Fertility and reproduction is one of the major events in every person's life. According to a report published by the World Health Organization, 10 to 30 percent of people are infertile in every society. 31% of cases were attributed to both women and men [1]. considering the importance of this issue, it is better to investigate the factors that cause disorders in related organs and prevent people from facing these factors. Acrylamide (ACR) is an unsaturated amide with the chemical formula (CH2=CH-CONH2) [2]. ACR is used in two forms of monomer and polymer in various industries such as textile, glue, paper, and in the laboratory [3]. This substance is one of the constituents of cigarette smoke, and ACR is Composed in foods fried at high temperatures [4]. In biochemical analysis, polyacrylamides are widely used for chromatography and electrophoresis, also ACR is widely used as an industrial product for the separation and purification of proteins and in the synthesis of polyacrylamide. This substance was obtained by combining water with acrylonitrile, it is also released into the environment during the production and use of polyacrylamide [5]. Recent research has shown that ACR is formed under physiological conditions (27 °C and pH = 7.4) when asparagine is formed in the presence of hydrogen peroxide. Due to its low molecular weight and high solubility of ACR in water, this substance can pass through the cell membrane. The chemical structure of ACR and its metabolic conversion ability make it able to react with various cells. Various studies of the effects of ACR on various tissues such as the testis, liver, kidney, and brain have been investigated and it has been observed that It has led to changes in the tissues of the organs [6-8]. ACR affects especially the enzymatic and endocrine function of the testis [9] ACR causes cystic changes in the ovary and structural changes in the granulosa layer of the follicles. It also reduces the number of follicles increases the number of atretic follicles, and causes atrophy of the overall ovarian tissue [10].
Vitamin C also known as Ascorbic acid is an antioxidant compound that protects the tissue from oxidative damage. It seems necessary in many biological functions of the body, including growth, collagen biosynthesis, and the normal functioning of organs. It also has cell protection effects that prevent the cell from death in some tissues [11]. Ascorbic acid is the most powerful antioxidant to prevent oxidative damage in biological systems and to organize the first line of defense in different oxidation conditions [11, 12]. It is an essential compound in the physiology of the gonads and it is considered a basic biochemical factor in the reproduction process and a potentially important factor in human fertility [13]. Stereology is one of the branches of applied mathematics that makes it possible to perform three-dimensional calculations according to two-dimensional studies obtained from microscopic sections, and with its help, it is possible to cut the tissue or a member of their geometric properties such as volume, and volume density. get the shape and spatial position, tissue area, length, and number of components in the tissue [14]. In stereology, a test system such as test points or test lines is used on a specific frame or test area. This science deals with voids to measure volume, one-dimensional lines and lengths (L) to measure area, two-dimensional areas to measure length, and three-dimensional volume (V) to estimate number. Sometimes it is considered a reference point for measurement [15]. The goal of this study was to examine the protective effects of vitamin C on histopathological changes induced by ACR on rat ovaries by the Cavalieri principle and the physical dissector.
Materials and Methods
In this experimental study, 28 adult female Wistar rats (200-250 grams) were used. Rats were kept at controlled room temperature (22±2 °C), 12 hours of light, and 12 hours of darkness. All the experiments were performed according to the guidelines for working with animals approved by the ethics committee of Shahid Sadoughi University of Medical Sciences, Yazd, Iran. The animals (n = 7) were divided into 4 groups as follows: 1- control group, do not undergo any intervention. 2- ACR is administered daily for five weeks with a dose of 10 mg/kg soluble in water. 3- vitamin C is administered soluble in water at a dose of 200 mg/kg daily for five weeks. 4- in addition to ACR 10 mg/kg, vitamin C with a dose of 200 mg/kg is administered soluble in water daily for five weeks. After the last oral dose for five weeks, the ovaries were removed weighed, and fixed.
Histopathological examinations
The ovaries were fixed in 10% formalin and placed in the tissue processor for tissue preparation and molded. Serial sections with a thickness of 5 and 20 microns were prepared from ovaries and after Hematoxylin-Eosin staining (H&E) staining using the Cavalieri technique, the total volume of the ovary, the volume of the medulla and cortex was calculated. The physical dissector technique was used to calculate the count of follicles [14, 16].
H & E staining
First, the slides were placed in two xylene containers for 10 minutes to deparaffinize. In the following, rehydration in 96%, 80%, 70%, and 50% ethanol (each one for 2 min). After that, the slides were stained with hematoxylin (sigma) for 5 minutes. and then washed in running water. After that, the slides were stained with eosin (sigma) for 5 minutes and then dehydrated in 50%, 70%, 80%, and 96% ethanol for 4 min and mounted in Entellan (Merck).
The volume of the ovary (mm3) sections were evaluated by light microscopy to evaluate histopathological changes. The total ovaries area was observed on a light microscope at a magnification of 40X with the image projected on a computer monitor. Volume calculations were performed using Cavalieri's principle. A point grid with a 100 μ distance between two points was overlaid on the images. Hitting points on the ovary were calculated and then the volumes were calculated using the following formula:
V= t × a (p) × Σ P/M2
V refers to the volume component, t is the section thickness, a (p) is the area of one point (1000 μ), ΣP is the total number of points counted in the component, and M is the linear magnification [19]. The total number of primordial, primary, secondary Graafian, and atresian follicles was estimated using the physical dissector counting method. A director is a 3D probe that consists of two parallel plates to count the number of follicles. The first selected sample is considered as the reference part and the next part is the search part. The distances between the selected slices will be 20 μ. In this study, twenty-five consecutive pairs of dissections from each sample were used for analysis. [22]. Objects that do not exist in the search area and do not touch the prohibited lines are counted. 80-100 microscopic fields were selected in each ovary. Finally, the number of types of follicles was obtained using the following formula:
Nv=∑p/∑p.a/f.h
N total =Nv × Vovary
∑Q is the total number of the counted follicles, h is the tissue thickness (10 μ), a/f is the frame area in the true tissue scale, and ∑p is the total number of the points superimposed on the selected fields (Fig 1 A and B).
Statistical analysis
The data obtained from this study were analyzed using SPSS version 18 software and P < 0.05 was considered significant. Normally distributed data were analyzed with one-way ANOVA followed by the post hoc Tukey test. The experimental data are represented as mean ± SEM.
Results
The mean ovary weight (mg) and total volume (mm3) in different groups
ACR increased the ovary weight of the ACR group compared to control and vitamin C groups but it was not significant. A reduction was observed in the ovary weight of the ACR+Vitamin C group but it was not significant (Fig. 2A). A significant increase was detected in the mean volume of the ovary (mm3) of ACR-treated and ACR+Vitamin C groups compared to the control group (p ≤ 0.05) (Fig. 2B).
The mean cortex and medullary volume (mm3) of the ovary in different groups
There was a significant decrease in the mean cortex volume (mm3) in the ACR group compared to the control group (p ≤ 0.01). The mean cortex volume of the ovary (mm3) in the ACR+Vitamin C group decreased compared to the control group (p ≤ 0.05), but the mean cortex volume of the ovary (mm3) in vitamin C treated group increased compared to the control group (p ≤ 0.05) (Fig. 3A).
There was a significant increase in the mean medullary volume of the ovary (mm3) in the ACR-treated group compared to the control group (p ≤ 0.01). The mean medullary volume of the ovary (mm3) in the ACR+Vitamin C group decreased compared to the ACR group (p ≤ 0.05) (Fig. 3B).
The mean number of primordial follicles in different group
The mean count of primordial follicles significantly decreased in the ACR-treated group compared to the control group (p ≤ 0.01). The mean count of primordial follicles increased in the ACR+Vitamin C group compared to the ACR group (p ≤ 0.05) (Fig. 4).
The mean number of primary follicles in four groups
The mean count of primary follicles decreased in the ACR group compared to the control group (p ≤ 0.05). This parameter increased in the ACR+Vitamin C group compared to the ACR group (p ≤ 0.05) (Fig. 5).
The mean number of secondary and Graafian follicles in different groups
The mean count of secondary follicles decreased in the ACR- group compared to the control group (p ≤ 0.01). This parameter increased in the ACR+Vitamin C group compared to the ACR group (p ≤ 0.05) (Fig. 6A). The mean number of Graafian follicles decreased in the ACR group compared to the control group (p ≤ 0.001). This parameter increased in the ACR+Vitamin C group compared to the ACR group (p ≤ 0.01) (Fig. 6B).
The mean number of follicular atresia in different groups
The mean count of follicular atresia increased significantly in the ACR group compared to the control group (p ≤ 0.01). This parameter decreased significantly in the ACR+Vitamin C group compared to the ACR-treated group (p ≤ 0.05) (Fig. 7).
The mean number of corpus luteum in different groups
The mean count of the corpus luteum decreased in the ACR group compared to the control group (p ≤ 0.05). This parameter increased in the ACR+Vitamin C group compared to the ACR group (p ≤ 0.05) (Fig. 8).
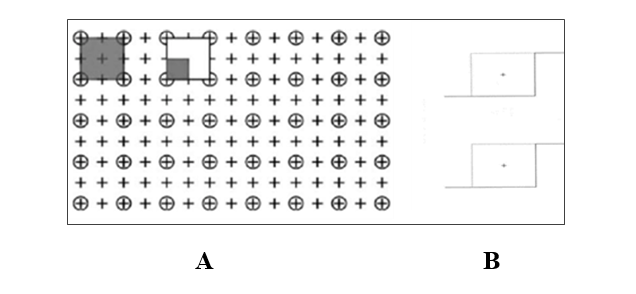
Fig. 1. Grid counting point for ovary volume (A) and physical dissector grid for counting the number of ovarian follicles (B)
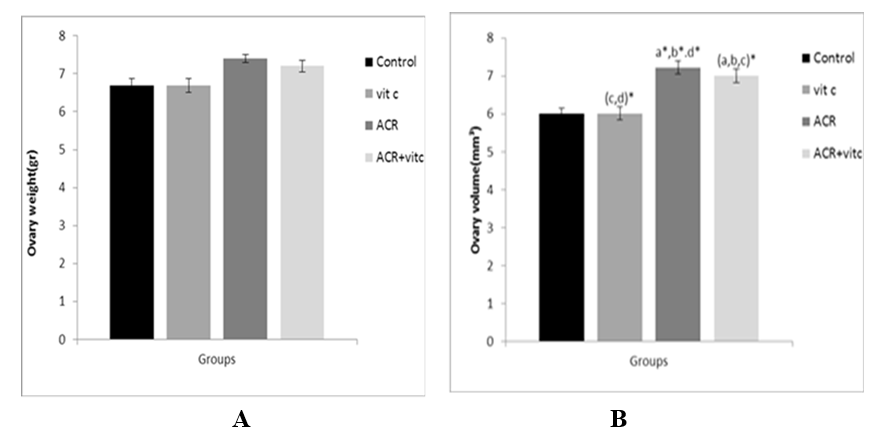
Fig. 2. Mean ovary weight (A), and volume (B) in four groups. p ≤ 0.05 is indicated by *. (a) Control group (b) Vitamin C (Vit C) group compared to other groups, (c) Acrylamide (ACR) group compared to other groups, and (d) ACR+Vit C group compared to other groups.
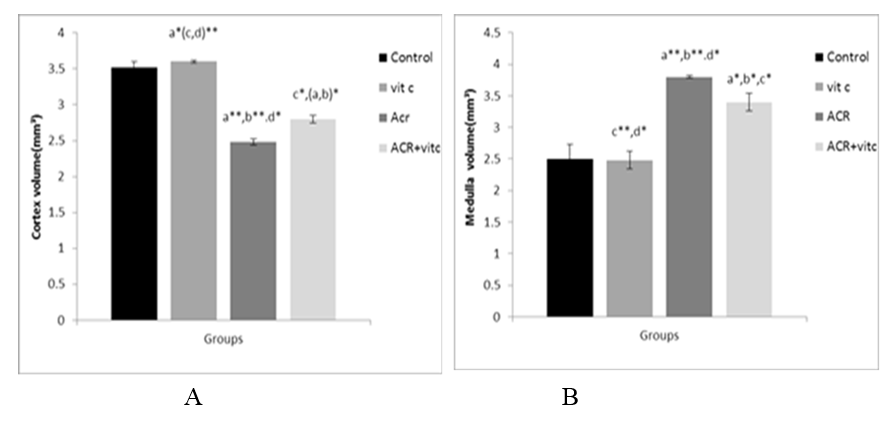
Fig. 3. Mean cortex (A) and medullary (B) volume of the ovary (mm3) in four groups.p ≤ 0.05 is indicated by *. (a) Control group compared to other groups, (b) Vitamin C (Vit C) group compared to other groups, (c) Acrylamide (ACR) group compared to other groups, and (d) ACR+Vit C group compared to other groups.
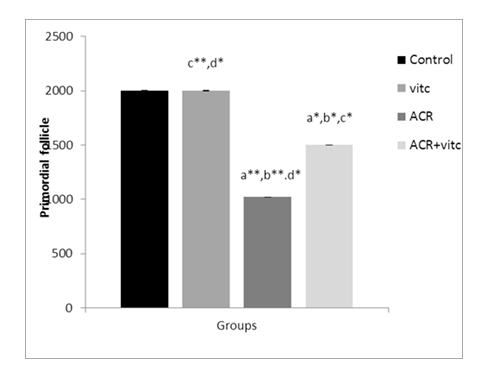
Fig. 4. The mean number of primordial follicles in four groups. p ≤ 0.05 is indicated by *. (a) Control group compared to other groups, (b) Vitamin C (Vit C) group compared to other groups, (c) Acrylamide (ACR) group compared to other groups, and (d) ACR+Vit C group compared to other groups.
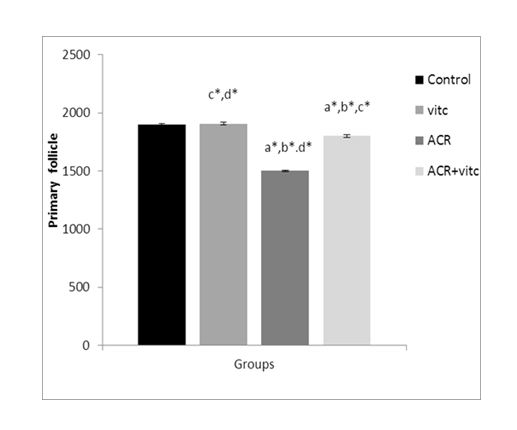
Fig. 5. The mean number of primary follicles in different groups. p ≤ 0.05 is indicated by *. (a) Control group compared to other groups, (b) Vitamin C (Vit C) group compared to other groups, (c) Acrylamide (ACR) group compared to other groups, and (d) ACR+Vit C group compared to other groups.
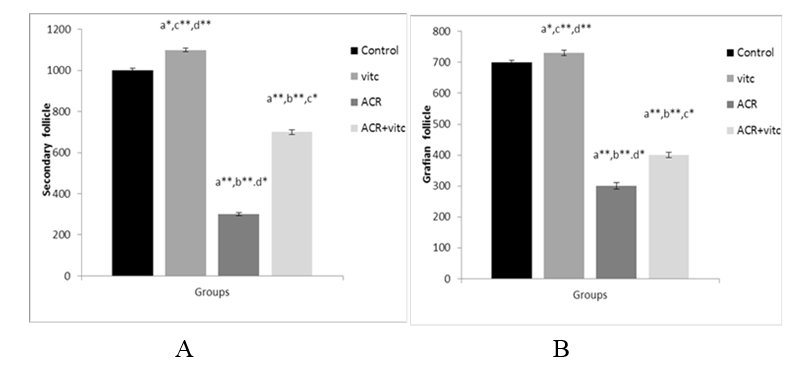
Fig. 6. The mean number of secondary follicles (A) and Graafian follicles (B) in four groups. p ≤0.05 is indicated by * and p ≤ 0.01 is indicated by **. (a) Control group compared to other groups, (b) Vitamin C (Vit C) group compared to other groups, (c) Acrylamide (ACR) group compared to other groups, and (d) ACR+Vit C group compared to other groups.
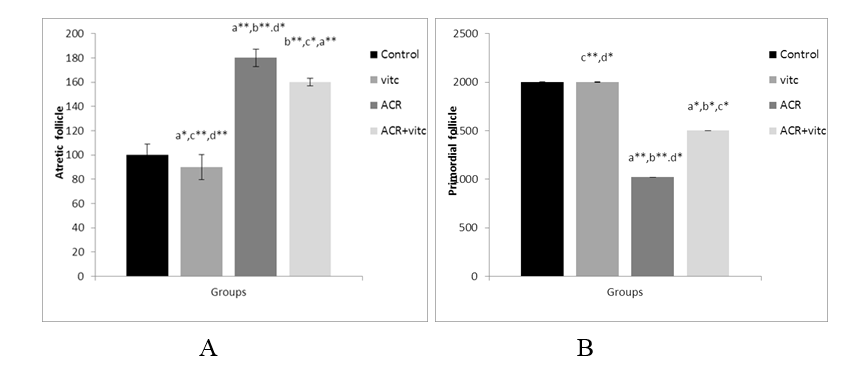
Fig. 7. The mean number of secondary follicles (A) and Graafian follicles (B) in four groups. p ≤0.05 is indicated by * and p ≤ 0.01 is indicated by **. (a) Control group compared to other groups, (b) Vitamin C (Vit C) group compared to other groups, (c) Acrylamide (ACR) group compared to other groups, and (d) ACR+Vit C group compared to other groups.
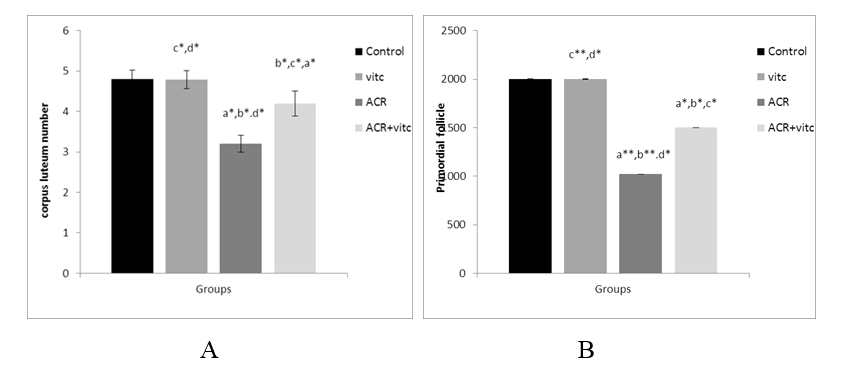
Fig. 8. The mean number of secondary follicles (A) and Graafian follicles (B) in four groups. p ≤0.05 is indicated by * and p ≤ 0.01 is indicated by **. (a) Control group compared to other groups, (b) Vitamin C (Vit C) group compared to other groups, (c) Acrylamide (ACR) group compared to other groups, and (d) ACR+Vit C group compared to other groups.
Discussion
In this study, the impact of ACR and vitamin C on tissue and stereological indicators of rat ovaries were investigated. In this study, the volume of the ovary increased noticeably in the ACR and ACR + Vitamin C groups compared to the control group. There was no significant change in ovarian volume in the vitamin C group compared to the control group. There was a significant decrease in the volume of the cortex in the ACR group compared to the control group. In the ACR + vitamin C group, compared to the control group, there was also a noticeable decrease in the volume of the cortex, but there was an increase compared to the ACR group only.
In the ovary, the antioxidant system consists of non-enzymatic antioxidants such as vitamins E, C, and A, and enzymatic antioxidants such as glutathione catalase, dismutase, superoxide, and peroxidase [17, 18]. According to the study of Abdollahifar and co-workers, the volume of the cortex increased in the ascorbic acid group compared to the control group, this increase is probably due to the increase of growing follicles [17]. The volume of the medulla was also noticeably increased in the ACR group compared to the control group. Perhaps the increase in volume is due to the edema that occurred in the medulla tissue in the ACR group. On the other hand, vitamin C with its antioxidant properties has reduced the volume of the medulla and total ovary by reducing the edema of the interstitial tissue. In line with our study, in a study, female rats were exposed to different doses of ACR, found that ACR causes a significant decrease in ovarian weight. This study also stated the possible reproductive toxic effects of ACR in female mice [19]. Petersson and colleagues stated the following effects of a diet containing ACR. Ovary weight was decreased in the ACR group [20]. ACR increased oxidative stress and apoptosis in the early stages of oocyte maturation. DNA methylation levels were decreased in oocytes treated with ACR. Histone methylation levels were also altered, as the levels of H3K9me2, H3K9me3, H3K4me2, and H3K27me3 decreased after ACR treatment. The results indicated that ACR may affect oocyte quality by affecting cytoskeletal integrity, reactive oxygen species production, induction of apoptosis, and epigenetic changes. and it was graphed and maybe this reduction can be explained based on the mechanism of this study [21].
In our study, an increase in the count of primary, secondary, and graft follicles, was observed in the vitamin C group compared to the ACR group and also in the ACR + Vitamin C group compared to the ACR group. Abdollahifar and co-workers stated in 2019 that ascorbic acid increased the count of follicles, but unlike our study, the number of graft follicles did not change significantly in their study [17]. In a study investigating the reduction of The number of ovarian follicles in newborn guinea pig offspring of mothers exposed to ACR concluded that the use of ACR before birth led to a decrease in the average number of primordial ovarian follicles in the newborns. In the study that Mehranjani et al. conducted for the stereological investigation of the effect of ascorbic acid in averting the adverse effects of bisphenol A on the ovaries of rats, the expression of ascorbic acid improves the adversarial properties of the bisphenol A on the follicles [22].
Conclusion
ACR causes oxidative stress affecting ovarian tissue, particularly the decreased ovulation, growing follicles, maturation of follicles, also edema of the medulla region, On the other hand, Vitamin C as an antioxidant reduces the harmful effects of ACR on ovarian tissue and follicles.
Ethical Consideration
Ethics code received from the Research Council of Shahid Sadoughi University of Medical Sciences, Yazd (IR.SSU.AEC.1401.029).
Funding
This work was supported by Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Conflict of Interests
There are no conflicts of interest based on the author's approval.
Acknowledgments
We would like to acknowledge Shahid Sadoughi University of Medical Sciences, Yazd, Iran for providing us with the necessary resources and facilities to carry out this project.
Authors’ Contributions
N.G, M.P and M.Y: Conceptualization, experimental set-up, data interpretation, manuscript writing, and figure preparation. M.A: Experimental work, data analysis, and interpretation. A.T: Data analysis, interpretation, manuscript, and figure formatting. All authors reviewed and approved the final version of the manuscript.
References
- Fallara G, Pozzi E, Belladelli F, Boeri L, Capogrosso P, Corona G, et al. A systematic review and meta-analysis on the impact of infertility on men’s general health. Eur Urol Focus. 2023; 23: 183-89.
- Sunarko B, Sulistinah N, editors. Acrylonitrile degradation by whole cells of Corynebacterium sp. D5, isolated from polluted industrial waste. IOP Conference Series: Earth and Environmental Science; 2019.
- Kumari A, Bhattacharya B, Agarwal T, Paul V, Maurya VK, Chakkaravarthi S, et al. Method development and validation for acrylamide in potato cutlet by UHPLC-MS/MS. Food Control 2023; 151: 109817.
- Wang B, Wang X, Yu L, Liu W, Song J, Fan L, et al. Acrylamide exposure increases cardiovascular risk of general adult population probably by inducing oxidative stress, inflammation, and TGF-β1: A prospective cohort study. Environment International 2022; 164: 107261.
- Yu Q, Yu Z, Song X, Cao X, Jiang W, Chu Y. The synthesis of an acrylamide copolymer and its synergistic effects on clay flocculation of red tide organisms. Journal of Environmental Management 2023; 332: 117326.
- Ahrari Roodi P, Moosavi Z, Afkhami Goli A, Azizzadeh M, Hosseinzadeh H. Histopathological study of protective effects of honey on subacute toxicity of acrylamide-induced tissue lesions in rats’ brain and liver. Iranian Journal of Toxicology 2018;12(3): 1-8.
- Gao JG, Jiang Y, Zheng JT, Nie LW. Pubertal exposure to acrylamide disrupts spermatogenesis by interfering with meiotic progression in male mice. Toxicology Letters 2022; 358(1): 80-7.
- Gelen V, Yıldırım S, Şengül E, Çınar A, Çelebi F, Küçükkalem M, et al. Naringin attenuates oxidative stress, inflammation, apoptosis, and oxidative DNA damage in acrylamide-induced nephrotoxicity in rats. Asian Pacific Journal of Tropical Biomedicine 2022; 12 (5): 223-32.
- Gram DY, Sexton B, Liman N, Müller L, Abay M, Gram A, et al. Testicular expression of antioxidant enzymes and changes in response to a slow-release deslorelin implant (Suprelorin® 4.7 mg) in the dog. Animals 2022; 12 (18): 2343.
- El Sawi NM. Acrylamide-induced ovarian toxicity in female wistar albino rats. Sohag Journal of Sciences 2022; 7 (3): 111-15.
- Arrigoni O, De Tullio MC. Ascorbic acid: much more than just an antioxidant. Biochimica et Biophysica Acta (BBA)-General Subjects 2002; 1569(1-3): 1-9.
- Bilska K, Wojciechowska N, Alipour S, Kalemba EM. Ascorbic acid: The little-known antioxidant in woody plants. Antioxidants 2019; 8(12): 645.
- Di Renzo L, De Lorenzo A, Fontanari M, Gualtieri P, Monsignore D, Schifano G, et al. Immunonutrients involved in the regulation of the inflammatory and oxidative processes: implication for gamete competence. Journal of Assisted Reproduction and Genetics. 2022; 39(4): 817-46.
- Yadegari Dehnavi M, Mirjalili A, Dortaj H, Abbasi Sarcheshmeh A, Zare Mehrjerdi F. The effects of caffeine on ovarian tissue in rats. Naunyn-Schmiedeberg's Archives of Pharmacology 2021; 394: 307-15.
- Belali Kharaji M, Yadegari M, Anvari M, Abbasi Sarcheshmeh A, Dortaj H. Stereological study on the effects of maternal caffeine consumption on histomorphometric changes of testis and prostate in rat offspring. Modern Medical Laboratory Journal 2020; 3(1): 46-59.
- Yadegari M, Zare Mehrjerdi F, Rezvani ME, Dortaj H, Pourentezari Zarch M. Effect of vitamin C on histomorphometric changes of testis, epididymis, prostate, and seminal vesicle induced by acrylamide in rat. Naunyn-Schmiedeberg's Archives of Pharmacology 2023(1): 1-9.
- Abdollahifar MA, Azad N, Sajadi E, Mofarahe ZS, Zare F, Moradi A, et al. Vitamin C restores ovarian follicular reservation in a mouse model of aging. Anatomy & Cell Biology 2019; 52 (2): 196-203.
- Ighodaro O, Akinloye O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria Journal of Medicine 2018; 54 (4): 287-93.
- Aldawood N, Jalouli M, Alrezaki A, Nahdi S, Alamri A, Alanazi M, et al. Fetal programming: in utero exposure to acrylamide leads to intergenerational disrupted ovarian function and accelerated ovarian aging. Aging 2022; 14 (17): 6887.
- Petersson EV. Analysis of acrylamide and anthocyanins in foods: Extraction optimization for challenging analytes [Doctoral dissertation]. Acta Universitatis: Upsaliensis; 2009.
- Duan X, Wang QC, Chen KL, Zhu CC, Liu J, Sun Svit cC. Acrylamide toxic effects on mouse oocyte quality and fertility in vivo. Scientific Reports 2015; 5 (1): 11562.
- Mehranjani MS, Mansoori T. Stereological study on the effect of vitamin C in preventing the adverse effects of bisphenol A on rat ovary. International Journal of Reproductive Biomedicine 2016; 14 (6): 403.
















































