandeh * 
 , Aliasghar Khaleghi
, Aliasghar Khaleghi 
 , Mohammad Saeed Gholami
, Mohammad Saeed Gholami 
 , Davood Zarei
, Davood Zarei 
 , Zahra Nasiri
, Zahra Nasiri 
 , Amir Abbas Asadi
, Amir Abbas Asadi 

Cellular and Molecular Research Center, Gerash University of Medical Sciences, Gerash, Iran
Full-Text [PDF 320 kb]
(511 Downloads)
| Abstract (HTML) (932 Views)


Discussion
References
Full-Text: (709 Views)
Introduction
Blood transfusions are an essential part of health care, saving millions of lives worldwide each year. Despite promising research, artificial blood substitutes could not be a viable alternative to human blood transfusion. Therefore, blood and blood products must be managed for patient consumption [1]. In recent years, the need for blood transfusions has been increasing. Various factors are involved in this matter, including increasing life expectancy and advances in medical technology [2]. The industrial world and health accidents are increasing the use of blood and blood products. All of this has raised concerns about the sufficiency of blood to meet growing demand, thus doubling the importance of managing blood consumption to avoid wasting resources [3]. In 1974, the Blood Transfusion Organization of Iran focused on all blood transfusion activities from donor recruitment to blood component production and delivery of blood and blood products. There are currently 283 blood donation centers throughout the country [1].
The blood donation process is such that the donor is first certified by a physician after consultation and physical examination, and then a Confidential Unit Exclusion (CUE) form is provided to the donor so that the donor can decide whether or not the donated blood is used for the patient. Screening tests that are routinely performed on all blood vessels after donation include hepatitis B surface antigen (HBsAg), hepatitis C virus antibody (Anti-HCV), syphilis, and human immunodeficiency virus (HIV) Ag/ Ab [4, 5]. Donated blood is then sent to the processing department and various products are prepared from whole blood. After determining the test results, the product is labelled and sent to the blood distribution department. During the various stages of preparation of blood products, from the stage of blood collection to blood distribution, these products may be damaged or expired [6]. Despite the country's self-sufficiency in the supply of blood and blood products, comprehensive information on the extent and causes of blood product wastage has not been published in the country. Collecting such information will be effective for the future planning of the blood transfusion organization for the preparation and distribution of blood products. Therefore, in this study, the rate and primary cause of wastage of blood and blood components in blood transfusion centers of Fars province from 2015 to 2020 were examined and evaluated.
Materials and Methods
The present article was a descriptive and retrospective study that examined the data related to the frequency and waste of blood component units and expiry units in blood transfusion centers of Fars province from 2015 to 2020. There are six blood collection centers, preparation and processing centers, and blood transfusion centers in Fars province. All of these centers' information related to blood collection, production of components, distribution of products, discarded units and outdated units are recorded in internal software or recorded and monitored in controlled forms. All this information is evaluated and checked at the province's and country's central blood transfusion headquarters.
The data relating to blood product wastage were extracted from the comprehensive software of the Blood Transfusion Organization (Negareh), and the cause and frequency of red blood cell (RBC), platelet (Plt), plasma and cryoprecipitate (CP) wastage from 2015 to 2020 were evaluated.
Definitions
Production units
Units from whole blood labeled and licensed for distribution and consumption.
Wastage units in blood transfusion centers
These units have been discarded for various reasons, such as hemolysis, rupture, or positive screening tests at blood transfusion centers, and have been removed from the distribution to hospitals. Screening tests were done on all samples and included HIV Ag / Ab, Anti-HCV, HBs Ag and syphilis. If the sample reacts in the screening tests, the donation units are lost according to the organization's algorithm, regardless of the repeat test result [1]. Confirmation tests, including HCV recombinant immunoblot assay (RIBA), HBsAg neutralization test, HIV western blot and fluorescent treponemal antibody (FTA), are performed on the samples that have reacted to the second time in the tests, depending on the type of reaction in the screening test.
Wasted units in hospitals (returning units from hospitals)
These units were discarded in the hospital, and their numbers were sent to blood transfusion centers in particular forms or units that were returned from the hospital to blood transfusion centers and wasted in these centers.
Expiration date units
These units have not been distributed in hospitals or used for patients during their storage in the hospital, and their expiration date has passed.
Statistical analysis
The data were analyzed using SPSS software version 20. All data were prepared as frequencies
and percentages for basic descriptive study. P-values of less than 0.05 were considered statistically significant.
Results
The total amount of waste was 164981 units (6.71%). Also, the annual amount of waste in 2015 was 34065 units (8.12%); 2016, 30991 units (7.15%); 2017, 28454 units (6.75%); 2018, 25449 units (6.19 %); 2019, 22327 units (5.44%) and 2020, 23695 units (6.46%).
The most discarded products were whole blood (WB) with 17757 units (93.62%) Percentage of waste to production (PWP)) and cryoprecipitate (CP) with 10246 units (9.20% PWP), respectively (table 1).
The total amount of WB product waste was 17757 units, and the highest WB waste was reported in 2018 with 3327 units (98.724% PWP). Also, after 2018, the WB wastage decreased (Table 1). The amount of leukocyte reduced RBC (RBC-LR) product waste was equal to 5974 units, and the highest amount of RBC-LR waste was reported in 2015 with 1502 units (2.87% PWP). After 2015, the production of RBC-LR waste was declining. The total amount of RBC product waste was 27813 units, and the highest RBC waste was reported in 2017, with 6983 units (6.75% PWP). After 2017, the wastage of RBC components was reduced (Table 1).
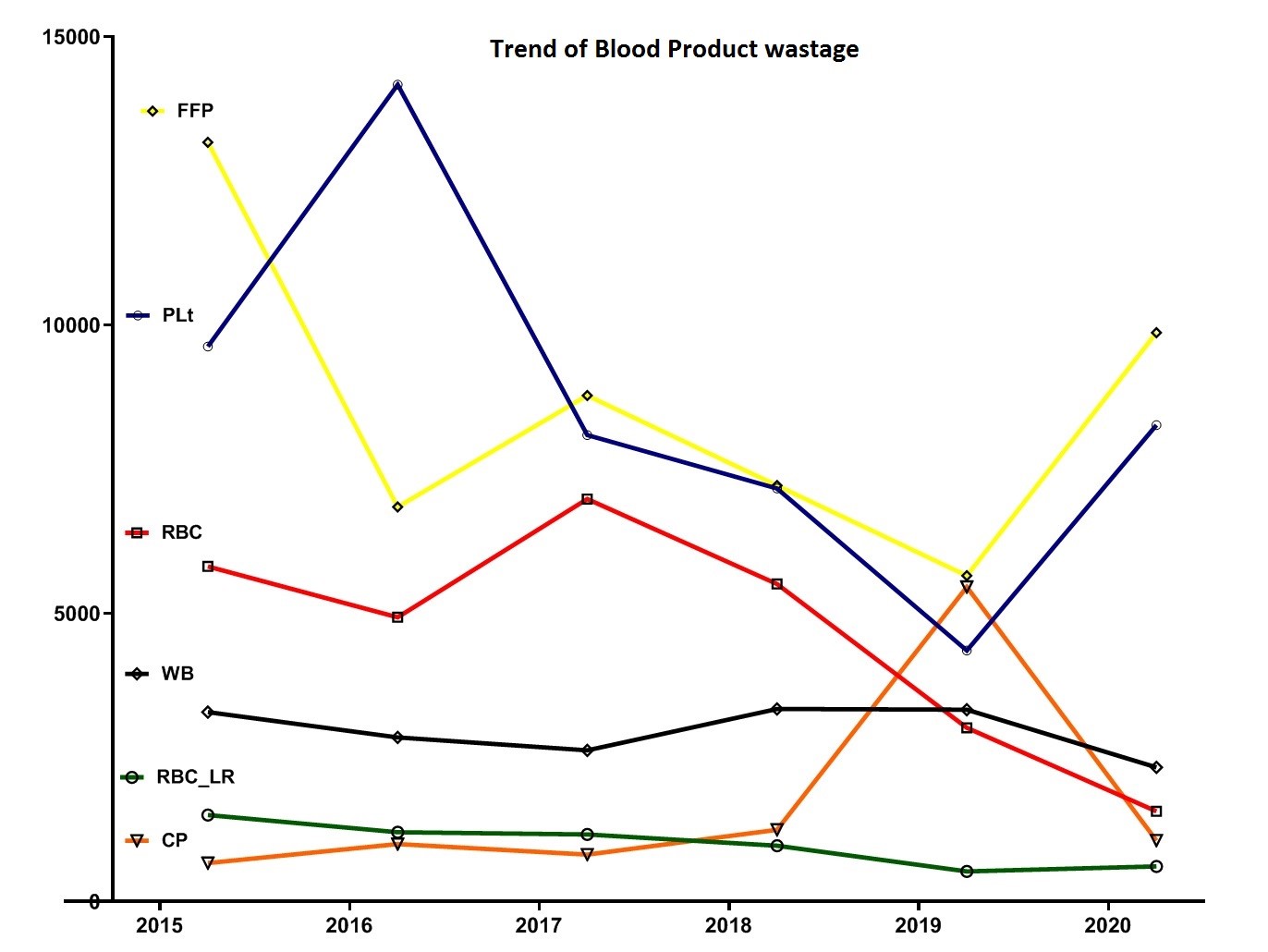
The total amount of fresh frozen plasma (FFP) waste was 51524 units, and the highest FFP waste was reported in 2015 with 13167 units (9.18% PWP). After 2015, the production of FFP waste showed a decreasing trend (Table 1 and Fig. 1).
The total amount of Plt wastage was equal to 51667 units, and the highest platelet wastage was reported in 2016 with 14167 units (12.7% PWP). After 2016, the production trend of Plt waste decreased (Table 1 and Fig. 1).
The total amount of CP product waste was equal to 10246 units, while the highest CP waste was reported in 2019 with 5457 units (19.68% PWP) (Table 1).
Reasons for blood component wasting
In this study, the causes of wasted products were also investigated. In the case of WB, the main reasons for discarding the products were low volume (5816), CUE (4042) and expiration date (1839) (Table 2).
In the case of RBC-LR, the main reasons for discarding products were positive screening test (1172), expiration date (1064) and high volume (964) (Table 2). Regarding RBC waste, the main reasons for discarding the products were the expiration date of consumption (14465), high volume (5801), and positive screening test (2825), respectively (Table 2). Regarding FFP products, the main reasons for discarding the products included low volume (18937), rupture and leakage (3580), abnormal color (Lipemic and bloody) (2906) and leakage from the seal site on the blood cord (2820), respectively (Table 2). Regarding the Plt product, the expiration date (35830), a positive screening test (521) and abnormal color (Lipemic and Bloody) (1791) were the prominent causes of product wastage, respectively (Table 2). Regarding CP products, the main reasons for discarding the products were the expiration date (6308), rupture and leakage (1661) and positive screening test (521), respectively (Table 2).
Evaluation of the trend of the wasting reasons
screening test-positive
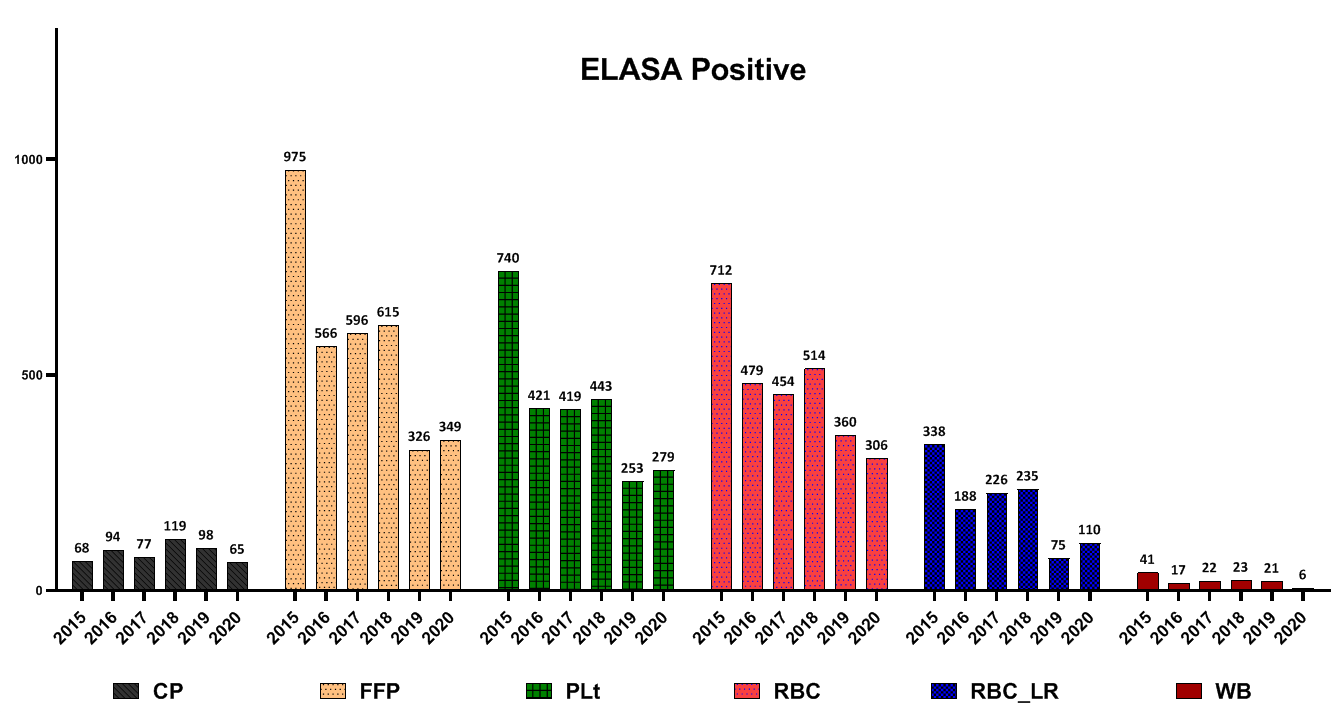
For most blood products, positive screening tests declined from 2015 to 2020 (Fig. 2).
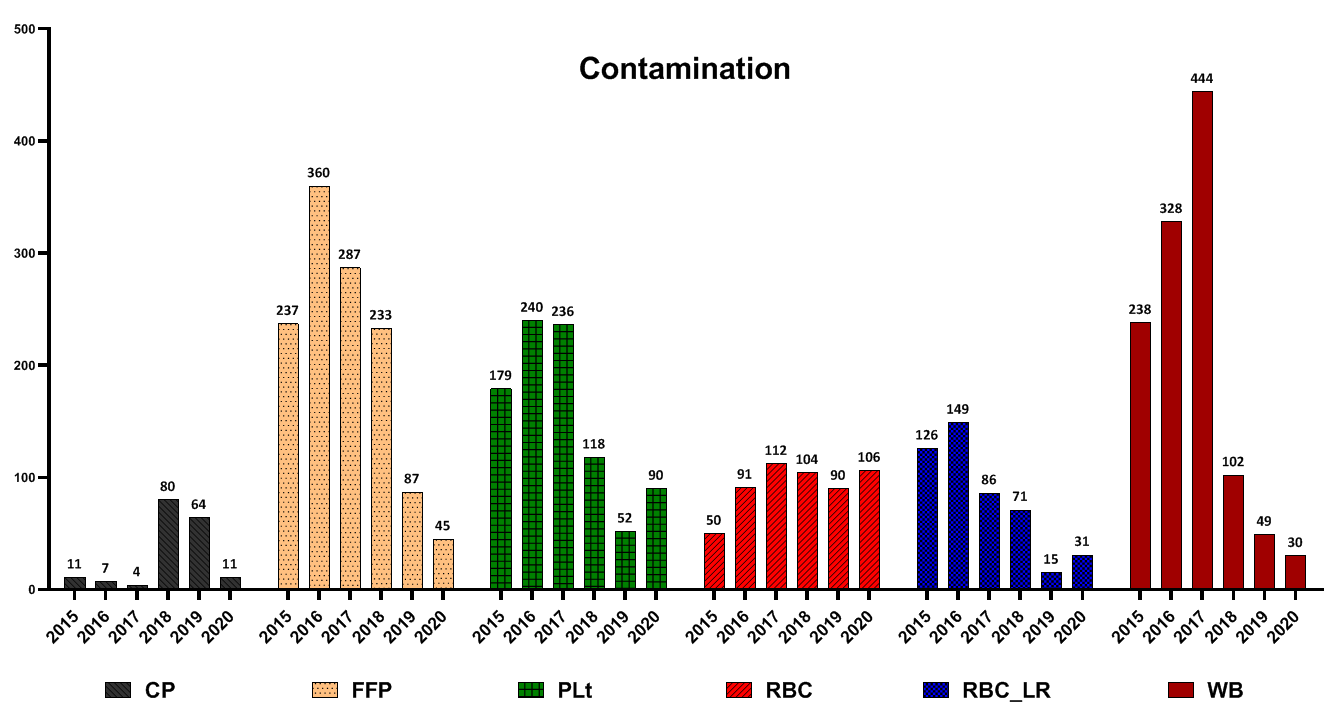
Contamination of blood products: In the case of FFP, Plt and RBC-LR, most contamination cases were reported in 2016. However, they decreased after that. Also, regarding WB and RBC products, most cases of wastage were reported in 2017; this trend declined after that (Fig. 3).
Expiration date
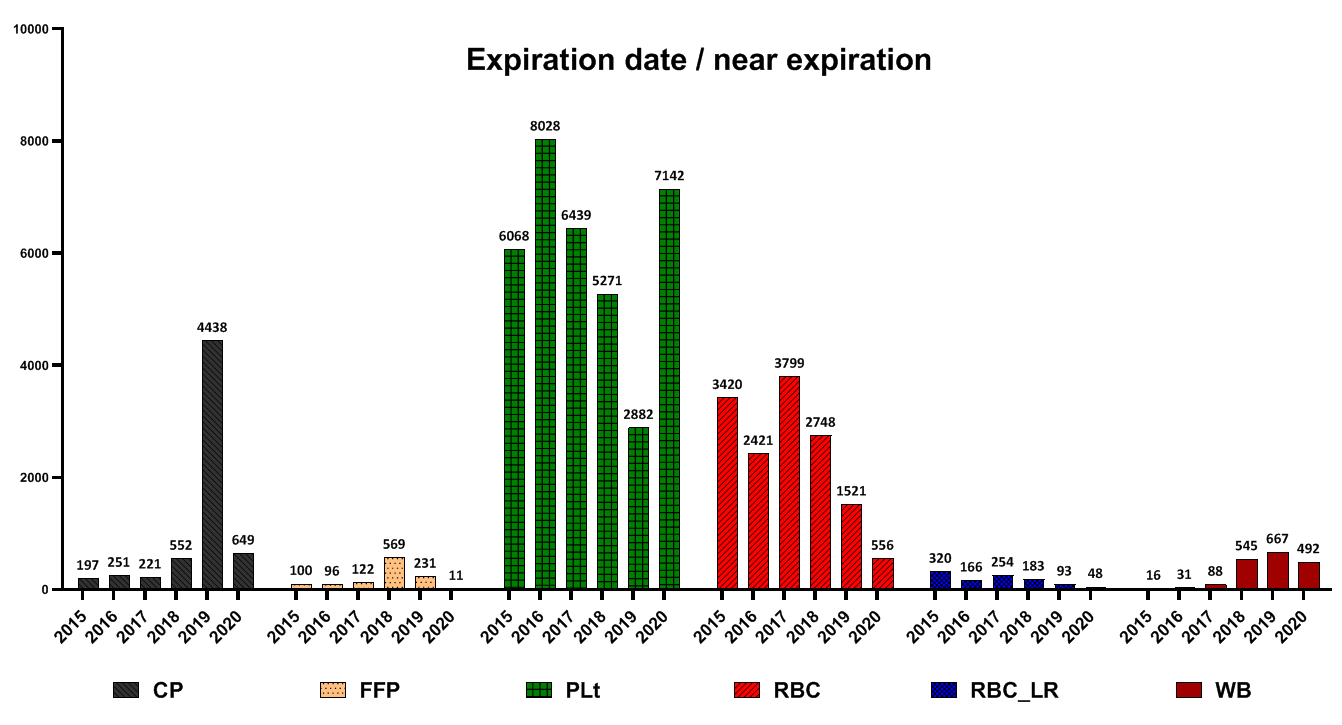
The highest number of FFP and CP wastage due to the expiration date was reported in 2019. Regarding Plt, the highest amount was in 2016 and then decreased, but in 2020, the rate of Plt wastage increased again due to the expiration date. Regarding RBC products, the highest amount of wastage due to the expiration date was reported in 2017 and has been declining ever since. Regarding RBC-LR products, from 2015 to 2020, the waste of components decreased due to the expiration date. Regarding WB products, the most waste due to the expiration date was reported in 2019 (Fig. 4).
The blood donation process is such that the donor is first certified by a physician after consultation and physical examination, and then a Confidential Unit Exclusion (CUE) form is provided to the donor so that the donor can decide whether or not the donated blood is used for the patient. Screening tests that are routinely performed on all blood vessels after donation include hepatitis B surface antigen (HBsAg), hepatitis C virus antibody (Anti-HCV), syphilis, and human immunodeficiency virus (HIV) Ag/ Ab [4, 5]. Donated blood is then sent to the processing department and various products are prepared from whole blood. After determining the test results, the product is labelled and sent to the blood distribution department. During the various stages of preparation of blood products, from the stage of blood collection to blood distribution, these products may be damaged or expired [6]. Despite the country's self-sufficiency in the supply of blood and blood products, comprehensive information on the extent and causes of blood product wastage has not been published in the country. Collecting such information will be effective for the future planning of the blood transfusion organization for the preparation and distribution of blood products. Therefore, in this study, the rate and primary cause of wastage of blood and blood components in blood transfusion centers of Fars province from 2015 to 2020 were examined and evaluated.
Materials and Methods
The present article was a descriptive and retrospective study that examined the data related to the frequency and waste of blood component units and expiry units in blood transfusion centers of Fars province from 2015 to 2020. There are six blood collection centers, preparation and processing centers, and blood transfusion centers in Fars province. All of these centers' information related to blood collection, production of components, distribution of products, discarded units and outdated units are recorded in internal software or recorded and monitored in controlled forms. All this information is evaluated and checked at the province's and country's central blood transfusion headquarters.
The data relating to blood product wastage were extracted from the comprehensive software of the Blood Transfusion Organization (Negareh), and the cause and frequency of red blood cell (RBC), platelet (Plt), plasma and cryoprecipitate (CP) wastage from 2015 to 2020 were evaluated.
Definitions
Production units
Units from whole blood labeled and licensed for distribution and consumption.
Wastage units in blood transfusion centers
These units have been discarded for various reasons, such as hemolysis, rupture, or positive screening tests at blood transfusion centers, and have been removed from the distribution to hospitals. Screening tests were done on all samples and included HIV Ag / Ab, Anti-HCV, HBs Ag and syphilis. If the sample reacts in the screening tests, the donation units are lost according to the organization's algorithm, regardless of the repeat test result [1]. Confirmation tests, including HCV recombinant immunoblot assay (RIBA), HBsAg neutralization test, HIV western blot and fluorescent treponemal antibody (FTA), are performed on the samples that have reacted to the second time in the tests, depending on the type of reaction in the screening test.
Wasted units in hospitals (returning units from hospitals)
These units were discarded in the hospital, and their numbers were sent to blood transfusion centers in particular forms or units that were returned from the hospital to blood transfusion centers and wasted in these centers.
Expiration date units
These units have not been distributed in hospitals or used for patients during their storage in the hospital, and their expiration date has passed.
Statistical analysis
The data were analyzed using SPSS software version 20. All data were prepared as frequencies
and percentages for basic descriptive study. P-values of less than 0.05 were considered statistically significant.
Results
The total amount of waste was 164981 units (6.71%). Also, the annual amount of waste in 2015 was 34065 units (8.12%); 2016, 30991 units (7.15%); 2017, 28454 units (6.75%); 2018, 25449 units (6.19 %); 2019, 22327 units (5.44%) and 2020, 23695 units (6.46%).
The most discarded products were whole blood (WB) with 17757 units (93.62%) Percentage of waste to production (PWP)) and cryoprecipitate (CP) with 10246 units (9.20% PWP), respectively (table 1).
The total amount of WB product waste was 17757 units, and the highest WB waste was reported in 2018 with 3327 units (98.724% PWP). Also, after 2018, the WB wastage decreased (Table 1). The amount of leukocyte reduced RBC (RBC-LR) product waste was equal to 5974 units, and the highest amount of RBC-LR waste was reported in 2015 with 1502 units (2.87% PWP). After 2015, the production of RBC-LR waste was declining. The total amount of RBC product waste was 27813 units, and the highest RBC waste was reported in 2017, with 6983 units (6.75% PWP). After 2017, the wastage of RBC components was reduced (Table 1).
The total amount of fresh frozen plasma (FFP) waste was 51524 units, and the highest FFP waste was reported in 2015 with 13167 units (9.18% PWP). After 2015, the production of FFP waste showed a decreasing trend (Table 1 and Fig. 1).
The total amount of Plt wastage was equal to 51667 units, and the highest platelet wastage was reported in 2016 with 14167 units (12.7% PWP). After 2016, the production trend of Plt waste decreased (Table 1 and Fig. 1).
The total amount of CP product waste was equal to 10246 units, while the highest CP waste was reported in 2019 with 5457 units (19.68% PWP) (Table 1).
Reasons for blood component wasting
In this study, the causes of wasted products were also investigated. In the case of WB, the main reasons for discarding the products were low volume (5816), CUE (4042) and expiration date (1839) (Table 2).
In the case of RBC-LR, the main reasons for discarding products were positive screening test (1172), expiration date (1064) and high volume (964) (Table 2). Regarding RBC waste, the main reasons for discarding the products were the expiration date of consumption (14465), high volume (5801), and positive screening test (2825), respectively (Table 2). Regarding FFP products, the main reasons for discarding the products included low volume (18937), rupture and leakage (3580), abnormal color (Lipemic and bloody) (2906) and leakage from the seal site on the blood cord (2820), respectively (Table 2). Regarding the Plt product, the expiration date (35830), a positive screening test (521) and abnormal color (Lipemic and Bloody) (1791) were the prominent causes of product wastage, respectively (Table 2). Regarding CP products, the main reasons for discarding the products were the expiration date (6308), rupture and leakage (1661) and positive screening test (521), respectively (Table 2).
Evaluation of the trend of the wasting reasons
screening test-positive
For most blood products, positive screening tests declined from 2015 to 2020 (Fig. 2).
Contamination of blood products: In the case of FFP, Plt and RBC-LR, most contamination cases were reported in 2016. However, they decreased after that. Also, regarding WB and RBC products, most cases of wastage were reported in 2017; this trend declined after that (Fig. 3).
Expiration date
The highest number of FFP and CP wastage due to the expiration date was reported in 2019. Regarding Plt, the highest amount was in 2016 and then decreased, but in 2020, the rate of Plt wastage increased again due to the expiration date. Regarding RBC products, the highest amount of wastage due to the expiration date was reported in 2017 and has been declining ever since. Regarding RBC-LR products, from 2015 to 2020, the waste of components decreased due to the expiration date. Regarding WB products, the most waste due to the expiration date was reported in 2019 (Fig. 4).
Table 1. Blood product waste
| Blood product | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | Total | |
| WB | Total WB waste | 3288 | 2849 | 2623 | 3341 | 3327 | 2329 | 17757 |
| % of waste to production WB | 92.62 | 90.07 | 89.74 | 93.717 | 98.724 | 97.204 | 93.62 | |
| RBC_LR | Total RBC-LR waste | 1502 | 1203 | 1164 | 971 | 524 | 610 | 5974 |
| % of waste to production RBC_LR | 2.87 | 2.28 | 2.13 | 1.76 | 1.33 | 1.29 | 1.98 | |
| RBC | Total RBC waste | 5811 | 4930 | 6983 | 5509 | 3013 | 1567 | 27813 |
| % of waste to production RBC | 5.76 | 4.69 | 6.75 | 5.54 | 2.63 | 1.72 | 4.53 | |
| Plt | Total Plt waste | 9628 | 14167 | 8090 | 7166 | 4353 | 8263 | 51667 |
| % of waste to production PLt | 8.7987 | 12.7 | 7.92 | 7.24 | 4.43 | 9.45 | 8.50 | |
| FFP | Total FFP waste | 13167 | 6843 | 8778 | 7216 | 5653 | 9867 | 51524 |
| % of waste to production FFP | 9.18 | 4.86 | 6.19 | 5.45 | 4.48 | 8.28 | 6.41 | |
| CP | Total CP waste | 669 | 999 | 816 | 1246 | 5457 | 1059 | 10246 |
| % of waste to production CP | 6.89 | 5.92 | 5.02 | 5.72 | 19.68 | 5.55 | 9.20 | |
CP= Cryoprecipitate; FFP= Fresh frozen plasma; Plt = Platelet; RBC= Red blood cells; RBC-LR= Leukocyte reduced red blood cells ; WB= Whole blood
Table 2. Prominent reason of blood products waste
| CP | FFP | Plt | RBC | RBC-LR | WB |
Products
Reason of waste
|
| 521 | 3427 | 2555 | 2825 | 1172 | 130 | Screening test positive |
| 6308 | 1129 | 35830 | 14465 | 1064 | 1839 | Expiry date |
| 94 | 1113 | 493 | 716 | 317 | 69 | Callback and callback history |
| 177 | 1249 | 915 | 553 | 478 | 1191 | Contamination |
| 9 | 29 | 11 | 45 | 14 | 1 | Blood type discrepancies |
| 243 | 2906 | 1791 | 165 | 67 | 153 | (Unnatural and bloody color (Icteric, Lipemic, Bloody |
| - | - | - | - | - | 4042 | Confidential Unit Exclusion |
| - | - | - | - | - | 700 | No filtration |
| 15 | 99 | 58 | 50 | 67 | 3 | Exemptions in other provinces |
| 130 | 2820 | 226 | 192 | 186 | 439 | Leakage from the place of seal and cord |
| 1661 | 3580 | 647 | 268 | 262 | 1738 | Rupture and leakage |
| - | 77 | 22 | 32 | 28 | 101 | Hemolysis |
| 24 | 18937 | 812 | 532 | 69 | 5816 | Low volume |
| 6 | 21 | 76 | 5801 | 964 | 764 | High volume |
| - | - | - | 1241 | 65 | - | No cord for RBC and cord finish |
| 228 | 274 | 69 | 43 | 47 | - | Label distortion after processing and pilot distorted label |
CP= Cryoprecipitate; FFP= Fresh frozen plasma; Plt = Platelet; RBC= Red blood cells; RBC-LR= Leukocyte reduced red blood cells ; WB= Whole blood
High volume
High volume is one of the main reasons for discarding RBC, RBC-LR and WB products. In the case of RBC and RBC-LR, most cases of wastage were reported in 2017 and have been declining since then. In the case of the WB, this trend has been declining since 2015 (Fig. 5).
Degradation and distortion of the label
For all blood products, the highest level of destruction and distortion of the label was reported in 2016, and after that, it had a decreasing trend (Fig. 6).
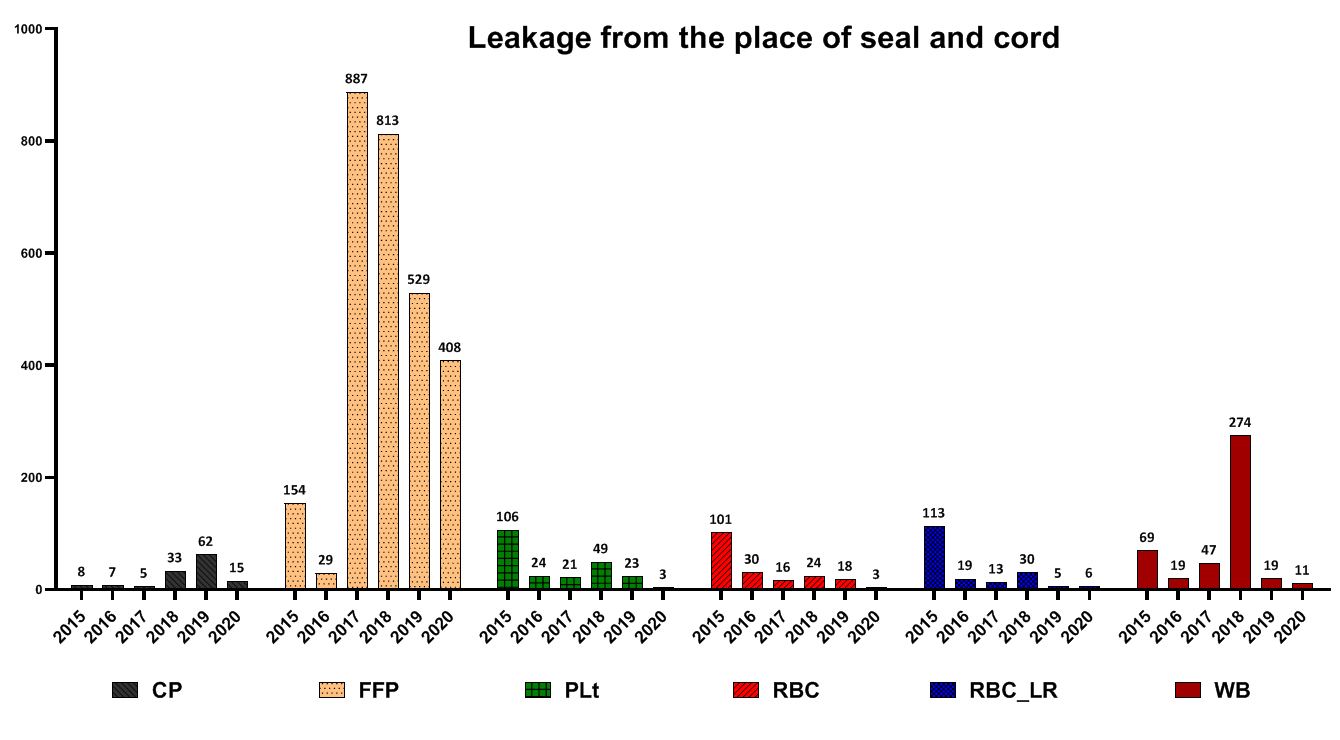
Leakage from seal site and cord
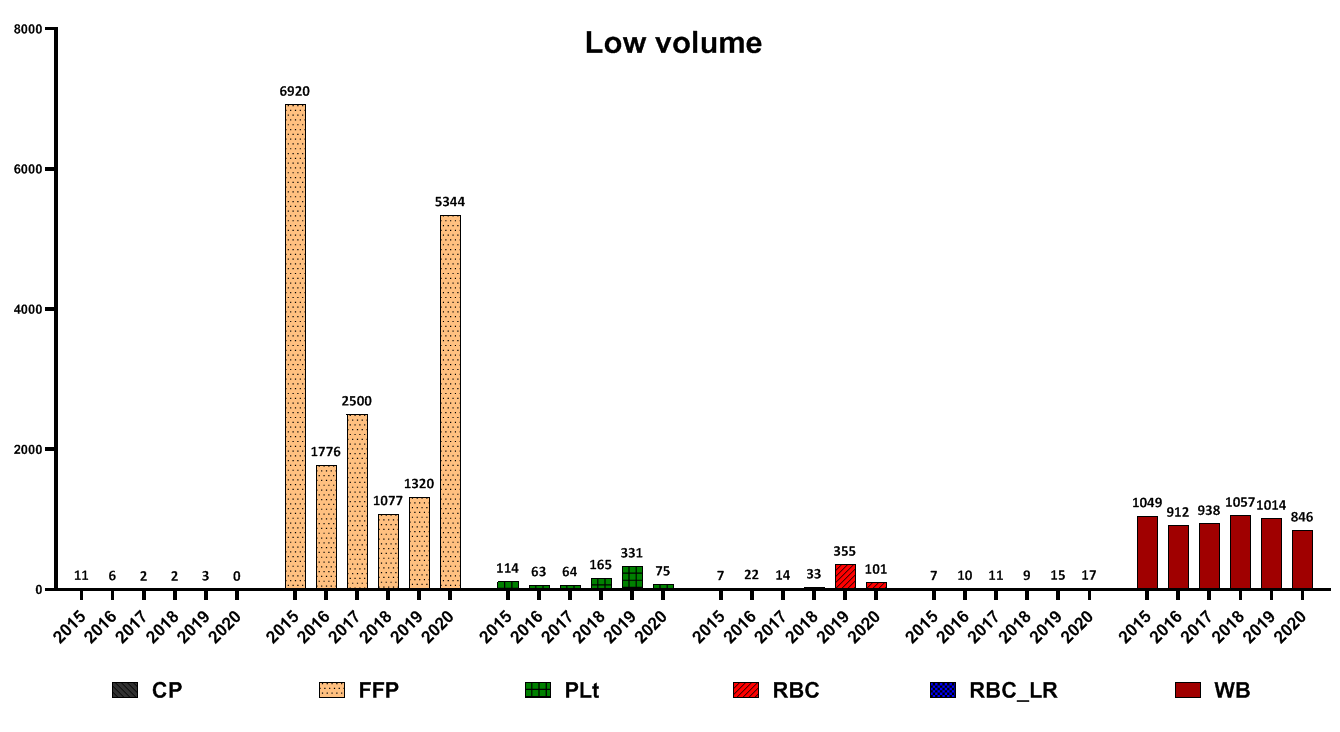
The highest number of FFP wastage for this reason was reported in 2017, CP in 2019 and WB in 2018 (Fig. 7). Low volume was the main reason for eliminating blood products such as FFP and WB (Fig. 8).
High volume is one of the main reasons for discarding RBC, RBC-LR and WB products. In the case of RBC and RBC-LR, most cases of wastage were reported in 2017 and have been declining since then. In the case of the WB, this trend has been declining since 2015 (Fig. 5).
Degradation and distortion of the label
For all blood products, the highest level of destruction and distortion of the label was reported in 2016, and after that, it had a decreasing trend (Fig. 6).
Leakage from seal site and cord
The highest number of FFP wastage for this reason was reported in 2017, CP in 2019 and WB in 2018 (Fig. 7). Low volume was the main reason for eliminating blood products such as FFP and WB (Fig. 8).

Fig. 1. Trend of different blood components wastage during 2015- 2020
CP= Cryoprecipitate; FFP= Fresh frozen plasma; Plt = Platelet; RBC= Red blood cells; RBC-LR= Leukocyte reduced red blood cells ; WB= Whole blood

Fig. 2. Frequency of different blood components wastage due to positive tests for blood-borne diseases (human immunodeficiency virus, hepatitis B surface antigen, hepatitis C virus, syphilis)
CP= Cryoprecipitate; FFP= Fresh frozen plasma; Plt = Platelet; RBC= Red blood cells; RBC-LR= Leukocyte reduced red blood cells ; WB= Whole blood

Fig. 3. Frequency of different blood components wastage due to contamination

Fig. 4. Frequency of different blood components wastage due to expiration date
CP= Cryoprecipitate; FFP= Fresh frozen plasma; Plt = Platelet; RBC= Red blood cells; RBC-LR= Leukocyte reduced red blood cells ; WB= Whole blood

Fig. 5. Frequency of different blood components wastage due to high volum
CP= Cryoprecipitate; FFP= Fresh frozen plasma; Plt = Platelet; RBC= Red blood cells; RBC-LR= Leukocyte reduced red blood cells ; WB= Whole blood

Fig. 6. Frequency of different blood components wastage due to lable distortion after processing or pilot distorted lable
CP= Cryoprecipitate; FFP= Fresh frozen plasma; Plt = Platelet; RBC= Red blood cells; RBC-LR= Leukocyte reduced red blood cells ; WB= Whole blood

Fig.7. Frequency of different blood components wastage due to leakage
CP= Cryoprecipitate; FFP= Fresh frozen plasma; PLt = Platelet; RBC= Red Blood Cells; RBC-LR= Leukocyte reduced Red Blood Cells ; WB= Whole blood
CP= Cryoprecipitate; FFP= Fresh frozen plasma; PLt = Platelet; RBC= Red Blood Cells; RBC-LR= Leukocyte reduced Red Blood Cells ; WB= Whole blood

Fig. 8. Frequency of different blood components wastage due to low volume
CP= Cryoprecipitate; FFP= Fresh frozen plasma; PLt = Platelet; RBC= Red Blood Cells; RBC-LR= Leukocyte reduced Red Blood Cells ; WB= Whole blood
CP= Cryoprecipitate; FFP= Fresh frozen plasma; PLt = Platelet; RBC= Red Blood Cells; RBC-LR= Leukocyte reduced Red Blood Cells ; WB= Whole blood
Discussion
Blood transfusions are an important part of health care. With the advent of newer treatments and accurate and better diagnosis, diseases requiring blood transfusions have increased; therefore, the need for blood and blood components has also increased [7]. So, in the current study, the situation related to blood component wastage between 2015 and 2020 was examined in blood transfusion centers of Fars Province. Our findings indicated that the total amount of waste was 164981 units (6.71%). Also, the highest percentage of waste to products was in 2015 (8.12%), 2016 (7.15 %) and 2017 (6.75 %), respectively. The most discarded products were related to WB with 17757 units (93.62%), CP with 10246 units (9.20%) and Plt (8.50 %), respectively.
A study by Dunbar et al. conducted at three trauma centers reported that CP had the highest rates of discarding units at these centers [8]. In the present study, CP was also one of the most common waste products.
However, a study by Desai et al. at the Intensive Care Hospital in Mumbai reported that random platelets were the most discarded product [9].
Evaluation of the trend of wastage rate during 2015- 2020 showed that the highest WB waste was reported in 2018, and after that, the WB wastage decreased.
The highest amount of RBC-LR and FFP wastage was reported in 2015. After 2015, the amount of FFP and RBC-LR wastage declined. The highest platelet wastage was reported in 2016. After 2016, the production trend of Plt waste decreased. The highest RBC wastage was reported in 2017. After 2017, the production of RBC waste decreased, and
the highest CP wastage was reported in 2019.
In this study, the main causes of waste included positive screening test results, expiration date, contamination, blood group incompatibility, abnormal and bloody color, confidential unit exclusion, impaired filtration, the exemption in other provinces, leakage from the seal site on the blood cord, leakage and rupture, hemolysis, low volume, high volume, absence of blood cord and label distortion after processing.
Other researchers evaluated the frequency and causes of blood product wastage in another province of Iran and another part of the world.
A study by Kurup et al. reported that the most discarded blood type was O-positive, and the most discarded product was platelets. Their study showed that the main causes of blood product wastage included expiration date, broken wax seal, cold chain disorder, bag rupture, return after 30 minutes and blood clots [10].
Moreover, the present study's findings showed that expiration date, positive screening test and low-volume components were the most common reasons for blood product wastage. These findings are consistent with other studies. For instance, a study conducted by Javadzadeh et al. and Kumar et al. reported that the expiration date was the most common cause of discarded blood units [11, 12]. A study conducted by Far et al. showed that about 77.9 % of the discarded pack cell units were due to expiration date [13]. In another study by Joshi et al., the reasons for blood component wastage were analyzed in the blood bank of Shri Krishna Hospital, India. They reported that the most common reason for wastage was the short expiry life of PLt, followed by Transfusion-transmitted infection (TTI) reactivity after the first run of the screening test [14].
In a study conducted by Suresh et al. and several other studies, seroreactivity due to TTIs was reported as the most common cause of blood unit discarding [15-17]. In another study by Shamshirian et al. at the Sari Heart Center, they reported that the most common cause of blood product waste was over-ordering of blood units [18]. The evaluation of the reasons for the wastage of blood products in this study showed that the positive results of the screening test had a declining trend from 2015 to 2020. Implementing some measures in the Iranian Blood Transfusion Organization has decreased positive screening tests and transfusion-transmitted infections (TTIs) in blood components. These measures included implementing CUE since 2002 for the identification and elimination of high-risk donors, data registry of all blood donors by computerized software and uniform donor deferral criteria since 1997, validating all procedures across the country, screening of first-time volunteer donors since 2017 and educational efforts of Iranian Blood Transfusion Organization (IBTO) to increase the public's knowledge on TTIs and routes of transmission of TTIs [4, 19]. Regarding FFP, Plt and RBC-LR, most contamination cases were reported in 2016 and then decreased. Also, regarding WB and RBC products, the most cases of wastage due to contamination were reported in 2017, which have been declining ever since. Evaluations of discarding blood product wastage due to the expiration date showed that the highest number of FFP and CP wastage due to the expiration date were reported in 2019. Regarding Plt, the highest amount was in 2016 and then decreased. However, in 2020, the rate of Plt wastage increased again due to the expiration date. Regarding RBC products, the highest amount of wastage was reported in 2017 and has declined since then. Regarding RBC-LR products, from 2015 to 2020, the waste of components had a decreasing trend. Regarding WB products, the highest number of waste was reported in 2019. Evaluation high volume components showed that this is a prominent reason for discarding RBC, RBC-LR and WB products. In the case of RBC and RBC-LR, most cases of wastage were reported in 2017 and have been declining since then. In the case of the WB, this trend has declined since 2015. For all blood products, the highest level of destruction and distortion of the label was reported in 2016, and after that, it had a decreasing trend. Evaluation of leakage from the seal site and blood cord indicated that the highest amount of FFP wastage, for this reason, was reported in 2017, CP in 2019 and WB in 2018. In a study by Roy et al., the rate of blood units discarding due to blood leakage was 25.7%. Moreover, they reported that the main cause of the leakage was the rupture of blood units during centrifugation. Regarding FFP, accidental fall after freezing has been reported as the main cause of leakage and rupture [7]. Also, low volume was primarily due to discarding blood products such as FFP and WB. In the present study, expired platelets, low-volume FFP, and rupture were the main causes of wastage. Regarding expired platelet wastes, these reasons can be mentioned: 1- Short consumption period ( 3 to 5 days) for platelets, 2- mismatch between hospital demand and platelet production rate, 3- Lack of coordination between the blood groups requested by the medical centers with the blood group of the produced products (processing unit has no involvement in selecting the blood type of whole blood as raw materials).
The main solution to manage the amount of expired platelet waste is to balance the demand of medical centers and the amount of platelet production. In addition, in cases where the number of blood donations is much more than requested by medical centers, we can prevent platelet overproduction by methods such as changing the type of blood bags in the donation unit as well as producing other products from triple bags such as cryoprecipitate with longer shelf life. The possible reasons for low volume FFP production are 1- Lack of hemoglobin check of donors in the donation unit and blood donation in cases with high hemoglobin concentration. To solve this problem, it is recommended that hemoglobin be checked for all donors and donors with elevated hemoglobin, and double or triple-filtered bags be used for blood collection. In cases where these bags are not available, blood bags taken from these donors should be labeled to produce a suitable product in the processing unit.
2- Lack of validation of parameters of refrigerated centrifuges (speed, temperature, time) to produce different products; to solve this problem, validation of centrifuge parameters (speed, temperature, time) reduces the high percentage of low volume FFP. Finally, reducing the low volume of FFP also reduces the high volume of RBC and RBC-LR products.
3- Failure to completely separate the platelet-rich plasma from packed red blood cells (packed cells) during platelet production at light speed (time: 4 minutes, temperature: 22 °C, speed: 2150 g) so that some platelet-rich plasma remains on the packed cells in the first stage.
To solve these problems, it is suggested that different stages of production be performed according to the appropriate instructions. Skill training and retraining should be done periodically. Also, in the case of new technical staff, the training process should be done completely to ensure the effectiveness of the training.
4- Inadequate volume of WB due to lack of hemoshaker calibration check in the donation unit. It is possible to prevent the production of low volume WB by using a hemoscale-hemoshaker device in all blood collection centers. Also, daily and periodic calibration of the hemoscale-hemoshaker with calibrated weights can ensure its accuracy. Regarding the reasons for leakage and rupture, which are some of the main causes of the waste in WB, FFP and CP products, the following reasons can be mentioned: 1- Defects of blood collection bags 2- Problems in sealing devices 3- Improper balance of bags of blood during centrifugation.
Conclusion
Our study showed the dropping pattern of blood wastage during this period in the Fars blood transfusion Centers. Also, the most discarded products were WB, CP and Plt, respectively. It was found that the main reason for the blood wastage in these centers included positive screening test results, expiration date, contamination, blood type incompatibility, and abnormal and bloody color products, respectively.
The authors of this article suggest some measures that can be implemented to minimize the wastage of blood components at the blood transfusion center, including strictly following the instructions for preparing blood products, maintaining and validating and accurately calibrating the instruments involved in the preparation of blood products, periodical training of technical staff and regular management meetings about blood component wastage.
In this study, the reason for the positive enzyme-linked immunosorbent assay test was not discussed in detail. Moreover, the details of the blood grouping problems, such as mismatches with the records, unclear RH, or the rate of O cell positive, were not determined.
Ethical Considerations
This study was approved and conducted according to the Ethics Committee Guidelines of the Gerash University of Medical Sciences, Gerash, Iran (Ethic code: IR.GERUMS.REC.1402.001).
Funding
This study was funded by the Deputy of Research of Gerash University of Medical Sciences, Gerash, Iran.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgments
The authors would like to thank the cellular and molecular research center of Gerash University of Medical Sciences and the Shiraz Blood Transfusion Center staff for their help in conducting this study.
Authors’ Contributions
M.A, M.A, A.K, Z.N and P.B: conceptualization, data collection, data interpretation. H.F: manuscript writing, and figure preparation. D.Z, MS.G and AB.A: data analysis, interpretation, manuscript revision, and figure formatting. All authors reviewed and approved the final version of the manuscript.
A study by Dunbar et al. conducted at three trauma centers reported that CP had the highest rates of discarding units at these centers [8]. In the present study, CP was also one of the most common waste products.
However, a study by Desai et al. at the Intensive Care Hospital in Mumbai reported that random platelets were the most discarded product [9].
Evaluation of the trend of wastage rate during 2015- 2020 showed that the highest WB waste was reported in 2018, and after that, the WB wastage decreased.
The highest amount of RBC-LR and FFP wastage was reported in 2015. After 2015, the amount of FFP and RBC-LR wastage declined. The highest platelet wastage was reported in 2016. After 2016, the production trend of Plt waste decreased. The highest RBC wastage was reported in 2017. After 2017, the production of RBC waste decreased, and
the highest CP wastage was reported in 2019.
In this study, the main causes of waste included positive screening test results, expiration date, contamination, blood group incompatibility, abnormal and bloody color, confidential unit exclusion, impaired filtration, the exemption in other provinces, leakage from the seal site on the blood cord, leakage and rupture, hemolysis, low volume, high volume, absence of blood cord and label distortion after processing.
Other researchers evaluated the frequency and causes of blood product wastage in another province of Iran and another part of the world.
A study by Kurup et al. reported that the most discarded blood type was O-positive, and the most discarded product was platelets. Their study showed that the main causes of blood product wastage included expiration date, broken wax seal, cold chain disorder, bag rupture, return after 30 minutes and blood clots [10].
Moreover, the present study's findings showed that expiration date, positive screening test and low-volume components were the most common reasons for blood product wastage. These findings are consistent with other studies. For instance, a study conducted by Javadzadeh et al. and Kumar et al. reported that the expiration date was the most common cause of discarded blood units [11, 12]. A study conducted by Far et al. showed that about 77.9 % of the discarded pack cell units were due to expiration date [13]. In another study by Joshi et al., the reasons for blood component wastage were analyzed in the blood bank of Shri Krishna Hospital, India. They reported that the most common reason for wastage was the short expiry life of PLt, followed by Transfusion-transmitted infection (TTI) reactivity after the first run of the screening test [14].
In a study conducted by Suresh et al. and several other studies, seroreactivity due to TTIs was reported as the most common cause of blood unit discarding [15-17]. In another study by Shamshirian et al. at the Sari Heart Center, they reported that the most common cause of blood product waste was over-ordering of blood units [18]. The evaluation of the reasons for the wastage of blood products in this study showed that the positive results of the screening test had a declining trend from 2015 to 2020. Implementing some measures in the Iranian Blood Transfusion Organization has decreased positive screening tests and transfusion-transmitted infections (TTIs) in blood components. These measures included implementing CUE since 2002 for the identification and elimination of high-risk donors, data registry of all blood donors by computerized software and uniform donor deferral criteria since 1997, validating all procedures across the country, screening of first-time volunteer donors since 2017 and educational efforts of Iranian Blood Transfusion Organization (IBTO) to increase the public's knowledge on TTIs and routes of transmission of TTIs [4, 19]. Regarding FFP, Plt and RBC-LR, most contamination cases were reported in 2016 and then decreased. Also, regarding WB and RBC products, the most cases of wastage due to contamination were reported in 2017, which have been declining ever since. Evaluations of discarding blood product wastage due to the expiration date showed that the highest number of FFP and CP wastage due to the expiration date were reported in 2019. Regarding Plt, the highest amount was in 2016 and then decreased. However, in 2020, the rate of Plt wastage increased again due to the expiration date. Regarding RBC products, the highest amount of wastage was reported in 2017 and has declined since then. Regarding RBC-LR products, from 2015 to 2020, the waste of components had a decreasing trend. Regarding WB products, the highest number of waste was reported in 2019. Evaluation high volume components showed that this is a prominent reason for discarding RBC, RBC-LR and WB products. In the case of RBC and RBC-LR, most cases of wastage were reported in 2017 and have been declining since then. In the case of the WB, this trend has declined since 2015. For all blood products, the highest level of destruction and distortion of the label was reported in 2016, and after that, it had a decreasing trend. Evaluation of leakage from the seal site and blood cord indicated that the highest amount of FFP wastage, for this reason, was reported in 2017, CP in 2019 and WB in 2018. In a study by Roy et al., the rate of blood units discarding due to blood leakage was 25.7%. Moreover, they reported that the main cause of the leakage was the rupture of blood units during centrifugation. Regarding FFP, accidental fall after freezing has been reported as the main cause of leakage and rupture [7]. Also, low volume was primarily due to discarding blood products such as FFP and WB. In the present study, expired platelets, low-volume FFP, and rupture were the main causes of wastage. Regarding expired platelet wastes, these reasons can be mentioned: 1- Short consumption period ( 3 to 5 days) for platelets, 2- mismatch between hospital demand and platelet production rate, 3- Lack of coordination between the blood groups requested by the medical centers with the blood group of the produced products (processing unit has no involvement in selecting the blood type of whole blood as raw materials).
The main solution to manage the amount of expired platelet waste is to balance the demand of medical centers and the amount of platelet production. In addition, in cases where the number of blood donations is much more than requested by medical centers, we can prevent platelet overproduction by methods such as changing the type of blood bags in the donation unit as well as producing other products from triple bags such as cryoprecipitate with longer shelf life. The possible reasons for low volume FFP production are 1- Lack of hemoglobin check of donors in the donation unit and blood donation in cases with high hemoglobin concentration. To solve this problem, it is recommended that hemoglobin be checked for all donors and donors with elevated hemoglobin, and double or triple-filtered bags be used for blood collection. In cases where these bags are not available, blood bags taken from these donors should be labeled to produce a suitable product in the processing unit.
2- Lack of validation of parameters of refrigerated centrifuges (speed, temperature, time) to produce different products; to solve this problem, validation of centrifuge parameters (speed, temperature, time) reduces the high percentage of low volume FFP. Finally, reducing the low volume of FFP also reduces the high volume of RBC and RBC-LR products.
3- Failure to completely separate the platelet-rich plasma from packed red blood cells (packed cells) during platelet production at light speed (time: 4 minutes, temperature: 22 °C, speed: 2150 g) so that some platelet-rich plasma remains on the packed cells in the first stage.
To solve these problems, it is suggested that different stages of production be performed according to the appropriate instructions. Skill training and retraining should be done periodically. Also, in the case of new technical staff, the training process should be done completely to ensure the effectiveness of the training.
4- Inadequate volume of WB due to lack of hemoshaker calibration check in the donation unit. It is possible to prevent the production of low volume WB by using a hemoscale-hemoshaker device in all blood collection centers. Also, daily and periodic calibration of the hemoscale-hemoshaker with calibrated weights can ensure its accuracy. Regarding the reasons for leakage and rupture, which are some of the main causes of the waste in WB, FFP and CP products, the following reasons can be mentioned: 1- Defects of blood collection bags 2- Problems in sealing devices 3- Improper balance of bags of blood during centrifugation.
Conclusion
Our study showed the dropping pattern of blood wastage during this period in the Fars blood transfusion Centers. Also, the most discarded products were WB, CP and Plt, respectively. It was found that the main reason for the blood wastage in these centers included positive screening test results, expiration date, contamination, blood type incompatibility, and abnormal and bloody color products, respectively.
The authors of this article suggest some measures that can be implemented to minimize the wastage of blood components at the blood transfusion center, including strictly following the instructions for preparing blood products, maintaining and validating and accurately calibrating the instruments involved in the preparation of blood products, periodical training of technical staff and regular management meetings about blood component wastage.
In this study, the reason for the positive enzyme-linked immunosorbent assay test was not discussed in detail. Moreover, the details of the blood grouping problems, such as mismatches with the records, unclear RH, or the rate of O cell positive, were not determined.
Ethical Considerations
This study was approved and conducted according to the Ethics Committee Guidelines of the Gerash University of Medical Sciences, Gerash, Iran (Ethic code: IR.GERUMS.REC.1402.001).
Funding
This study was funded by the Deputy of Research of Gerash University of Medical Sciences, Gerash, Iran.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgments
The authors would like to thank the cellular and molecular research center of Gerash University of Medical Sciences and the Shiraz Blood Transfusion Center staff for their help in conducting this study.
Authors’ Contributions
M.A, M.A, A.K, Z.N and P.B: conceptualization, data collection, data interpretation. H.F: manuscript writing, and figure preparation. D.Z, MS.G and AB.A: data analysis, interpretation, manuscript revision, and figure formatting. All authors reviewed and approved the final version of the manuscript.
References
[1]. Cheraghali A. Overview of blood transfusion system of Iran: 2002–2011. Iranian Journal of Public Health 2012; 41(8): 89-94.
[2]. American Association Of Blood Banks (AABB); 2008. Standards for blood banks and transfusion services; p. 20-7.
[3]. Sack D. Spotlight BSMS, Blood Stocks Management Scheme. Merthyr Tydfil, UK, Prince Charles Hospital. 2010.
[4]. Azadbakht M, Ardakani MT, Delirakbariazar M, Kasraian L, Khaledi A, Foruozandeh H, et al. Seroprevalence and trend of HBV, HCV, and HIV infections among blood donors of Fars Province, Iran (2006-2018). Ethiopian Journal of Health Sciences 2020; 30(3): 32-40.
[5]. Cheraghali AM. Blood safety concerns in the Eastern Mediterranean region. Hepatitis monthly 2011; 11(6): 422-30.
[6]. Beliën J, Forcé H. Supply chain management of blood products: A literature review. European Journal of Operational Research 2012; 217(1): 1-16.
[7]. Roy N, Shah A. Retrospective analysis of reasons for discarding whole blood and blood components at tertiary care hospital blood bank in South Gujarat. National Journal of Community Medicine 2017; 8(9): 525-29.
[8]. Dunbar NM, Olson NJ, Szczepiorkowski ZM, Martin ED, Tysarcyk RM, Triulzi DJ, et al. Blood component transfusion and wastage rates in the setting of massive transfusion in three regional trauma centers. Transfusion 2017; 57(1): 45-52.
[9]. Desai N, Shukla K, Ahire N. Utilization pattern of blood and blood components in a blood bank of a tertiary care hospital in Mumbai. Indian Journal of Public Health Research & Development 2018; 9(5): 91-5.
[10]. Kurup R, Anderson A, Boston C, Burns L, George M, Frank M. A study on blood product usage and wastage at the public hospital, Guyana. BMC research notes. 2016; 9(1): 1-6.
[11]. Javadzadeh Shahshahani H, Taghvaee N, Akhavan Tafti F. Frequency of blood components wastage and associated factors in Yazd healthcare centers. Iranian Journal of Blood and Cancer 2016; 8(4): 112-16.
[12]. Kumar A, Sharma SM, Ingole NS, Gangane N. Analysis of reasons for discarding blood and blood components in a blood bank of tertiary care hospital in central India: A prospective study. International Journal of Medicine and Public Health 2014;4 (1): 71-4.
[13]. Far RM, Rad FS, Abdolazimi Z, Kohan MMD. Determination of rate and causes of wastage of blood and blood products in Iranian hospitals. Turkish Journal of Hematology 2014; 31(2): 161-66.
[14]. Joshi HJ, Patel KB, Dholu M. An analysis of wastage of blood components in blood bank at tertiary care hospital. International Journal of Clinical and Diagnostic Pathology 2021; 4(1): 138-42.
[15]. Suresh B, Sreedhar Babu K, Arun R, Chandramouli P, Jothibai D. Reasons for discarding whole blood and its components in a tertiary care teaching hospital blood bank in South India. J Clin Sci Res. 2015; 4: 213-19.
[16]. Kora S, Kulkarni K. An analysis Of donor blood wastage in a blood bank in rural Karnataka. J Clin Diagn Res. 2011; 5(7): 1393-396.
[17]. Thakare M, Dixit J, Goel N. Reasons for discarding blood from blood bank of government medical college, Aurangabad. Asian journal of transfusion science. 2011; 5(1): 59-64.
[18]. Shamshirian A, Mohseni AR, Pourfathollah AA, Mehdipour S, Hosseini S, Ghorbanpour A, et al. A review of blood usage and wastage in a tertiary heart center. Acta Clinica Belgica. 2020; 75(2): 96-103.
[19]. Farshadpour F, Taherkhani R, Tajbakhsh S, Gholizadeh Tangestani M, Hajiani G, Sharifi N, et al. Prevalence and trends of transfusion-transmissible viral infections among blood donors in south of Iran: an eleven-year retrospective study. PloS one. 2016; 11(6): 157-65.
[2]. American Association Of Blood Banks (AABB); 2008. Standards for blood banks and transfusion services; p. 20-7.
[3]. Sack D. Spotlight BSMS, Blood Stocks Management Scheme. Merthyr Tydfil, UK, Prince Charles Hospital. 2010.
[4]. Azadbakht M, Ardakani MT, Delirakbariazar M, Kasraian L, Khaledi A, Foruozandeh H, et al. Seroprevalence and trend of HBV, HCV, and HIV infections among blood donors of Fars Province, Iran (2006-2018). Ethiopian Journal of Health Sciences 2020; 30(3): 32-40.
[5]. Cheraghali AM. Blood safety concerns in the Eastern Mediterranean region. Hepatitis monthly 2011; 11(6): 422-30.
[6]. Beliën J, Forcé H. Supply chain management of blood products: A literature review. European Journal of Operational Research 2012; 217(1): 1-16.
[7]. Roy N, Shah A. Retrospective analysis of reasons for discarding whole blood and blood components at tertiary care hospital blood bank in South Gujarat. National Journal of Community Medicine 2017; 8(9): 525-29.
[8]. Dunbar NM, Olson NJ, Szczepiorkowski ZM, Martin ED, Tysarcyk RM, Triulzi DJ, et al. Blood component transfusion and wastage rates in the setting of massive transfusion in three regional trauma centers. Transfusion 2017; 57(1): 45-52.
[9]. Desai N, Shukla K, Ahire N. Utilization pattern of blood and blood components in a blood bank of a tertiary care hospital in Mumbai. Indian Journal of Public Health Research & Development 2018; 9(5): 91-5.
[10]. Kurup R, Anderson A, Boston C, Burns L, George M, Frank M. A study on blood product usage and wastage at the public hospital, Guyana. BMC research notes. 2016; 9(1): 1-6.
[11]. Javadzadeh Shahshahani H, Taghvaee N, Akhavan Tafti F. Frequency of blood components wastage and associated factors in Yazd healthcare centers. Iranian Journal of Blood and Cancer 2016; 8(4): 112-16.
[12]. Kumar A, Sharma SM, Ingole NS, Gangane N. Analysis of reasons for discarding blood and blood components in a blood bank of tertiary care hospital in central India: A prospective study. International Journal of Medicine and Public Health 2014;4 (1): 71-4.
[13]. Far RM, Rad FS, Abdolazimi Z, Kohan MMD. Determination of rate and causes of wastage of blood and blood products in Iranian hospitals. Turkish Journal of Hematology 2014; 31(2): 161-66.
[14]. Joshi HJ, Patel KB, Dholu M. An analysis of wastage of blood components in blood bank at tertiary care hospital. International Journal of Clinical and Diagnostic Pathology 2021; 4(1): 138-42.
[15]. Suresh B, Sreedhar Babu K, Arun R, Chandramouli P, Jothibai D. Reasons for discarding whole blood and its components in a tertiary care teaching hospital blood bank in South India. J Clin Sci Res. 2015; 4: 213-19.
[16]. Kora S, Kulkarni K. An analysis Of donor blood wastage in a blood bank in rural Karnataka. J Clin Diagn Res. 2011; 5(7): 1393-396.
[17]. Thakare M, Dixit J, Goel N. Reasons for discarding blood from blood bank of government medical college, Aurangabad. Asian journal of transfusion science. 2011; 5(1): 59-64.
[18]. Shamshirian A, Mohseni AR, Pourfathollah AA, Mehdipour S, Hosseini S, Ghorbanpour A, et al. A review of blood usage and wastage in a tertiary heart center. Acta Clinica Belgica. 2020; 75(2): 96-103.
[19]. Farshadpour F, Taherkhani R, Tajbakhsh S, Gholizadeh Tangestani M, Hajiani G, Sharifi N, et al. Prevalence and trends of transfusion-transmissible viral infections among blood donors in south of Iran: an eleven-year retrospective study. PloS one. 2016; 11(6): 157-65.
Type of Study: Research |
Subject:
Hematology & Blood Banking
Received: 2023/09/10 | Accepted: 2024/06/23 | Published: 2024/10/1
Received: 2023/09/10 | Accepted: 2024/06/23 | Published: 2024/10/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




