Thu, Feb 5, 2026
[Archive]
Volume 11, Issue 1 (February 2024)
IJML 2024, 11(1): 15-27 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Bakhshi A, Savaee M, Goodarzvand Chegini K, Hedayati M. A Simple and Rapid Colorimetric Method for Methanol Determination Using Sodium Nitroprusside. IJML 2024; 11 (1) :15-27
URL: http://ijml.ssu.ac.ir/article-1-512-en.html
URL: http://ijml.ssu.ac.ir/article-1-512-en.html
Cellular and Molecular Endocrine Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Full-Text [PDF 315 kb]
(1688 Downloads)
| Abstract (HTML) (1433 Views)



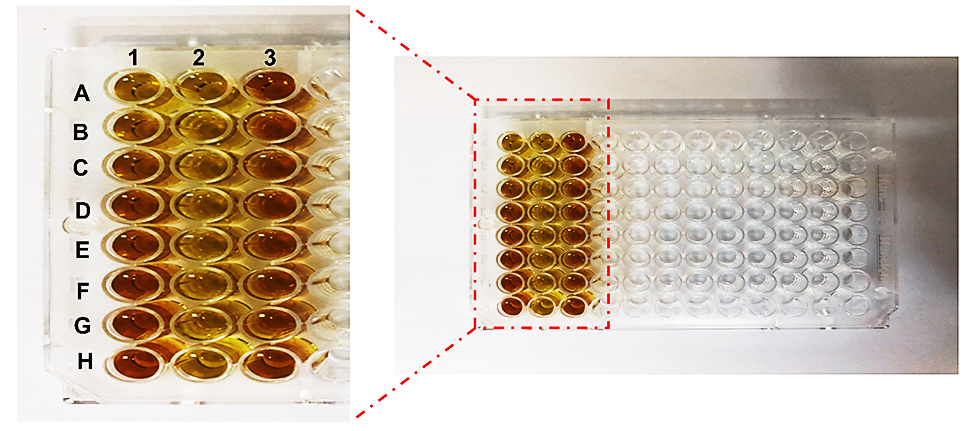
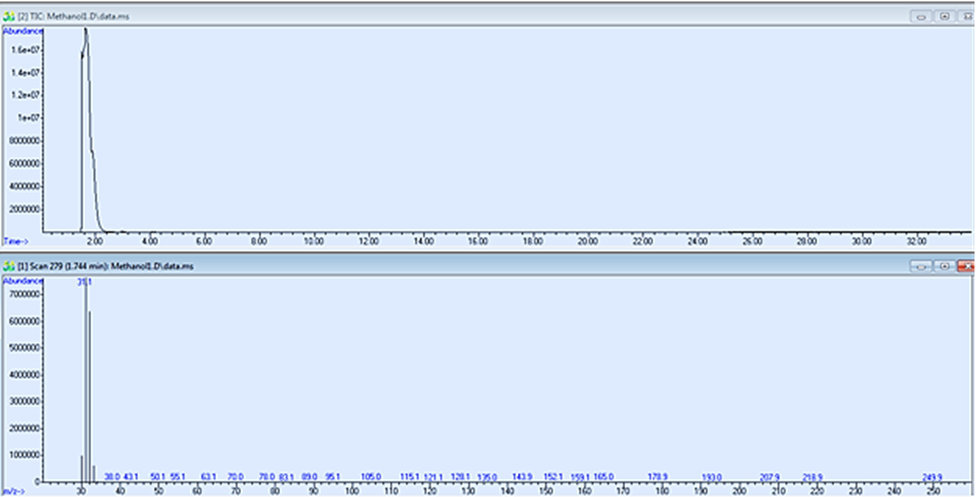

Fig. 6. Total ion chromatogram (TIC) of A) alcoholic beverages, B) alcoholic beverages + methanol (1%), C) herbal distillate, D) herbal distillate + methanol (1%), E) hand sanitizer, and F) hand sanitizer + methanol (1%). The red arrow indicates the methanol peak.
References
Full-Text: (1214 Views)
Introduction
Methanol (CH3OH), also known as methyl alcohol, is generated naturally from the hydrolysis of pectin by pectin-methylesterase during the fermentation of fruit sugar by yeast (Figure 1) [1]. It is a popular organic solvent used for various household and industrial purposes. Methanol intoxication can arise via ingestion, inhalation, or skin exposure to different formulations that contain methanol as a base in their components [2]. In the body, methanol is typically oxidized by alcohol dehydrogenase into formaldehyde and then into formic acid, which can cause irreversible consequences such as permanent neurological damage, severe vision loss, metabolic disturbances, and even death [3]. Although the intoxication of methanol is relatively unusual, it customarily involves a large number of victims at the same time and potentially causes a series of serious medical consequences. Accordingly, methanol poisoning occupies a particular place in the field of clinical toxicology [4].
Due to herbal distillates' beneficial and therapeutic properties, many kinds of them are constantly being utilized in some countries' food regimens, such as Iran [2]. In general, herbal distillates are transparent and colorless liquid products mainly containing water and various organic compounds, and methanol is also detected as an unwanted chemical [5]. Many reports reveal that different kinds of herbal distillates are typically prone to methanol contamination. Therefore, quantifying methanol contents in commonly used brands of herbal distillates is highly important, especially in Iran [6]. On the other hand, a low percentage of methanol may be produced in most alcoholic beverages during the natural fermentation process without causing any harmful effects [7].
Nevertheless, methanol toxicity can occur due to the illegal sale of alcohol products containing methanol, causing severe health problems or even death [8]. Moreover, other substances like commercial alcohol-based hand sanitizers may also be contaminated [9]. According to the World Health Organization (WHO) recommendation, only ethanol and 2-propanol are acceptable alcohols in commercial alcohol-based hand sanitizers for antisepsis purposes. However, the Food and Drug Administration (FDA) stated that several hand sanitizers consisted of 81% methanol in November 2020, warning about specific hand sanitizer products containing methanol [9]. Hence, since determining methanol concentration in these compounds is an important parameter in quality control and safety of their production processes, methods that can easily estimate the methanol quantity in these products are highly desired. Recently, the gold standard method for determining methanol content is gas-liquid chromatography [10]. However, it is a bulky and expensive instrument that requires trained personnel. It is usually applicable only in specialized laboratories and is unsuitable for public laboratories and industries [11]. Moreover, the National Association for Occupational Safety and Health (NIOSH) confirmed a chromotropic acid (CA) spectrophotometric method for quantitative measurement of formaldehyde. Nevertheless, a large amount of hot, concentrated sulfuric acid is needed to react with CA, which is potentially unsafe [12]. Therefore, low-cost, safe, rapid methanol detection methods are required to check product safety. Accordingly, the current research was designed to establish a rapid, simple, and low-cost sodium nitroprusside (SNP)-based colorimetric method for the determination of methanol quantity in different samples of hand sanitizers, herbal distillates, and alcoholic beverages. Subsequently, the results were compared to a gas chromatography-mass spectrometry (GC–MS) apparatus to evaluate the Kit's efficacy.
Materials and Methods
In this research, a simple colorimetric method was developed to detect the samples' methanol levels with high sensitivity.
Reagents and solutions
All chemicals used in the experiments, including sodium hydroxide (NaOH), ethanol (C₂H₅OH), CH₃OH, SNP, and potassium ferricyanide, were obtained from Sigma-Aldrich Company (USA).
Sample preparation
Several types of the most commonly used herbal distillates [peppermint (Mentha piperita), musk willow (Salix aegyptiaca), chicory (Cichorii flos), fenugreek (Trigonella foenum-graecum), and camel thorn (Alhagi Maurorum)], hand sanitizers, and alcoholic drinks were purchased from local markets in Tehran and transferred to the laboratory for determination of methanol concentrations. The samples were kept at room temperature until the test. The samples were diluted 1: 20 with distilled water to prepare the working solution.
Methanol standard preparation
To prepare methanol standard stock solution (20% v/v), 200 μl methanol (99.95%) and 800 µl distilled water were mixed into an Eppendorf microtube to make 1 ml of methanol standard stock solution (20% v/v). According to Table 1, the working standard solutions were prepared at 0-2.4% v/v concentrations by diluting the standard stock solution with an appropriate amount of distilled water. Different concentrations of methanol (0-2.4%) were prepared and read in the 575 nm to prepare the standard curve.
Due to herbal distillates' beneficial and therapeutic properties, many kinds of them are constantly being utilized in some countries' food regimens, such as Iran [2]. In general, herbal distillates are transparent and colorless liquid products mainly containing water and various organic compounds, and methanol is also detected as an unwanted chemical [5]. Many reports reveal that different kinds of herbal distillates are typically prone to methanol contamination. Therefore, quantifying methanol contents in commonly used brands of herbal distillates is highly important, especially in Iran [6]. On the other hand, a low percentage of methanol may be produced in most alcoholic beverages during the natural fermentation process without causing any harmful effects [7].
Nevertheless, methanol toxicity can occur due to the illegal sale of alcohol products containing methanol, causing severe health problems or even death [8]. Moreover, other substances like commercial alcohol-based hand sanitizers may also be contaminated [9]. According to the World Health Organization (WHO) recommendation, only ethanol and 2-propanol are acceptable alcohols in commercial alcohol-based hand sanitizers for antisepsis purposes. However, the Food and Drug Administration (FDA) stated that several hand sanitizers consisted of 81% methanol in November 2020, warning about specific hand sanitizer products containing methanol [9]. Hence, since determining methanol concentration in these compounds is an important parameter in quality control and safety of their production processes, methods that can easily estimate the methanol quantity in these products are highly desired. Recently, the gold standard method for determining methanol content is gas-liquid chromatography [10]. However, it is a bulky and expensive instrument that requires trained personnel. It is usually applicable only in specialized laboratories and is unsuitable for public laboratories and industries [11]. Moreover, the National Association for Occupational Safety and Health (NIOSH) confirmed a chromotropic acid (CA) spectrophotometric method for quantitative measurement of formaldehyde. Nevertheless, a large amount of hot, concentrated sulfuric acid is needed to react with CA, which is potentially unsafe [12]. Therefore, low-cost, safe, rapid methanol detection methods are required to check product safety. Accordingly, the current research was designed to establish a rapid, simple, and low-cost sodium nitroprusside (SNP)-based colorimetric method for the determination of methanol quantity in different samples of hand sanitizers, herbal distillates, and alcoholic beverages. Subsequently, the results were compared to a gas chromatography-mass spectrometry (GC–MS) apparatus to evaluate the Kit's efficacy.
Materials and Methods
In this research, a simple colorimetric method was developed to detect the samples' methanol levels with high sensitivity.
Reagents and solutions
All chemicals used in the experiments, including sodium hydroxide (NaOH), ethanol (C₂H₅OH), CH₃OH, SNP, and potassium ferricyanide, were obtained from Sigma-Aldrich Company (USA).
Sample preparation
Several types of the most commonly used herbal distillates [peppermint (Mentha piperita), musk willow (Salix aegyptiaca), chicory (Cichorii flos), fenugreek (Trigonella foenum-graecum), and camel thorn (Alhagi Maurorum)], hand sanitizers, and alcoholic drinks were purchased from local markets in Tehran and transferred to the laboratory for determination of methanol concentrations. The samples were kept at room temperature until the test. The samples were diluted 1: 20 with distilled water to prepare the working solution.
Methanol standard preparation
To prepare methanol standard stock solution (20% v/v), 200 μl methanol (99.95%) and 800 µl distilled water were mixed into an Eppendorf microtube to make 1 ml of methanol standard stock solution (20% v/v). According to Table 1, the working standard solutions were prepared at 0-2.4% v/v concentrations by diluting the standard stock solution with an appropriate amount of distilled water. Different concentrations of methanol (0-2.4%) were prepared and read in the 575 nm to prepare the standard curve.

Fig. 1. Methanol is generated naturally through the enzymatic activity of pectin-methylesterase (TME) on pectin
Preparation of chromogenic reagent
In brief, 10 g SNP, 10 g potassium ferricyanide, and 5 g NaOH were added into a volumetric balloon and dissolved in distilled water to prepare 100 ml of SNP stock solution. The mixture was stored at 4 °C (in the dark) until its use. Subsequently, different dilutions (1: 5 to 1: 12) were prepared from SNP stock solution using distilled water (diluent). Different concentrations of methanol standards were mixed with varying dilutions of SNP and read at 474-484 nm. Based on the results, 1.6 dilution was chosen as the optimal dilution.
Determination of optimal reaction time
To determine the optimal reaction time, different concentrations of methanol standards were mixed with varying dilutions of SNP. Subsequently, the absorbance of reactions was measured at different time points (2, 5, 10, 15, 20, 25, and 30 minutes) at 474-484 nm. Based on the results, 15 minutes was chosen as the optimal reaction time.
The procedure of the Kit
In brief, 60 µl of each methanol standard (0-2.4%) and 60 µl of each diluted sample were pipetted into a 96-well microplate. Subsequently, 240 µl of SNP solution was added to each well. The microplate was shaken gently and incubated at 25 °C for 15 minutes to complete the process. Finally, the absorbance of each sample was measured by a microplate reader at 478-484 nm. The methanol level was assessed by using the standard curve. A schematic illustration of the procedure is depicted in Figure 2.
In brief, 10 g SNP, 10 g potassium ferricyanide, and 5 g NaOH were added into a volumetric balloon and dissolved in distilled water to prepare 100 ml of SNP stock solution. The mixture was stored at 4 °C (in the dark) until its use. Subsequently, different dilutions (1: 5 to 1: 12) were prepared from SNP stock solution using distilled water (diluent). Different concentrations of methanol standards were mixed with varying dilutions of SNP and read at 474-484 nm. Based on the results, 1.6 dilution was chosen as the optimal dilution.
Determination of optimal reaction time
To determine the optimal reaction time, different concentrations of methanol standards were mixed with varying dilutions of SNP. Subsequently, the absorbance of reactions was measured at different time points (2, 5, 10, 15, 20, 25, and 30 minutes) at 474-484 nm. Based on the results, 15 minutes was chosen as the optimal reaction time.
The procedure of the Kit
In brief, 60 µl of each methanol standard (0-2.4%) and 60 µl of each diluted sample were pipetted into a 96-well microplate. Subsequently, 240 µl of SNP solution was added to each well. The microplate was shaken gently and incubated at 25 °C for 15 minutes to complete the process. Finally, the absorbance of each sample was measured by a microplate reader at 478-484 nm. The methanol level was assessed by using the standard curve. A schematic illustration of the procedure is depicted in Figure 2.
Table 1. Preparation of methanol standard solutions
| Standard (%) | Distilled water (μl) | Methanol 20% (μl) |
| 0 | 500 | 0 |
| 0.2 | 495 | 5 |
| 0.4 | 490 | 10 |
| 0.6 | 485 | 15 |
| 0.8 | 480 | 20 |
| 1 | 475 | 25 |
| 1.2 | 470 | 30 |
| 1.4 | 465 | 35 |
| 1.6 | 460 | 40 |
| 1.8 | 455 | 45 |
| 2 | 450 | 50 |
| 2.2 | 445 | 55 |
| 2.4 | 440 | 60 |

Fig. 2. Schematic illustration of the designed method to estimate the methanol amounts in alcoholic and non-alcoholic solutions.
Apparatus
The methanol content of all samples was determined using a 7890A/5975C GC/MS system (Agilent Technologies, Palo Alto, CA).
Method validation and statistical analyses
The validation parameters for estimating methanol in samples include sensitivity, accuracy, and precision. Sensitivity is the lowest amount that can be measured in an assay. Here, the sensitivity was calculated based on the standard curve, and the minimum value with the statistical difference was considered as sensitivity. The method's sensitivity was computed based on the
mean zero standard signals ± two standard deviations (mean ± 2SD).
To check the method's precision, three samples were chosen in the low, medium, and high concentrations range to assess the intra-
and inter-assay. The reproducibility was determined based on the coefficient of variations (CV %) and replication number 8 in intra-assay and 14 in inter-assay. Intra and inter-assay CV% were calculated with the following formula:
CV% = [standard deviation (SD)/Mean)] × 100
Therefore, the parallelism and recovery tests were performed to determine the relative accuracy of the designed method.
According to the parallelism test, the developed method assessed the methanol amount in the samples. Then, the samples were diluted 2, 4, and 8 times. Finally, the ratio of the parallelism test was calculated using the expected and the measured data. A known sample volume was added to the standard solutions for the recovery test. Subsequently, recovery was computed using the expected and the estimated data.
Finally, three random samples of common commercial sanitizers, herbal distillates, and alcoholic drinks were measured for methanol levels. Microsoft Excel software was executed to perform data analysis. The results were reported as mean ± SD.
Results
Optimization of the standard curve
Different methanol concentrations ranging from 0.4 to 2.4% (v/v) were first prepared to optimize the methanol standard curve. By comparing the absorbance of standards and the obtained graphs, eight concentrations of methanol standard solutions were set as the main standard points (Figures 3A and B). As shown in Figure 3B, the standard curve is fully linear with a good slope and equation.
Method validation
Intra- and inter-assay CV%
Intra- and inter-assay CV% were determined to specify the precision of the colorimetric method (Table 2). Eight replicates of three samples with low, medium, and high concentration ranges were chosen to evaluate the proposed method's precision. Subsequently, the replicates of three samples were assessed in different working periods, and the intra-assay accuracy was checked. The CV% were reported as mean ± SD. As shown in Table 2, all CV% were less than 10%, indicating that the method's precision was acceptable.
Method sensitivity
In this study, the sensitivity was evaluated based on mean ± 2SD. As shown in Table 3, the method's sensitivity was 0.077% (Table 3).
Method relative accuracy
The parallelism and recovery tests were utilized to check the method's accuracy, and their results are shown in Tables 4 and 5, respectively. According to parallelism and recovery tests, ratio and recovery ranged from 96.5-107.5% and 99.3-108%, respectively.
The methanol content of all samples was determined using a 7890A/5975C GC/MS system (Agilent Technologies, Palo Alto, CA).
Method validation and statistical analyses
The validation parameters for estimating methanol in samples include sensitivity, accuracy, and precision. Sensitivity is the lowest amount that can be measured in an assay. Here, the sensitivity was calculated based on the standard curve, and the minimum value with the statistical difference was considered as sensitivity. The method's sensitivity was computed based on the
mean zero standard signals ± two standard deviations (mean ± 2SD).
To check the method's precision, three samples were chosen in the low, medium, and high concentrations range to assess the intra-
and inter-assay. The reproducibility was determined based on the coefficient of variations (CV %) and replication number 8 in intra-assay and 14 in inter-assay. Intra and inter-assay CV% were calculated with the following formula:
CV% = [standard deviation (SD)/Mean)] × 100
Therefore, the parallelism and recovery tests were performed to determine the relative accuracy of the designed method.
According to the parallelism test, the developed method assessed the methanol amount in the samples. Then, the samples were diluted 2, 4, and 8 times. Finally, the ratio of the parallelism test was calculated using the expected and the measured data. A known sample volume was added to the standard solutions for the recovery test. Subsequently, recovery was computed using the expected and the estimated data.
Finally, three random samples of common commercial sanitizers, herbal distillates, and alcoholic drinks were measured for methanol levels. Microsoft Excel software was executed to perform data analysis. The results were reported as mean ± SD.
Results
Optimization of the standard curve
Different methanol concentrations ranging from 0.4 to 2.4% (v/v) were first prepared to optimize the methanol standard curve. By comparing the absorbance of standards and the obtained graphs, eight concentrations of methanol standard solutions were set as the main standard points (Figures 3A and B). As shown in Figure 3B, the standard curve is fully linear with a good slope and equation.
Method validation
Intra- and inter-assay CV%
Intra- and inter-assay CV% were determined to specify the precision of the colorimetric method (Table 2). Eight replicates of three samples with low, medium, and high concentration ranges were chosen to evaluate the proposed method's precision. Subsequently, the replicates of three samples were assessed in different working periods, and the intra-assay accuracy was checked. The CV% were reported as mean ± SD. As shown in Table 2, all CV% were less than 10%, indicating that the method's precision was acceptable.
Method sensitivity
In this study, the sensitivity was evaluated based on mean ± 2SD. As shown in Table 3, the method's sensitivity was 0.077% (Table 3).
Method relative accuracy
The parallelism and recovery tests were utilized to check the method's accuracy, and their results are shown in Tables 4 and 5, respectively. According to parallelism and recovery tests, ratio and recovery ranged from 96.5-107.5% and 99.3-108%, respectively.

Fig. 3. A) The methanol standard solutions. B) Methanol standard curve.
Measurement of samples using the established method
The methanol levels in three samples, including common commercial sanitizers, herbal distillates, and alcoholic drinks, were randomly assessed, and their results are presented in Table 6. The methanol can react with the sodium nitroprusside in the basic solution to generate a colored product (Figure 4). As given in Figure 4 and Table 6,
the established method detected methanol in
alcoholic and non-alcoholic samples.
GC result
The GC/MS chromatograms are shown in Figures 5 and 6. The area under the curve of each peak was calculated to determine the presence of methanol in each sample (Table 7). According to the findings, comparing methanol content between GC/MS and the established colorimetric methods revealed that the designed method could detect methanol.
The methanol levels in three samples, including common commercial sanitizers, herbal distillates, and alcoholic drinks, were randomly assessed, and their results are presented in Table 6. The methanol can react with the sodium nitroprusside in the basic solution to generate a colored product (Figure 4). As given in Figure 4 and Table 6,
the established method detected methanol in
alcoholic and non-alcoholic samples.
GC result
The GC/MS chromatograms are shown in Figures 5 and 6. The area under the curve of each peak was calculated to determine the presence of methanol in each sample (Table 7). According to the findings, comparing methanol content between GC/MS and the established colorimetric methods revealed that the designed method could detect methanol.
Table 2. Coefficients of variation results for precision assessment
| Test | Concentration range | Replication number | Mean ± SD | CV% |
Intra-assay |
Low | 8 | 0.36±0.02 | 4.94 |
| Medium | 8 | 1.31±0.02 | 3.29 | |
| High | 8 | 2.35±0.04 | 1.65 | |
Inter-assay |
Low | 12 | 0.36±0.03 | 8.13 |
| Medium | 14 | 1.31±0.06 | 4.37 | |
| High | 14 | 2.35±0.03 | 1.3 |
SD= Standard deviation; CV= Coefficients of variation
Table 3. Sensitivity of the designed method.
| Concentration | Replication number | Signal (Mean) | SD | 2SD | Mean ± 2SD |
| Sensitivity (%) | 8 | 2.750 | 0.014 | 0.028 | 2.778 |
| 0.077 | |||||
Table 4. Accuracy assessment results from parallelism test
| Sample | Dilution | Expected amount | Measured amount | Ratio % |
| 1 | 1 | 2.24 | 2.16 | 96.5 |
| 2 | 2 | 1.12 | 1.11 | 99.3 |
| 3 | 4 | 0.56 | 0.54 | 96.6 |
| 4 | 8 | 0.28 | 0.3 | 107.8 |
Table 5. Accuracy assessment results from recovery test
| Sample | Standard (%) | Expected amount | Measured amount | Recovery % |
| 1 | 0.0 | 0.64 | 0.6 | 99.8 |
| 2 | 0.4 | 0.84 | 0.9 | 102.8 |
| 3 | 0.8 | 1.04 | 1.0 | 99.3 |
| 4 | 1.2 | 1.24 | 1.3 | 102.3 |
| 5 | 1.3 | 1.28 | 1.3 | 101.3 |
| 6 | 1.4 | 1.34 | 1.4 | 108.0 |
| 7 | 1.6 | 1.44 | 1.5 | 104.9 |
| 8 | 2.0 | 1.64 | 1.7 | 105.8 |
| 9 | 2.4 | 1.84 | 1.9 | 105.4 |

Fig. 4. The microplate view of samples after the complete setup of the method. 1: A to H show standards. 2: A to H shows the samples diluted (l: 20) with distilled water. 3: A to H shows the samples containing 1% methanol.
Table 6. Methanol level in random alcoholic and non-alcoholic samples
| No. | Sample type | Methanol (Mean ± SD) |
| 1 | Beverage | 0.452 ± 0.149 |
| 2 | Beverage | 0.671 ± 0.225 |
| 3 | Beverage | 0.988 ± 0.294 |
| 4 | Sanitizer | 0.848 ± 0.926 |
| 5 | Sanitizer | 0.883 ± 0.170 |
| 6 | Sanitizer | 0.418 ± 0.311 |
| 7 | Herbal Ext. | 0.179 ± 0.019 |
| 8 | Herbal Ext. | 0.152 ± 0.022 |
| 9 | Herbal Ext. | 0.160 ± 0.009 |
SD= standard deviation

Fig. 5. Total ion chromatogram (TIC) of pure methanol

Fig. 6. Total ion chromatogram (TIC) of A) alcoholic beverages, B) alcoholic beverages + methanol (1%), C) herbal distillate, D) herbal distillate + methanol (1%), E) hand sanitizer, and F) hand sanitizer + methanol (1%). The red arrow indicates the methanol peak.
Table 7. Comparison of methanol concentration between GC/MS and colorimetric methods
| Methanol % (colorimetric assay) | GC/MS | Samples |
| 0.86% | 0% | Herbal distillates |
| 1.51% | 1.82% | Hand sanitizers |
| 1.07% | 1.39% | Alcoholic beverages |
Discussion
The current work designed an inexpensive, simple, and reliable methanol detection method based on a reaction between methanol and SNP. The established colorimetric method revealed a good sensitivity (0.077%), high precision, and acceptable accuracy for methanol determination in different samples.
Drinking or dermal exposure to nonstandard solutions contaminated with methanol may cause methanol poisoning. As seen in the recent events regarding the COVID-19 pandemic, grave concerns were uncovered about some hand sanitizers contaminated with methanol globally [13]. Therefore, estimating methanol in different alcohol-based hand sanitizers is imperative to certify their quality and safety [9].
Moreover, it is indispensable to note that many investigations have authenticated the contamination of methanol in alcoholic beverages and even fruit juices and herbal distillates, which reveals the seriousness of this issue for further research [14]. Herbal distillates have been consumed extensively as beverages and herbal medicine in Iran for a long time [6]. The production of large amounts of herbal distillates is growing yearly by factories and even houses worldwide, and the products are frequently marketed. Methanol generation is unavoidable during the production process of herbal distillates [6]. The pollution of some herbal distillates, such as mint, rose water, and plant forty water, with methanol, may cause blurred vision and blindness in people who consume these products for a long time [15]. There are more than 4000 small and large producers of herbal distillates in Iran, and this observation needs to be considered due to the inaccessibility of appropriate tools for methanol monitoring in these products [16]. Besides, early diagnosis of methanol content in alcoholic and non-alcoholic drinks is extremely pivotal owing to the hazardous impressions of methanol on the human body [17]. Undoubtedly, methanol intoxication is accompanied by blindness and even death when identified too late [2]. Therefore, estimating methanol content in alcoholic or non-alcoholic beverages, herbal distillates, and hand sensitizers is a critical parameter in the quality control of these products. Determining the methanol quantity requires expensive and highly specialized equipment and high technical knowledge [18]. The most accurate and reliable methods, such as HPLC and GC, estimate methanol quantity in different fluids [19]. Previously, Nisbar and colleagues developed a GC/MS-based analytical method to simultaneously determine isopropyl alcohol as the active ingredient in alcohol-based hand sanitizers and methanol as an impurity [13]. In a cross-sectional study, Zamani and collegues determined the contents of methanol in illegal alcoholic beverages in Iran's black market using GC and a modified colorimetric CA method. Of 1221 samples, methanol was identified in 160 samples (13.1%) using the colorimetric CA method and in 128 (10.48%) samples using the GC method with 100% sensitivity and 97.07% specificity [20]. Unfortunately, the gold standard methods of methanol detection are not easily available in developing countries with limited financial and trained personnel. Hence, establishing and using a feasible and reliable strategy for determining methanol in these countries is an advantage [19]. In the colorimetric CA method recommended as the reference method for detecting alcoholic drink-derived methanol by the Association of Official Analytical Chemists (AOAC), methanol is changed to formaldehyde. Then, this compound is measured indirectly through its reaction with CA in hot, concentrated sulfuric acid media [12]. Saadat et al. (2020) appraised the methanol level in some herbal distillates using a new kit based on the modified CA method. According to their findings, different methanol concentrations were detected in all examined samples of herbal distillates ranging from 21 to 770 mg/L [16]. These findings revealed that some of the available herbal distillates in Iran contain enough amounts of methanol, which can cause chronic methanol intoxication in consumers. However, Rafizadeh et al. [21] and Saadat and Rafizadeh [5] previously announced that applying the AOAC-recommended chromotropic acid method for methanol quantification in herbal distillates can give rise to erroneous findings. Besides, the amounts of formaldehyde and formic acid in the examined samples can affect the methanol content if they are present in the sample. Therefore, designing an accurate, safe, and low-cost kit that can determine the methanol contents in any herbal distillates is highly desired [12].
The current research reports a simple method for quantifying methanol quantity in some herbal distillates and hand sensitizer samples by a modified colorimetric method. This proposed method has been shown to have 0.077% sensitivity. Moreover, this colorimetric Kit was precise and accurate, with intra- and inter-assay CV% of less than 10% within the acceptable ranges. Besides, the recovery percentages ranged from 96.5 to 108, indicating an acceptable accuracy of the studied method. We found few kinds of literature to date on the reaction of SNP and alcohol. Zhan et al. developed a SNP-based spectrophotometric method for the direct determination of methanol. This colorimetric method exhibits that the sodium nitroprusside can react with the methanol to create methoxy nitroprusside in the basic solution, a colored product with absorption at 481 nm. Meanwhile, the methanol content can also be calculated based on the absorbance [22].
Additionally, we measured the methanol quantity in different samples using the GC–MS apparatus (a gold standard method) to evaluate the Kit's efficacy. According to the findings, comparing methanol content between GC/MS and the established colorimetric method revealed that the designed method could detect methanol.
The results of the present study showed that this colorimetric method could directly measure the methanol content using sodium nitroprusside reagent without using modern equipment and experts. However, the current research was not specifically designed to evaluate other factors that reacted with SNP solution. Indeed, herbal extract and fermented products may have some compounds that react with SNP solution and decrease the specificity of the output.
Conclusion
This study was undertaken to design a novel chemical method and evaluate methanol in various alcoholic and non-alcoholic products. The established colorimetric method exhibited good sensitivity, high precision, and acceptable accuracy for methanol estimation in various samples. The results of this proposed method have indicated that the newly developed method can quantify methanol with high sensitivity and accuracy by being used through distributors, local authorities, and consumers with no need for appropriate laboratory equipment and increased knowledge and skills.
Ethical Considerations
The Institutional Review Board and Ethics Committee of Shahid Sadoughi University of Medical Sciences, Yazd, Iran (the committee approval number: IR.SSU.MEDICINE.REC.1399.205) approved the study.
Funding
Not applicable.
Conflict of Interest
The authors declared no conflict of interest.
Acknowledgments
This article was extracted from Ali Bakhshi's thesis on the fulfillment of a Master of Science (MSc) degree. The authors thank the Cellular and Molecular Endocrine Research Center's staff for their collaboration.
Authors' Contributions
Study design, data analysis and interpretation: Mehdi Hedayati, Koorosh Goodarzvand Chegini, Ali Bakhshi and Mohamadreza Savaee; Data collection and initial draft preparation: Ali Bakhshi and Mohamadreza Savaee; Editing, supervision and review: Mehdi Hedayati and Koorosh Goodarzvand Chegini; Final approval: All authors.
The current work designed an inexpensive, simple, and reliable methanol detection method based on a reaction between methanol and SNP. The established colorimetric method revealed a good sensitivity (0.077%), high precision, and acceptable accuracy for methanol determination in different samples.
Drinking or dermal exposure to nonstandard solutions contaminated with methanol may cause methanol poisoning. As seen in the recent events regarding the COVID-19 pandemic, grave concerns were uncovered about some hand sanitizers contaminated with methanol globally [13]. Therefore, estimating methanol in different alcohol-based hand sanitizers is imperative to certify their quality and safety [9].
Moreover, it is indispensable to note that many investigations have authenticated the contamination of methanol in alcoholic beverages and even fruit juices and herbal distillates, which reveals the seriousness of this issue for further research [14]. Herbal distillates have been consumed extensively as beverages and herbal medicine in Iran for a long time [6]. The production of large amounts of herbal distillates is growing yearly by factories and even houses worldwide, and the products are frequently marketed. Methanol generation is unavoidable during the production process of herbal distillates [6]. The pollution of some herbal distillates, such as mint, rose water, and plant forty water, with methanol, may cause blurred vision and blindness in people who consume these products for a long time [15]. There are more than 4000 small and large producers of herbal distillates in Iran, and this observation needs to be considered due to the inaccessibility of appropriate tools for methanol monitoring in these products [16]. Besides, early diagnosis of methanol content in alcoholic and non-alcoholic drinks is extremely pivotal owing to the hazardous impressions of methanol on the human body [17]. Undoubtedly, methanol intoxication is accompanied by blindness and even death when identified too late [2]. Therefore, estimating methanol content in alcoholic or non-alcoholic beverages, herbal distillates, and hand sensitizers is a critical parameter in the quality control of these products. Determining the methanol quantity requires expensive and highly specialized equipment and high technical knowledge [18]. The most accurate and reliable methods, such as HPLC and GC, estimate methanol quantity in different fluids [19]. Previously, Nisbar and colleagues developed a GC/MS-based analytical method to simultaneously determine isopropyl alcohol as the active ingredient in alcohol-based hand sanitizers and methanol as an impurity [13]. In a cross-sectional study, Zamani and collegues determined the contents of methanol in illegal alcoholic beverages in Iran's black market using GC and a modified colorimetric CA method. Of 1221 samples, methanol was identified in 160 samples (13.1%) using the colorimetric CA method and in 128 (10.48%) samples using the GC method with 100% sensitivity and 97.07% specificity [20]. Unfortunately, the gold standard methods of methanol detection are not easily available in developing countries with limited financial and trained personnel. Hence, establishing and using a feasible and reliable strategy for determining methanol in these countries is an advantage [19]. In the colorimetric CA method recommended as the reference method for detecting alcoholic drink-derived methanol by the Association of Official Analytical Chemists (AOAC), methanol is changed to formaldehyde. Then, this compound is measured indirectly through its reaction with CA in hot, concentrated sulfuric acid media [12]. Saadat et al. (2020) appraised the methanol level in some herbal distillates using a new kit based on the modified CA method. According to their findings, different methanol concentrations were detected in all examined samples of herbal distillates ranging from 21 to 770 mg/L [16]. These findings revealed that some of the available herbal distillates in Iran contain enough amounts of methanol, which can cause chronic methanol intoxication in consumers. However, Rafizadeh et al. [21] and Saadat and Rafizadeh [5] previously announced that applying the AOAC-recommended chromotropic acid method for methanol quantification in herbal distillates can give rise to erroneous findings. Besides, the amounts of formaldehyde and formic acid in the examined samples can affect the methanol content if they are present in the sample. Therefore, designing an accurate, safe, and low-cost kit that can determine the methanol contents in any herbal distillates is highly desired [12].
The current research reports a simple method for quantifying methanol quantity in some herbal distillates and hand sensitizer samples by a modified colorimetric method. This proposed method has been shown to have 0.077% sensitivity. Moreover, this colorimetric Kit was precise and accurate, with intra- and inter-assay CV% of less than 10% within the acceptable ranges. Besides, the recovery percentages ranged from 96.5 to 108, indicating an acceptable accuracy of the studied method. We found few kinds of literature to date on the reaction of SNP and alcohol. Zhan et al. developed a SNP-based spectrophotometric method for the direct determination of methanol. This colorimetric method exhibits that the sodium nitroprusside can react with the methanol to create methoxy nitroprusside in the basic solution, a colored product with absorption at 481 nm. Meanwhile, the methanol content can also be calculated based on the absorbance [22].
Additionally, we measured the methanol quantity in different samples using the GC–MS apparatus (a gold standard method) to evaluate the Kit's efficacy. According to the findings, comparing methanol content between GC/MS and the established colorimetric method revealed that the designed method could detect methanol.
The results of the present study showed that this colorimetric method could directly measure the methanol content using sodium nitroprusside reagent without using modern equipment and experts. However, the current research was not specifically designed to evaluate other factors that reacted with SNP solution. Indeed, herbal extract and fermented products may have some compounds that react with SNP solution and decrease the specificity of the output.
Conclusion
This study was undertaken to design a novel chemical method and evaluate methanol in various alcoholic and non-alcoholic products. The established colorimetric method exhibited good sensitivity, high precision, and acceptable accuracy for methanol estimation in various samples. The results of this proposed method have indicated that the newly developed method can quantify methanol with high sensitivity and accuracy by being used through distributors, local authorities, and consumers with no need for appropriate laboratory equipment and increased knowledge and skills.
Ethical Considerations
The Institutional Review Board and Ethics Committee of Shahid Sadoughi University of Medical Sciences, Yazd, Iran (the committee approval number: IR.SSU.MEDICINE.REC.1399.205) approved the study.
Funding
Not applicable.
Conflict of Interest
The authors declared no conflict of interest.
Acknowledgments
This article was extracted from Ali Bakhshi's thesis on the fulfillment of a Master of Science (MSc) degree. The authors thank the Cellular and Molecular Endocrine Research Center's staff for their collaboration.
Authors' Contributions
Study design, data analysis and interpretation: Mehdi Hedayati, Koorosh Goodarzvand Chegini, Ali Bakhshi and Mohamadreza Savaee; Data collection and initial draft preparation: Ali Bakhshi and Mohamadreza Savaee; Editing, supervision and review: Mehdi Hedayati and Koorosh Goodarzvand Chegini; Final approval: All authors.
References
[1]. Pineau NJ, Magro L, van den Broek J, Anderhub P, Güntner AT, Pratsinis SE. Spirit distillation: monitoring methanol formation with a hand-held device. ACS Food Sci Technol. 2021; 1(5): 839-44.
[2]. Jangjou A, Moqadas M, Mohsenian L, Kamyab H, Chelliapan S, Alshehery S, et al. Awareness raising and dealing with methanol poisoning based on effective strategies. Environ Res. 2023; 228: 115886.
[3]. Tephly TR, McMartin KE. Methanol metabolism and toxicity. In: Aspartame. CRC Press; 2020. p. 111-40.
[4]. Nekoukar Z, Zakariaei Z, Taghizadeh F, Musavi F, Banimostafavi ES, Sharifpour A, et al. Methanol poisoning as a new world challenge: A review. Ann Med Surg. 2021; 66: 102445.
[5]. Saadat F, Rafizadeh A. Rapid determination of methanol in herbaceous distillates for their safety evaluation by a new modified chromotropic acid method. Iran J Pharm Res IJPR. 2019; 18(2): 696.
[6]. Shirani K, Hassani FV, Azar-Khiavi KR, Moghaddam ZS, Karimi G. Determination of methanol in Iranian herbal distillates. J Complement Integr Med. 2016; 13(2): 123-27.
[7]. Ohimain EI. Methanol contamination in traditionally fermented alcoholic beverages: the microbial dimension. Springerplus 2016; 5(1): 1607.
[8]. Manning L, Kowalska A. Illicit alcohol: Public health risk of methanol poisoning and policy mitigation strategies. Foods. 2021; 10(7): 1625.
[9]. Güntner AT, Magro L, van den Broek J, Pratsinis SE. Detecting methanol in hand sanitizers. Iscience. 2021; 24(2): 102050.
[10]. Kraut JA. Diagnosis of toxic alcohols: limitations of present methods. Clin Toxicol. 2015; 53(7): 589-95.
[11]. van den Broek J, Abegg S, Pratsinis SE, Güntner AT. Highly selective detection of methanol over ethanol by a handheld gas sensor. Nat Commun. 2019; 10(1): 4220.
[12]. Fagnani E, Melios CB, Pezza L, Pezza HR. Chromotropic acid–formaldehyde reaction in strongly acidic media. The role of dissolved oxygen and replacement of concentrated sulphuric acid. Talanta. 2003; 60(1): 171-76.
[13]. Nisbar ND, Jamal Khair SK, Bujang NB, Mohd Yusop AY. Determination of ethanol, isopropyl alcohol and methanol in alcohol-based hand sanitiser to ensure product quality, safety and efficacy. Sci Rep. 2023; 13(1): 9478.
[14]. Tulashie SK, Appiah AP, Torku GD, Darko AY, Wiredu A. Determination of methanol and ethanol concentrations in local and foreign alcoholic drinks and food products (Banku, Ga kenkey, Fante kenkey and Hausa koko) in Ghana. Int J food Contam. 2017; 4(1): 1-5.
[15]. Mousavi SR, Namaei-Ghassemi M, Layegh M, AfzalAghaee M, Zare G, Moghiman T, et al. Determination of methanol concentrations in traditional herbal waters of different brands in Iran. Iran J Basic Med Sci. 2011; 14(4): 361.
[16]. Saadat F, Hassanian Moghadam H, Zamani N, Rafizadeh A. Quantification of some herbal distillates’ methanol to evaluate a new diagnostic kit. J Food Qual. 2020; 2020(1): 1-7.
[17]. Jones AW. Comment on ‘Estimates of non-alcoholic food-derived ethanol and methanol in human. J Anal Toxicol. 2022; 46(1): 48-51.
[18]. Jang M, Yang H, Shin G, Koo JM, Hwang SY, Park J, et al. Determination of methanol in commercialized alcohol-based hand sanitizing and other similar products using headspace GC-MS. Curr Anal Chem. 2022; 18(7): 774-80.
[19]. Rafizadeh A, Bhalla A, Sharma N, Kumar K, Zamani N, McDonald R, et al. Evaluating new simplified assays for harm reduction from methanol poisoning using chromotropic acid kits: an analytical study on indian and iranian alcoholic beverages. Front Public Heal. 2022; 10: 983663.
[20]. Zamani N, Rafizadeh A, Hassanian-Moghaddam H, Akhavan-Tavakoli A, Ghorbani-Samin M, Akhgari M, et al. Evaluation of methanol content of illegal beverages using GC and an easier modified Chromotropic acid method; a cross sectional study. Subst Abuse Treat Prev Policy. 2019; 14: 1-7.
[21]. Rafizadeh A, Shariati SH, Safarzadeh V. Determination of herbal distillates methanol using a new diagnostic kit. J Guilan Univ Med Sci. 2016; 24(96): 61-7.
[22]. Zhan Y, Zhang Y, Li Q, Du X. A novel visible spectrophotometric method for the determination of methanol using sodium nitroprusside as spectroscopic probe. J Chinese Chem Soc. 2010; 57(2): 230-35.
[2]. Jangjou A, Moqadas M, Mohsenian L, Kamyab H, Chelliapan S, Alshehery S, et al. Awareness raising and dealing with methanol poisoning based on effective strategies. Environ Res. 2023; 228: 115886.
[3]. Tephly TR, McMartin KE. Methanol metabolism and toxicity. In: Aspartame. CRC Press; 2020. p. 111-40.
[4]. Nekoukar Z, Zakariaei Z, Taghizadeh F, Musavi F, Banimostafavi ES, Sharifpour A, et al. Methanol poisoning as a new world challenge: A review. Ann Med Surg. 2021; 66: 102445.
[5]. Saadat F, Rafizadeh A. Rapid determination of methanol in herbaceous distillates for their safety evaluation by a new modified chromotropic acid method. Iran J Pharm Res IJPR. 2019; 18(2): 696.
[6]. Shirani K, Hassani FV, Azar-Khiavi KR, Moghaddam ZS, Karimi G. Determination of methanol in Iranian herbal distillates. J Complement Integr Med. 2016; 13(2): 123-27.
[7]. Ohimain EI. Methanol contamination in traditionally fermented alcoholic beverages: the microbial dimension. Springerplus 2016; 5(1): 1607.
[8]. Manning L, Kowalska A. Illicit alcohol: Public health risk of methanol poisoning and policy mitigation strategies. Foods. 2021; 10(7): 1625.
[9]. Güntner AT, Magro L, van den Broek J, Pratsinis SE. Detecting methanol in hand sanitizers. Iscience. 2021; 24(2): 102050.
[10]. Kraut JA. Diagnosis of toxic alcohols: limitations of present methods. Clin Toxicol. 2015; 53(7): 589-95.
[11]. van den Broek J, Abegg S, Pratsinis SE, Güntner AT. Highly selective detection of methanol over ethanol by a handheld gas sensor. Nat Commun. 2019; 10(1): 4220.
[12]. Fagnani E, Melios CB, Pezza L, Pezza HR. Chromotropic acid–formaldehyde reaction in strongly acidic media. The role of dissolved oxygen and replacement of concentrated sulphuric acid. Talanta. 2003; 60(1): 171-76.
[13]. Nisbar ND, Jamal Khair SK, Bujang NB, Mohd Yusop AY. Determination of ethanol, isopropyl alcohol and methanol in alcohol-based hand sanitiser to ensure product quality, safety and efficacy. Sci Rep. 2023; 13(1): 9478.
[14]. Tulashie SK, Appiah AP, Torku GD, Darko AY, Wiredu A. Determination of methanol and ethanol concentrations in local and foreign alcoholic drinks and food products (Banku, Ga kenkey, Fante kenkey and Hausa koko) in Ghana. Int J food Contam. 2017; 4(1): 1-5.
[15]. Mousavi SR, Namaei-Ghassemi M, Layegh M, AfzalAghaee M, Zare G, Moghiman T, et al. Determination of methanol concentrations in traditional herbal waters of different brands in Iran. Iran J Basic Med Sci. 2011; 14(4): 361.
[16]. Saadat F, Hassanian Moghadam H, Zamani N, Rafizadeh A. Quantification of some herbal distillates’ methanol to evaluate a new diagnostic kit. J Food Qual. 2020; 2020(1): 1-7.
[17]. Jones AW. Comment on ‘Estimates of non-alcoholic food-derived ethanol and methanol in human. J Anal Toxicol. 2022; 46(1): 48-51.
[18]. Jang M, Yang H, Shin G, Koo JM, Hwang SY, Park J, et al. Determination of methanol in commercialized alcohol-based hand sanitizing and other similar products using headspace GC-MS. Curr Anal Chem. 2022; 18(7): 774-80.
[19]. Rafizadeh A, Bhalla A, Sharma N, Kumar K, Zamani N, McDonald R, et al. Evaluating new simplified assays for harm reduction from methanol poisoning using chromotropic acid kits: an analytical study on indian and iranian alcoholic beverages. Front Public Heal. 2022; 10: 983663.
[20]. Zamani N, Rafizadeh A, Hassanian-Moghaddam H, Akhavan-Tavakoli A, Ghorbani-Samin M, Akhgari M, et al. Evaluation of methanol content of illegal beverages using GC and an easier modified Chromotropic acid method; a cross sectional study. Subst Abuse Treat Prev Policy. 2019; 14: 1-7.
[21]. Rafizadeh A, Shariati SH, Safarzadeh V. Determination of herbal distillates methanol using a new diagnostic kit. J Guilan Univ Med Sci. 2016; 24(96): 61-7.
[22]. Zhan Y, Zhang Y, Li Q, Du X. A novel visible spectrophotometric method for the determination of methanol using sodium nitroprusside as spectroscopic probe. J Chinese Chem Soc. 2010; 57(2): 230-35.
Type of Study: Research |
Subject:
Biochemistry
Received: 2023/12/31 | Accepted: 2024/06/23 | Published: 2024/10/1
Received: 2023/12/31 | Accepted: 2024/06/23 | Published: 2024/10/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







