In December 2019, an outbreak of a novel coronavirus in Wuhan, China, caused viral pneumonia, ultimately leading to a global health emergency [1]. The virus was subsequently recognized as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the International Committee on Taxonomy of Viruses (ICTV). The disease was named Corona virus disease 2019 (COVID-19) by the World Health Organization (WHO) [2]. SARS-CoV-2, which belongs to the Coronaviridae family and Coronavirinae subfamily, is known to be a positive-sense, non-segmented RNA-enveloped virus [3]. Apart from three epidemics caused by coronaviruses during the past two decades, including Severe Acute Respiratory Syndrome (SARS), Middle East Respiratory Syndrome (MERS), and COVID-19, the majority of human coronavirus infections have been known to cause mild diseases [2, 3]. Lungs are believed to be the primary target for SARS-CoV-2; however, secondary effects on multiple organs such as the heart, stomach, liver, kidneys, intestines, blood, and immune system have also been reported [2]. Using angiotensin-converting-enzyme-2 (ACE2) as a binding receptor, which is abundantly found in the respiratory system (lung alveolar epithelial cells, oral mucosa, and nasopharynx), as well as in the gut and peripheral organs including the kidneys and liver, SARS-CoV-2 initiates its replication [1, 2]. This indicates that COVID-19 is not merely a localized pneumonia but a multisystem illness that affects various organs and may lead to systemic complications [1]. Patients have exhibited symptoms during and after their recovery from COVID-19, such as fatigue, diarrhea, renal failure, dyspnea, hepatic damage, joint pain, neurological manifestations, and chest pain. These symptoms have been clinically observed to persist [4]. A commonly used animal model for experimental purposes is the golden Syrian hamster (Mesocricetus auratus), which has been shown to support the replication of SARS-CoV-1 and SARS-CoV-2 (but not MERS-CoV). The golden Syrian hamster has been widely used as an animal model for studying SARS-CoV-2 infection and developing potential countermeasures. The proliferation of SARS-CoV-2 in the lung of the golden Syrian hamster and its pathological symptoms are similar to that of the human.
Furthermore, it has even been discovered that the injection of serum from other hamsters previously infected with SARS-Cov-2 into the golden Syrian hamster can prevent proliferation in the lungs of this specific animal model [4]. This study briefly discusses the histopathological manifestations of multiple organ damage in Syrian hamsters after infection with SARS-CoV-2. Quantitative reverse transcription polymerase chain reaction (qRT-PCR) was used to determine the organs in which the genome of SARS-CoV-2 can be found in Syrian hamsters.
Materials and Methods
Ethics Statement
The approved standard operating procedures of the Razi Vaccine and Serum Research Institute Biosafety Level 3 Facility (ABSL3) were followed in all experiments involving the handling of live SARS-CoV-2.
Animal model
This experiment was conducted to determine the presence of SARS-CoV-2 in four organs of golden Syrian hamsters, namely the heart, lungs, kidneys, and liver. Three groups of hamsters [1-3], each containing four Syrian hamsters (A, B, C, and D), were allocated for the experiment. All the hamsters in this study were held under the same conditions and away from stress. They were all the same age of six weeks. One hamster in each group was mock-infected with phosphate buffered saline (PBS), and the others were infected with SARS-CoV-2 at a 50% tissue culture infectious dose (TCID50) of 105.5, which was administered intranasally at a volume of 100 µl. Groups 1, 2 and 3 were euthanized with a steam of Ketamine at 3, 6, and 10 days post-inoculation (dpi). (Table 1).
Histopathology analysis
All the collected tissues were placed in 10% neutral buffered formalin for fixation. The tissues were then embedded in paraffin blocks, and the blocks were sectioned into thicknesses of 5-10 µm. Sections were placed onto a glass slide and stained with hematoxylin and eosin (H&E). A veterinary pathologist performed histopathologic analysis.
RNA extraction
Total RNA was extracted from all organs (lung, heart, kidney, and liver) using a commercial kit (BehGene Biotechnology, Iran) following the manufacturer's protocol. Briefly, 200 μL of lysis buffer, 25 μL Proteinase K and 6 μL of carrier RNA were mixed with each sample. After incubating at 56 °C for 10 minutes, the solution was applied to a mini spin column with a silica matrix. The column was washed twice with wash buffers, and then the RNA was eluted in 60 μL of elution buffer, following the manufacturer's instructions.
One-step RT-PCR
Extracted RNA was subjected to a one-step real-time RT-PCR assay for the detection of SARS-CoV-2 in samples. According to the manufacturer's instructions, the assay was performed using the COVID-19 One-Step RT-PCR Kit (Pishtaz Teb Diagnostic, Iran). The reaction mixture was prepared by mixing 9 μL of resuspended master mix, 1 μL of primers-probes mix, 5 μL of distilled water, and 5 μL of the extracted RNA. The PCR machine was programmed to follow the cycling profile: 15 minutes hold at 50 °C for reverse transcription, 3 min at 95 °C for cDNA denaturation, and finally, 45 cycles of 15 seconds at 95 °C and 40 seconds at 55 °C. Data acquisition was programmed at the end of the annealing step to detect the viral RdRP gene (FAM), N gene (HEX), and RNase P gene (ROX). ). A cycle threshold of less than 35 was considered as true amplification.
Results
Virus detection by RT-PCR in different tissues
RT-PCR was used to detect viral SARS-CoV-2 RNA in four hamster tissues, including the lung and accessory lobes, kidney, liver, and heart. RT-PCR is a sensitive method for viral detection, but it is not an indicator of infectivity. The positive test result indicates the presence of a virus in the tissue. While the test yielded negative results for the control group tissues, the lung and accessory lobes of the case groups showed the presence of viral SARS-CoV-2 RNA. As opposed to lung tissues, viral RNA was not detected in any of the heart tissues, except for one sample from a hamster euthanized at 6 dpi. Although viral RNA was not detected in kidney tissues, the RT-PCR test produced positive results for liver tissues of group B and C hamsters, but not for group D (Table 2).
Histopathological change in the respiratory system
At 3, 6, and 10 dpi, the lung tissues of hamsters were collected and stained with hematoxylin and eosin (H&E). Mock-inoculated animals (internasal PBS alone), serving as uninfected controls, showed no unusual sign of histopathology in their lung tissue. In contrast, all the SARS-CoV-2 infected animals displayed a range of lung involvement. The lung tissue exhibited pneumonia in hamsters infected with SARS-CoV-2 at a TCID50 of 105.5. In the histopathologic investigation of the lung tissue of these hamsters, inflammatory cells were observed (Fig. 1).
Histopathology of organ system
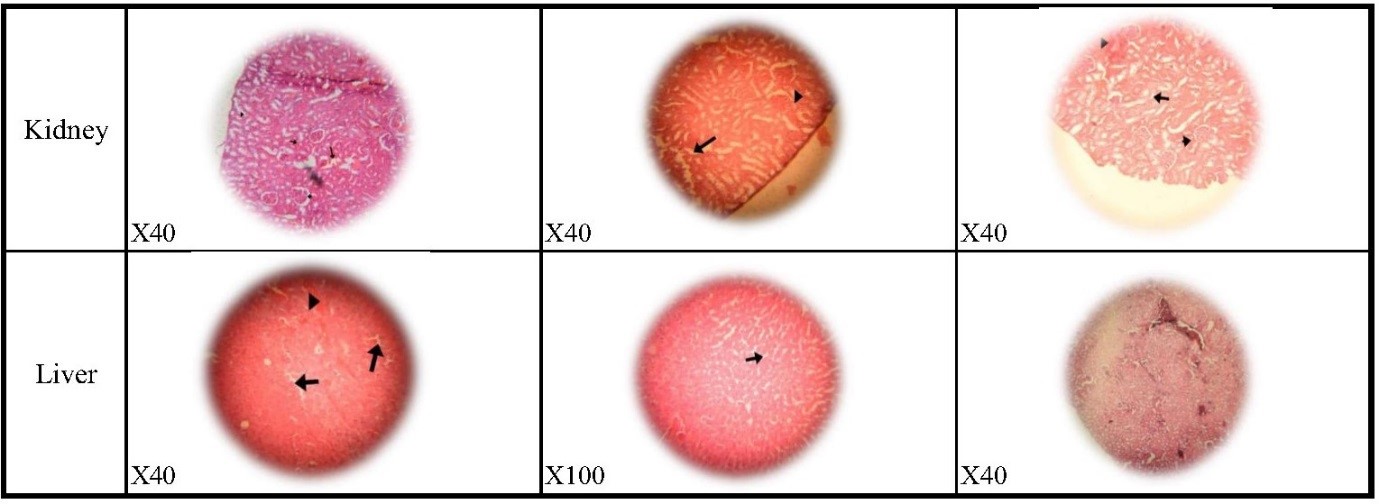
Three other organ tissues, including the kidney, liver, and heart, of all groups of hamsters were histopathologically examined. Kidney tissue exhibited the absence of some glomeruli, intertubular edema, glomerular hyperemia, and, in certain cases, intertubular hemorrhage. Liver tissue showed infiltration of lymphocytes in the parenchyma and, in some cases, foci of hyperemia and edema. Heart tissue did not show any significant changes in the histopathological examination (Fig. 2).
Table 1. Study groups of the present investigation
| Group 1 (3 dpi) |
Group 2 (6 dpi) |
Group 3 (10 dpi) |
| A1 |
Mock-infected |
A2 |
Mock-infected |
A3 |
Mock-infected |
| B1 |
Challenged with 105.5 TCID50 |
B2 |
Challenged with 105.5 TCID50 |
B3 |
Challenged with 105.5 TCID50 |
| C1 |
Challenged with 105.5 TCID50 |
C2 |
Challenged with 105.5 TCID50 |
C3 |
Challenged with 105.5 TCID50 |
| D1 |
Challenged with 105.5 TCID50 |
D2 |
Challenged with 105.5 TCID50 |
D3 |
Challenged with 105.5 TCID50 |
dpi= Days post-inoculation
Table 2. RT-PCR results based on CT in four hamster tissues: CTs less than 35 show positive results
|
CTs of hamsters |
| Tissues |
B1 |
B2 |
B3 |
C1 |
C2 |
C3 |
D1 |
D2 |
D3 |
| Lungs accessory lobes |
22 |
18 |
28 |
19 |
21 |
30 |
25 |
24 |
28 |
| Heart |
36 |
36 |
37 |
38 |
31 |
38 |
36 |
38 |
38 |
| Liver |
30 |
26 |
30 |
32 |
36 |
32 |
36 |
36 |
36 |
| Kidney |
35 |
35 |
36 |
38 |
38 |
38 |
36 |
37 |
38 |
CT= Cycle threshold
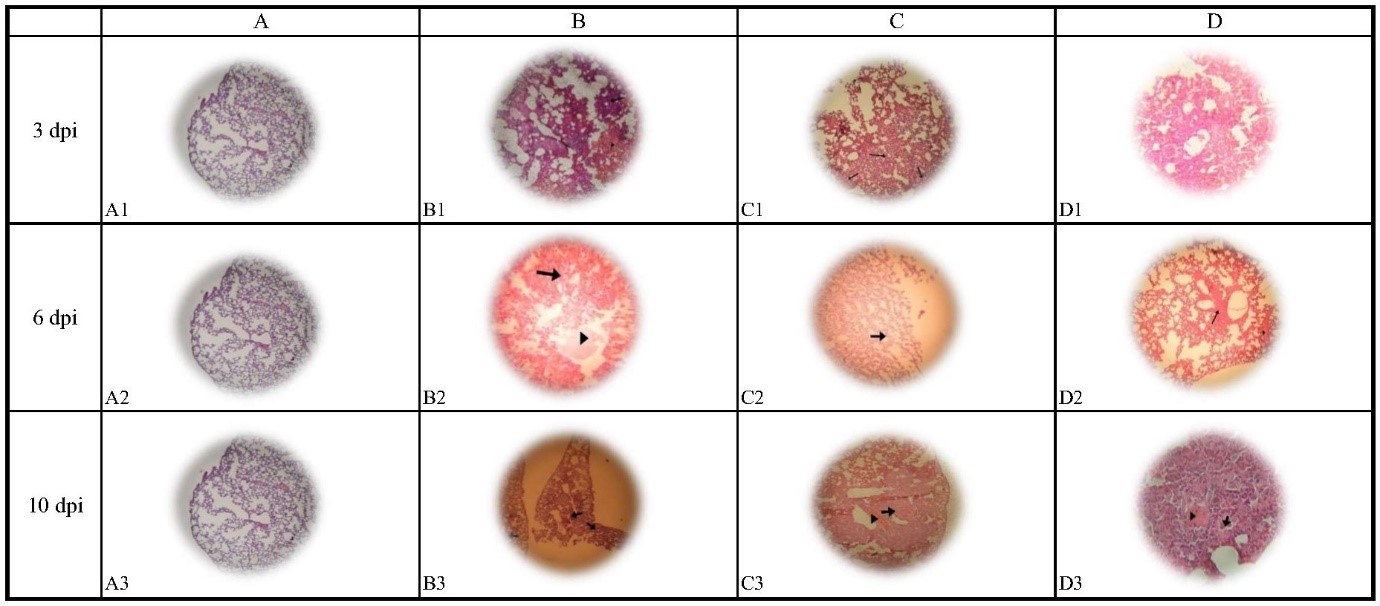
Fig. 1. Histopathologic effects in lung tissues
dpi= Days post-infection
Fig. 2. Histopathologic effects in kidney tissues and liver tissues
Discussion
Clinical findings showed that, in most cases, SARS-CoV-2 can cause respiratory diseases. However, it has also been shown that this virus can also affect other organs in addition to pathological effects on the lungs [5]. Not only can this virus cause respiratory symptoms such as cough, sore throat, and pneumonia, but it can also lead to other symptoms including headache, diarrhea, and chest pain [6]. Even a large number of cases infected with SARS-CoV-2 are asymptomatic, requiring no treatment [7]. In addition to the respiratory tract, SARS-CoV-2 has tissue tropism for myocardial, renal, gastrointestinal, pharyngeal, and neurological tissues [8–10]. SARS-CoV-2 can infect these organs through the Angiotensin-Converting Enzyme-2 (ACE2) receptor [11]. ACE2 is not only a receptor-binding domain for SARS-CoV-2 in humans but also in Syrian hamsters.
Syrian hamsters can be considered an animal model for SARS-CoV-2 studies [12]. In this animal model, SARS-CoV-2 is capable of replicating in lung tissue, which can subsequently lead to pneumonia [13, 14]. To better understand the pathological effects of SARS-CoV-2 in our study, Syrian hamsters were first infected with a viral titer of 105.5 TCID50, and after being euthanized by ethical guidelines, four organs - the lung, liver, kidney, and heart - were examined for pathological changes. The examination results indicated varying degrees of involvement in these four organs at 3, 6 and 10 days post-infection. For instance, RT-PCR results may indicate a higher abundance of the virus in the lung tissues.
Furthermore, histopathologic examination of the lung tissues revealed pneumonia in these hamsters. Several histopathological results in liver and kidney tissues included lymphocyte infiltration and the absence of certain glomeruli, respectively. Finally, the heart tissues showed no histopathological abnormalities. A variety of parameters, such as the time of infection, can affect the histopathological results of Syrian hamsters that are infected [15]. It appears that severe clinical symptoms are not solely caused by the presence of the virus in the organ tissues. Dysregulation of the immune response can also play a crucial role [16, 17]. It is not yet clear whether an active infection with SARS-CoV-2 can exist in extrapulmonary organs without involving the respiratory system [4].
Conclusion
Despite the studies conducted regarding the effects of SARS-CoV-2 on organ tissues, further research is still required to understand this virus's pathological effects better. The present study had several limitations. The small number of hamsters in each group may have influenced the evaluation and interpretation of the results. Additionally, time constraints limited the duration of observation, preventing longer-term screening of the hamsters. Extending the study period could have yielded more conclusive data to strengthen the findings and support the conclusions.
Ethical Considerations
All ethical considerations were followed when compiling this work. Ethical approval for this study was obtained from the Ethics Committee of Iran University of Medical Sciences under the Ethic code (IR.IUMS.FMD.REC.1402.047).
Funding
This study was financially supported by the Iran University of Medical Sciences (Grant Number: 26402).
Conflict of Interests
The authors declare that there is no conflict of interest.
Acknowledgements
Not applicable.
Authors’ Contributions
SHR.M: designed the study. SJ. K and M.T: performed all statistical analyses. Z.S: wrote, reviewed, and edited the manuscript. All authors read and approved the final draft.
References
- Robba C, Battaglini D, Pelosi P, Rocco PRM. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expert Rev Respir Med. 2020; 14(9): 865-68.
- Loganathan S, Kuppusamy M, Wankhar W, Gurugubelli KR, Mahadevappa VH, Lepcha L, et al. Angiotensin-converting enzyme 2 (ACE2): COVID 19 gate way to multiple organ failure syndromes. Respir Physiol Neurobiol. 2021; 283: 103548.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395(10223): 497-506.
- Song Z, Bao L, Yu P, Qi F, Gong S, Wang J, et al. SARS-CoV-2 Causes a systemically multiple organs damages and dissemination in hamsters. Front Microbiol. 2021; 11: 618891.
- Carvalho T. Extrapulmonary SARS-CoV-2 manifestations. Nat Med. 2020; 26(12): 1806.
- Weng LM, Su X, Wang XQ. Pain symptoms in patients with coronavirus disease (COVID-19): A literature review. J Pain Res. 2021; 14: 147-59.
- Rizvi ZA, Tripathy MR, Sharma N, Goswami S, Srikanth N, Sastry JLN, et al. Effect of prophylactic use of intranasal oil formulations in the hamster model of COVID-19. Front Pharmacol. 2021; 12: 746729.
- Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020; 26(7): 1017-1032.
- Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 2020; 158(6): 1831-833.
- Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020; 383(6): 590-92.
- Mokhtari T, Hassani F, Ghaffari N, Ebrahimi B, Yarahmadi A, Hassanzadeh G. COVID-19 and multiorgan failure: A narrative review on potential mechanisms. J Mol Histol. 2020; 51(6): 613-28.
- Roberts A, Vogel L, Guarner J, Hayes N, Murphy B, Zaki S, et al. Severe acute respiratory syndrome coronavirus infection of golden Syrian hamsters. J Virol. 2005; 79(1): 503-11.
- Imai M, Iwatsuki-Horimoto K, Hatta M, Loeber S, Halfmann PJ, Nakajima N, et al. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc Natl Acad Sci USA. 2020; 117(28): 16587-6595.
- Mohandas S, Yadav PD, Shete A, Nyayanit D, Sapkal G, Lole K, et al. SARS-CoV-2 delta variant pathogenesis and host response in Syrian Hamsters. Viruses 2021; 13(9): 1773.
- Gruber AD, Osterrieder N, Bertzbach LD, Vladimirova D, Greuel S, Ihlow J, et al. Standardization of reporting criteria for lung pathology in SARS-CoV-2-infected Hamsters: What Matters? Am J Respir Cell Mol Biol. 2020; 63(6): 856-59.
- Francis ME, Goncin U, Kroeker A, Swan C, Ralph R, Lu Y, et al. SARS-CoV-2 infection in the Syrian hamster model causes inflammation as well as type I interferon dysregulation in both respiratory and non-respiratory tissues including the heart and kidney. PLoS Pathog. 2021; 17(7): 1009705.
- Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020; 71(15): 762-68.









































 , Morteza Taghizadeh
, Morteza Taghizadeh 
 , Seyed Jalal Kiani
, Seyed Jalal Kiani 
 , Farah Bokharaei-Salim
, Farah Bokharaei-Salim 
 , Ahmad Tavakoli
, Ahmad Tavakoli 
 , Seyed Hamidreza Monavari *
, Seyed Hamidreza Monavari *