Wed, Feb 4, 2026
[Archive]
Volume 11, Issue 3 (August 2024)
IJML 2024, 11(3): 251-258 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Daneshvar A, Teymuri Kheravi M, Mohsenzadeh M, Shojaeian N. The effect of Endurance and Cognitive-Endurance Training on Brain Natriuretic Peptide in Aged Rats after Induction of Dementia by Trihexyphenidyl. IJML 2024; 11 (3) :251-258
URL: http://ijml.ssu.ac.ir/article-1-515-en.html
URL: http://ijml.ssu.ac.ir/article-1-515-en.html
Department of Sports Sciences, Faculty of Humanities, Bojnourd Branch, Islamic Azad University, Bojnourd, Iran
Full-Text [PDF 232 kb]
(181 Downloads)
| Abstract (HTML) (398 Views)
References
[1]. Malik R, Kalra S, Bhatia S, Al Harrasi A, Singh G, Mohan S, et al. Overview of therapeutic targets in management of dementia. Biomedicine & Pharmacotherapy 2022; 152: 113168.
[2]. Fayosse A, Nguyen DP, Dugravot A, Dumurgier J, Tabak AG, Kivimäki M, et al. Risk prediction models for dementia: role of age and cardiometabolic risk factors. BMC Medicine 2020; 18: 1-10.
[3]. Hildreth KL, Church S. Evaluation and management of the elderly patient presenting with cognitive complaints. The Medical Clinics of North America 2014; 99(2): 311-13.
[4]. Rubattu S, Volpe M. Natriuretic peptides in the cardiovascular system: multifaceted roles in physiology, pathology and therapeutics. International Journal of Molecular Sciences 2019; 20(16): 3991.
[5]. Gallo G, Bianchi F, Cotugno M, Volpe M, Rubattu S. Natriuretic peptides, cognitive impairment and dementia: an intriguing pathogenic link with implications in hypertension. Journal of Clinical Medicine 2020; 9(7): 2265.
[6]. Çavuşoğlu Y, Alper A, Altay H, Çelik A, Demirkan B, Guvenc T, et al. Natriuretic peptides in clinical practice. Anatolian Journal of Cardiology 2019; 21.
[7]. McGurran H, Glenn JM, Madero EN, Bott NT. Prevention and treatment of Alzheimer’s disease: biological mechanisms of exercise. Journal of Alzheimer’s Disease 2019; 69(2): 311-38.
[8]. Malandish A, Ghadamyari N, Karimi A, Naderi M. The role of exercise training on cardiovascular peptides in patients with heart failure: a systematic review and meta-analysis. Current Research in Physiology 2022; 5: 270-86.
[9]. Núñez-Marín G, Iraola D, Lorenzo M, De La Espriella R, Villar S, Santas E, et al. An update on utilising brain natriuretic peptide for risk stratification, monitoring and guiding therapy in heart failure. Expert Review of Molecular Diagnostics 2023; 23(6): 521-33.
[10]. Mirdar S, Arab A, Hedayati M, Hajizade A. The effect of pregnant rat swimming on hypoxia-inducible factor-1α levels of neonatal lung. Tehran University Medical Journal 2012; 69(12): 754.
[11]. Talaei Zavareh SA, Davari S, Gholami M, Salami M. Change in visual experience impairs rat’s spatial learning in morris water maze. Journal of Isfahan Medical School 2010; 28(111): 551-62.
[12]. Najizadeh MH, Mokhber Dezfouli MR, Nikhbakht Borujeni G, Vajhi A, Jabbari Fakhr M, Mehrara M, et al. Evaluation of plasma concentration of atrial natriuretic peptide (ANP) in horses with pulmonic valve regurgitation. Veterinary Clinical Pathology 2020; 14(55): 251-61.
[13]. Ostovaneh MR, Moazzami K, Yoneyama KA, Venkatesh B, Heckbert SR, Wu CO, et al. Change in NT-proBNP (N-terminal pro-B-type natriuretic peptide) level and risk of dementia in multi-ethnic study of atherosclerosis (MESA). Hypertension 2020; 75(2): 316-23.
[14]. Di Daniele N, Celotto R, Alunni Fegatelli D, Gabriele M, Rovella V, Scuteri A. Common carotid artery calcification impacts on cognitive function in older patients. High Blood Pressure & Cardiovascular Prevention 2019; 26: 127-34.
[15]. Rizzoni D, Rizzoni M, Nardin M, Chiarini G, Agabiti-Rosei C, Aggiusti C, et al. Vascular aging and disease of the small vessels. High Blood Pressure & Cardiovascular Prevention 2019; 26: 183-89.
[16]. Hilal S, Chai YL, Van Veluw S, Shaik MA, Ikram MK, Venketasubramanian N et al. Association between subclinical cardiac biomarkers and clinically manifest cardiac diseases with cortical cerebral microinfarcts. JAMA Neurology 2017; 74(4): 403-10.
[17]. Kondziella D, Göthlin M, Fu M, Zetterberg H, Wallin A. B-type natriuretic peptide plasma levels are elevated in subcortical vascular dementia. Neuroreport 2009; 20(9): 825-27.
[18]. Hu WT. Erratum: Plasma multianalyte profiling in mild cognitive impairment and Alzheimer disease. Neurology 2012; 79(18): 1935.
[19]. Tynkkynen J, Hernesniemi JA, Laatikainen T, Havulinna AS, Salo P, Blankenberg S, et al. High-sensitivity cardiac troponin I and NT-proBNP as predictors of incident dementia and Alzheimer’s disease: the FINRISK Study. Journal of Neurology 2017; 264: 503-11.
[20]. Ferguson IT, Elbejjani M, Sabayan B, Jacobs Jr DR, Meirelles O, Sanchez OA, et al. N-terminal pro-brain natriuretic peptide and associations with brain magnetic resonance imaging (MRI) features in middle age: The CARDIA brain MRI study. Frontiers in Neurology 2018; 9: 307.
[21]. Sabayan B, van Buchem MA, Sigurdsson S, Zhang Q, Harris TB, Gudnason V, et al. Cardiac hemodynamics are linked with structural and functional features of brain aging: the age, gene/ environment susceptibility (AGES)‐ Reykjavik Study. Journal of the American Heart Association 2015; 4(1): 1294.
[22]. Mirza SS, de Bruijn RF, Koudstaal PJ, van den Meiracker AH, Franco OH, Hofman A, et al. The N-terminal pro B-type natriuretic peptide, and risk of dementia and cognitive decline: a 10-year follow-up study in the general population. Journal of Neurology, Neurosurgery & Psychiatry 2016; 87(4): 356-62.
[23]. Ohba H, Takada H, Musha H, Nagashima J, Mori N, Awaya T, et al. Effects of prolonged strenuous exercise on plasma levels of atrial natriuretic peptide and brain natriuretic peptide in healthy men. American Heart Journal 2001; 141(5): 751-58.
[24]. Aengevaeren VL, Hopman MT, Thijssen DH, van Kimmenade RR, de Boer MJ, Eijsvogels TM. Endurance exercise-induced changes in BNP concentrations in cardiovascular patients versus healthy controls. International Journal of Cardiology 2017; 227: 430-35.
Full-Text: (113 Views)
Introduction
The clinical term for dementia is characterized as progressive cognitive deterioration that impeds an individual's ability to function independently [1]. The number of people with dementia worldwide stands at around 47 million, and it is estimated to reach 131 million by 2050. The symptoms of dementia are gradual, continuous, and progressive [2]. Dementia patients sustain changes in their cognition, performance, and behavior. The symptoms of dementia exhibit considerable variation among individuals, leading to cognitive deficits, including memory loss, speech and language challenges, agnosia (insufficient capacity to identify objects), apraxia (incapacity to execute formerly acquired activities), and impairment in executive function (reasoning, evaluation, and planning). Damage to the cerebral cortex is the cause of cognitive impairment, which can be attributed to synaptic dysfunction, inflammatory processes, and alterations in brain metabolism [3].
Natriuretic peptides (NPs) are defined as a group of cardiovascular hormones primarily released by the atrium (ANP), brain (BNP), and also by the endothelium or C-type natriuretic peptide (CNP). These hormones have defensive performance and play a critical role in the cardiovascular system. Beyond their significant participation in the pathophysiology, diagnosis, prognostication, and treatment of cardiovascular diseases (CVD), the role of BNP and ANP on CVD has been robustly validated through various population studies [4]. Higher amino-terminal natriuretic peptides (NT-proBNP and NT-proANP), considered more stable forms, could signify future cardiovascular disasters. The risk of cognitive decline increases with high levels of BNP, a serum marker of congestive heart failure. However, few studies have examined this marker in various forms of dementia [5].
As a non-pharmacological promising treatment, exercise has been instrumental in mitigating cognitive decline and improving the quality of life of patients with cognitive impairment. Several randomized controlled trials have shown desirable outcomes of exercise on cognitive function and neuropsychiatric symptoms in cognitive impairment patients [6]. Neuroimaging studies have revealed that exercise can enhance the functional flexibility of the brain. Exercise can enhance growth factors such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor 1 (IGF-1). This, in turn, helps regulate inflammatory cytokines, decrease oxidative stress, improve cerebral blood flow, lower Aβ concentration, and prevent tau phosphorylation. These protective effects of exercise positively impact cognitive function [7]. Prior research has demonstrated that specific forms of physical activity, including aerobic exercise and resistance exercise, may function via separate molecular processes and result in varying magnitudes of effects. The type of exercise prescribed by clinicians should be considered while prescribing exercise to prevent or slow down dementia [8, 9]. Due to the lack of studies that compare various types of simultaneous exercise interventions, it is ambiguous which exercise therapy is most effective in preventing or slowing down dementia. In this study, the effects of endurance training and cognitive endurance training on plasma BNP levels in dementia sample rats were investigated.
Materials and Methods
Animal preparation
A total of 40 adult male Wistar rats, all in good health, with an average weight of 250 grams, and of the same weight and age, were acquired from the Pasteur Institute-Iran. The animals were transferred to the animal facility at the college, where they were subjected to a temperature range of 20 to 22 °C, a humidity range of 56% to 60%, and a light cycle consisting of 12/12 hours of light/darkness. Unrestricted access to water and rat food was available to all animals. The animals had a one-week acclimation period to prevent any negative effects from environmental factors such as relocation, temperature, light, and humidity on the test outcomes. Daily handling was provided to the animals throughout the week to reduce any stress caused by their involvement in the experiment.
Rat models of dementia
Following one week of acclimating the animals to their new surroundings, the mice were randomly allocated into four groups, each consisting of ten individuals. There were four groups: healthy controls, dementia groups, endurance groups, and endurance-cognitive groups. The chemical trihexyphenidyl was used to impair the memory of mice and induce dementia temporarily. The Rats were given 250 mg per kg of their body weight intravenously for five days.
Endurance training
Rat models of dementia were subjected to an endurance training protocol, which involved swimming in a rodent pool for 3 weeks, 5 days per week. The endurance training group rats utilized a unique pool of 50×50×100 cm to engage in daily swimming sessions five days a week. The initial swimming training program commenced with a duration of 30 minutes, which was progressively augmented by an additional five minutes each day, culminating in a total of 60 minutes during the second week. The 60-minute time limit was in place until the end of the third week. Training overload during swimming was achieved by manipulating the intensity and velocity of the water. This approach was consistently maintained throughout the entire training adaptation week. Throughout the training weeks, the velocity and intensity of the water current escalated from 7 to 15 liters per minute while maintaining a consistent duration of 60 minutes [10].
Cognitive-endurance training
The cognitive-endurance training group implemented a training protocol of administering Morris water maze training sessions to each rat for three weeks. The training took place biweekly, consisting of four daily sessions, with a ten-minute interval between sessions. The Morris water maze device used contained a cylindrical basin with a diameter of 136 cm and a height of 60 cm. Water with a temperature of 20 °C was added to the basin until it reached a height of 25 cm. The center of the southwest quadrant of the circle had a black-painted metal platform that was 10 cm in diameter and 1 cm below the water's surface. The studies were conducted in a dimly lit space with visible markers on all four sides. The animal used these markers to locate the hidden platform. The mouse was released haphazardly from one of the quadrants of the pond, and the scientist recorded the time it took for the mouse to locate the platform. In each experiment, the animal was introduced into the water from a specific beginning point (north, south, east, or west) while its face was directed towards the wall of the cylinder. In every rotation, one of the four initial spots was utilized. The experiment concludes when the mouse reaches the platform or when 90 seconds have elapsed. Subsequently, 30 seconds was assigned to the animal, followed by the commencement of the subsequent experiment. The experimenter relocated the rats that could not locate the platform to the platform itself, where they were permitted to remain for 30 seconds. Upon completion of the fourth trial, the mice were removed from the pond [11].
Measurement of plasma BNP
The concentration of BNP in the plasma of rats was quantified using the sandwich enzyme-linked immunosorbent assay (ELISA) approach. To achieve this objective, blood samples were collected from various groups' rats after the training session concluded. The blood samples were then transferred into tubes containing Ethylenediaminetetraacetic acid. Separating plasma was achieved by centrifuging the blood samples for 10 minutes at 400 ×g. The plasma sample was kept in a freezer at -80 °C until further tests were carried out. The sandwich ELISA assay was conducted using the specialized kit [E-EL-M0204] to quantify BNP. To achieve this objective, 40 μl of each plasma sample was added to the wells containing the specific monoclonal antibody for brain natriuretic factor. The wells were poured with 10 μl of BNP antibody conjugated with biotin and 50 μl of streptavidin-HRP. After incubating the wells at 37 °C for an hour, they were rinsed and treated with 50 μl of two chromogen solvents. Following a 10-minute re-incubation at 37 °C, the reaction-blocking solvent was introduced into the wells. In the end, light absorption was measured at 450 nm wavelength using an ELISA reader [12].
Results
Plasma measurement of BNP
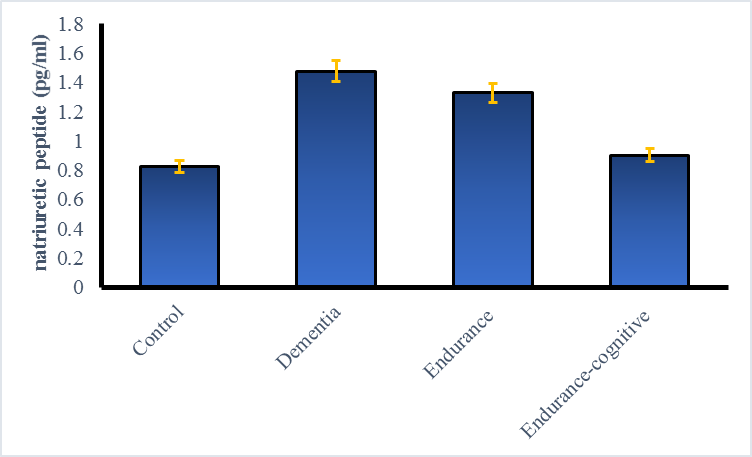
The findings demonstrated a significant increase in plasma BNP levels in demented rats belonging to the sham group as compared to the control group (p < 0.05). It has been found that engaging in endurance training and endurance-cognitive training leads to decreased BNP levels. Endurance-cognitive training yielded superior outcomes in this aspect, leading to a more significant reduction in BNP level (Fig. 1).
Discussion
In this study, it was found that the level of BNP in rats of the sham (dementia) group was significantly higher than that in the control group. Both endurance and cognitive-endurance exercise led to a decrease in BNP.
Natriuretic peptides (NPs) are defined as a group of cardiovascular hormones primarily released by the atrium (ANP), brain (BNP), and also by the endothelium or C-type natriuretic peptide (CNP). These hormones have defensive performance and play a critical role in the cardiovascular system. Beyond their significant participation in the pathophysiology, diagnosis, prognostication, and treatment of cardiovascular diseases (CVD), the role of BNP and ANP on CVD has been robustly validated through various population studies [4]. Higher amino-terminal natriuretic peptides (NT-proBNP and NT-proANP), considered more stable forms, could signify future cardiovascular disasters. The risk of cognitive decline increases with high levels of BNP, a serum marker of congestive heart failure. However, few studies have examined this marker in various forms of dementia [5].
As a non-pharmacological promising treatment, exercise has been instrumental in mitigating cognitive decline and improving the quality of life of patients with cognitive impairment. Several randomized controlled trials have shown desirable outcomes of exercise on cognitive function and neuropsychiatric symptoms in cognitive impairment patients [6]. Neuroimaging studies have revealed that exercise can enhance the functional flexibility of the brain. Exercise can enhance growth factors such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor 1 (IGF-1). This, in turn, helps regulate inflammatory cytokines, decrease oxidative stress, improve cerebral blood flow, lower Aβ concentration, and prevent tau phosphorylation. These protective effects of exercise positively impact cognitive function [7]. Prior research has demonstrated that specific forms of physical activity, including aerobic exercise and resistance exercise, may function via separate molecular processes and result in varying magnitudes of effects. The type of exercise prescribed by clinicians should be considered while prescribing exercise to prevent or slow down dementia [8, 9]. Due to the lack of studies that compare various types of simultaneous exercise interventions, it is ambiguous which exercise therapy is most effective in preventing or slowing down dementia. In this study, the effects of endurance training and cognitive endurance training on plasma BNP levels in dementia sample rats were investigated.
Materials and Methods
Animal preparation
A total of 40 adult male Wistar rats, all in good health, with an average weight of 250 grams, and of the same weight and age, were acquired from the Pasteur Institute-Iran. The animals were transferred to the animal facility at the college, where they were subjected to a temperature range of 20 to 22 °C, a humidity range of 56% to 60%, and a light cycle consisting of 12/12 hours of light/darkness. Unrestricted access to water and rat food was available to all animals. The animals had a one-week acclimation period to prevent any negative effects from environmental factors such as relocation, temperature, light, and humidity on the test outcomes. Daily handling was provided to the animals throughout the week to reduce any stress caused by their involvement in the experiment.
Rat models of dementia
Following one week of acclimating the animals to their new surroundings, the mice were randomly allocated into four groups, each consisting of ten individuals. There were four groups: healthy controls, dementia groups, endurance groups, and endurance-cognitive groups. The chemical trihexyphenidyl was used to impair the memory of mice and induce dementia temporarily. The Rats were given 250 mg per kg of their body weight intravenously for five days.
Endurance training
Rat models of dementia were subjected to an endurance training protocol, which involved swimming in a rodent pool for 3 weeks, 5 days per week. The endurance training group rats utilized a unique pool of 50×50×100 cm to engage in daily swimming sessions five days a week. The initial swimming training program commenced with a duration of 30 minutes, which was progressively augmented by an additional five minutes each day, culminating in a total of 60 minutes during the second week. The 60-minute time limit was in place until the end of the third week. Training overload during swimming was achieved by manipulating the intensity and velocity of the water. This approach was consistently maintained throughout the entire training adaptation week. Throughout the training weeks, the velocity and intensity of the water current escalated from 7 to 15 liters per minute while maintaining a consistent duration of 60 minutes [10].
Cognitive-endurance training
The cognitive-endurance training group implemented a training protocol of administering Morris water maze training sessions to each rat for three weeks. The training took place biweekly, consisting of four daily sessions, with a ten-minute interval between sessions. The Morris water maze device used contained a cylindrical basin with a diameter of 136 cm and a height of 60 cm. Water with a temperature of 20 °C was added to the basin until it reached a height of 25 cm. The center of the southwest quadrant of the circle had a black-painted metal platform that was 10 cm in diameter and 1 cm below the water's surface. The studies were conducted in a dimly lit space with visible markers on all four sides. The animal used these markers to locate the hidden platform. The mouse was released haphazardly from one of the quadrants of the pond, and the scientist recorded the time it took for the mouse to locate the platform. In each experiment, the animal was introduced into the water from a specific beginning point (north, south, east, or west) while its face was directed towards the wall of the cylinder. In every rotation, one of the four initial spots was utilized. The experiment concludes when the mouse reaches the platform or when 90 seconds have elapsed. Subsequently, 30 seconds was assigned to the animal, followed by the commencement of the subsequent experiment. The experimenter relocated the rats that could not locate the platform to the platform itself, where they were permitted to remain for 30 seconds. Upon completion of the fourth trial, the mice were removed from the pond [11].
Measurement of plasma BNP
The concentration of BNP in the plasma of rats was quantified using the sandwich enzyme-linked immunosorbent assay (ELISA) approach. To achieve this objective, blood samples were collected from various groups' rats after the training session concluded. The blood samples were then transferred into tubes containing Ethylenediaminetetraacetic acid. Separating plasma was achieved by centrifuging the blood samples for 10 minutes at 400 ×g. The plasma sample was kept in a freezer at -80 °C until further tests were carried out. The sandwich ELISA assay was conducted using the specialized kit [E-EL-M0204] to quantify BNP. To achieve this objective, 40 μl of each plasma sample was added to the wells containing the specific monoclonal antibody for brain natriuretic factor. The wells were poured with 10 μl of BNP antibody conjugated with biotin and 50 μl of streptavidin-HRP. After incubating the wells at 37 °C for an hour, they were rinsed and treated with 50 μl of two chromogen solvents. Following a 10-minute re-incubation at 37 °C, the reaction-blocking solvent was introduced into the wells. In the end, light absorption was measured at 450 nm wavelength using an ELISA reader [12].
Results
Plasma measurement of BNP
The findings demonstrated a significant increase in plasma BNP levels in demented rats belonging to the sham group as compared to the control group (p < 0.05). It has been found that engaging in endurance training and endurance-cognitive training leads to decreased BNP levels. Endurance-cognitive training yielded superior outcomes in this aspect, leading to a more significant reduction in BNP level (Fig. 1).
Discussion
In this study, it was found that the level of BNP in rats of the sham (dementia) group was significantly higher than that in the control group. Both endurance and cognitive-endurance exercise led to a decrease in BNP.

Fig. 1. Comparison of BNP plasma level in different groups (*p < 0.001)
The effect of endurance-cognitive training on reducing BNP was somewhat higher than that of endurance training, but this difference was not statistically significant. In a recent study, it was demonstrated, for the first time, that gradual variations in NT-proBNP levels over time can be used to forecast the onset of dementia in a Caucasian population [13]. They found that baseline NT-proBNP levels were linked to the progression of cognitive dysfunction in the future. Significantly, an increase in NT-proBNP levels during 3 years was correlated with a higher risk of developing dementia in the future, while a decline in NT-proBNP levels was related to a lower danger of dementia. When cardiovascular risk factors and comorbidities such as renal dysfunction, hypertension, diabetes mellitus, smoking, and atherosclerosis are present, having high levels of NT-proBNP could be a possible focus for preventing dementia [14, 15]. This research established that prompt intervention of these risk conditions and adequate preventive measures for cardiovascular diseases can prevent the onset of dementia in the elderly. Significantly, an elevated level of circulating BNP appears to be a reliable indicator for implementing effective intervention strategies for these risk factors and for effectively preventing the progression of cognitive impairment and dementia. Hilal et al.'s study shows a significant correlation between NT-proBNP and cognitive impairment only when cerebrovascular disease is present. It is important to consider NT-proBNP as a marker of ischemic brain damage [16]. These studies confirm that circulating markers of cardiac dysfunction can indicate silent brain damage or systemic vascular damage.
Kondziella et al. tested the hypothesis that BNP significantly correlates with vascular dementia. Plasma BNP was measured in 15 subcortical vascular dementia patients, 19 Alzheimer's patients without evidence of vascular disorder, and in the matched control group. According to their findings, BNP was elevated in subcortical vascular dementia when compared to the control group, but there was no modification in Alzheimer's disease. Ultimately, subcortical vascular dementia is indeed linked to high BNP levels, which cannot be demonstrated for Alzheimer's disease. This is probably due to the greater cardiovascular burden of BNP in patients with subcortical vascular dementia [17].
Conversely, few studies have indicated that elevated NPs in dementia may occur independently of cardiovascular disease risk factors. Additionally, higher NT-proBNP levels have been identified as an independent risk marker for dementia, particularly among men. This evidence indicates that BNP may be a direct marker of neuronal damage and a brain pathogenic process. This hypothesis was supported by the correlation of plasma BNP levels with amyloid beta levels in cerebrospinal fluid [18]. The study by Tynkkynen et al. [19] indicates a correlation between NT-proBNP levels and dementia in a population without CVD. It shows that neurodegenerative alterations begin early in the development of CVD, as seen by the early changes in NT-proBNP levels. The information demonstrated by Ferguson et al. in a middle-aged population further supports, at least to some extent, a positive relationship between BNP and brain damage [20]. Based on the Sabayan et al. study, higher levels of NT-proBNP were correlated to changes in brain conformation and function, independent of cardiac output and its damage risk factors, proffering that NT-proBNP may be directly related to structural changes and age-related brain function, including reduction of brain volume, cognitive impairment, and depression [21]. Therefore, the BNP level may serve as a potential biomarker for timely preventive and therapeutic interventions aimed at averting the future onset of dementia [22].
In the current study, it was demonstrated that rats with dementia exhibit an elevated plasma level of BNP. Additionally, it was observed that engaging in endurance and cognitive endurance exercises can lower BNP levels in these rats to match those of healthy control rats. The role of exercise on BNP levels in individuals suffering from dementia has not been extensively studied. Ohba et al. (2001) conducted a study on 10 healthy men to examine the impact of exercise on the levels of plasma ANP, BNP, catecholamines, lactate, and serum cardiac troponin T (cTnT) before and after a 100 km marathon. All variables experienced a significant increase after the race. However, there was a particularly strong correlation between the increase in ANP and BNP levels and the increase in cTnT levels. The blood levels of cTnT exceeded the upper reference limit in 9 out of 10 males after the race. The activity led to a substantial increase in ANP and BNP levels in healthy males, with this increase being partly linked to myocardial injury during the race [23].
Aengevaeren et al. (2017) conducted a study to examine the exercise-induced increases in BNP levels between healthy individuals and individuals with cardiovascular risk factors CVD. Their findings demonstrated that engaging in regular, moderate-intensity walking exercise for a prolonged period leads to an elevation in BNP levels among individuals with CVD. However, in individuals with cardiovascular risk factors and healthy individuals, the increase in BNP was minimal and did not progressively accrue with consecutive days of activity [24].
Conclusions
The type of exercise may play a role in BNP levels. In the present research, endurance and cognitive endurance exercises were used, and the results confirmed that these exercises reduce BNP in rats with dementia. This study is believed to be the first to examine the impact of exercise training on BNP levels in dementia. The results of the present research report that endurance and cognitive endurance exercises are non-pharmacological solutions to reduce BNP levels. These exercises can be combined with other treatment solutions. Also, further research is required to explore the underlying mechanisms and to validate these findings in human subjects.
Ethical Consideration
This study was approved by the Gerash University of Medical Sciences ethics committee and performed according to standard animal study instructions.
Funding
All expenses for this study were supported by contract No. IR.GERUMS.REC.1403.006 from Gerash University of Medical Sciences.
Conflict of Interests
The authors approve that there is no conflict of interest.
Acknowledgments
The authors would like to acknowledge the supportive assistance of Gerash University of Medical Sciences.
Authors’ Contributions
A.D, M.M and NA.S: Laboratory works, data interpretation, manuscript writing. M.TK: Manuscript editor and project manager. All authors reviewed and approved the final version of the manuscript.
Kondziella et al. tested the hypothesis that BNP significantly correlates with vascular dementia. Plasma BNP was measured in 15 subcortical vascular dementia patients, 19 Alzheimer's patients without evidence of vascular disorder, and in the matched control group. According to their findings, BNP was elevated in subcortical vascular dementia when compared to the control group, but there was no modification in Alzheimer's disease. Ultimately, subcortical vascular dementia is indeed linked to high BNP levels, which cannot be demonstrated for Alzheimer's disease. This is probably due to the greater cardiovascular burden of BNP in patients with subcortical vascular dementia [17].
Conversely, few studies have indicated that elevated NPs in dementia may occur independently of cardiovascular disease risk factors. Additionally, higher NT-proBNP levels have been identified as an independent risk marker for dementia, particularly among men. This evidence indicates that BNP may be a direct marker of neuronal damage and a brain pathogenic process. This hypothesis was supported by the correlation of plasma BNP levels with amyloid beta levels in cerebrospinal fluid [18]. The study by Tynkkynen et al. [19] indicates a correlation between NT-proBNP levels and dementia in a population without CVD. It shows that neurodegenerative alterations begin early in the development of CVD, as seen by the early changes in NT-proBNP levels. The information demonstrated by Ferguson et al. in a middle-aged population further supports, at least to some extent, a positive relationship between BNP and brain damage [20]. Based on the Sabayan et al. study, higher levels of NT-proBNP were correlated to changes in brain conformation and function, independent of cardiac output and its damage risk factors, proffering that NT-proBNP may be directly related to structural changes and age-related brain function, including reduction of brain volume, cognitive impairment, and depression [21]. Therefore, the BNP level may serve as a potential biomarker for timely preventive and therapeutic interventions aimed at averting the future onset of dementia [22].
In the current study, it was demonstrated that rats with dementia exhibit an elevated plasma level of BNP. Additionally, it was observed that engaging in endurance and cognitive endurance exercises can lower BNP levels in these rats to match those of healthy control rats. The role of exercise on BNP levels in individuals suffering from dementia has not been extensively studied. Ohba et al. (2001) conducted a study on 10 healthy men to examine the impact of exercise on the levels of plasma ANP, BNP, catecholamines, lactate, and serum cardiac troponin T (cTnT) before and after a 100 km marathon. All variables experienced a significant increase after the race. However, there was a particularly strong correlation between the increase in ANP and BNP levels and the increase in cTnT levels. The blood levels of cTnT exceeded the upper reference limit in 9 out of 10 males after the race. The activity led to a substantial increase in ANP and BNP levels in healthy males, with this increase being partly linked to myocardial injury during the race [23].
Aengevaeren et al. (2017) conducted a study to examine the exercise-induced increases in BNP levels between healthy individuals and individuals with cardiovascular risk factors CVD. Their findings demonstrated that engaging in regular, moderate-intensity walking exercise for a prolonged period leads to an elevation in BNP levels among individuals with CVD. However, in individuals with cardiovascular risk factors and healthy individuals, the increase in BNP was minimal and did not progressively accrue with consecutive days of activity [24].
Conclusions
The type of exercise may play a role in BNP levels. In the present research, endurance and cognitive endurance exercises were used, and the results confirmed that these exercises reduce BNP in rats with dementia. This study is believed to be the first to examine the impact of exercise training on BNP levels in dementia. The results of the present research report that endurance and cognitive endurance exercises are non-pharmacological solutions to reduce BNP levels. These exercises can be combined with other treatment solutions. Also, further research is required to explore the underlying mechanisms and to validate these findings in human subjects.
Ethical Consideration
This study was approved by the Gerash University of Medical Sciences ethics committee and performed according to standard animal study instructions.
Funding
All expenses for this study were supported by contract No. IR.GERUMS.REC.1403.006 from Gerash University of Medical Sciences.
Conflict of Interests
The authors approve that there is no conflict of interest.
Acknowledgments
The authors would like to acknowledge the supportive assistance of Gerash University of Medical Sciences.
Authors’ Contributions
A.D, M.M and NA.S: Laboratory works, data interpretation, manuscript writing. M.TK: Manuscript editor and project manager. All authors reviewed and approved the final version of the manuscript.
References
[1]. Malik R, Kalra S, Bhatia S, Al Harrasi A, Singh G, Mohan S, et al. Overview of therapeutic targets in management of dementia. Biomedicine & Pharmacotherapy 2022; 152: 113168.
[2]. Fayosse A, Nguyen DP, Dugravot A, Dumurgier J, Tabak AG, Kivimäki M, et al. Risk prediction models for dementia: role of age and cardiometabolic risk factors. BMC Medicine 2020; 18: 1-10.
[3]. Hildreth KL, Church S. Evaluation and management of the elderly patient presenting with cognitive complaints. The Medical Clinics of North America 2014; 99(2): 311-13.
[4]. Rubattu S, Volpe M. Natriuretic peptides in the cardiovascular system: multifaceted roles in physiology, pathology and therapeutics. International Journal of Molecular Sciences 2019; 20(16): 3991.
[5]. Gallo G, Bianchi F, Cotugno M, Volpe M, Rubattu S. Natriuretic peptides, cognitive impairment and dementia: an intriguing pathogenic link with implications in hypertension. Journal of Clinical Medicine 2020; 9(7): 2265.
[6]. Çavuşoğlu Y, Alper A, Altay H, Çelik A, Demirkan B, Guvenc T, et al. Natriuretic peptides in clinical practice. Anatolian Journal of Cardiology 2019; 21.
[7]. McGurran H, Glenn JM, Madero EN, Bott NT. Prevention and treatment of Alzheimer’s disease: biological mechanisms of exercise. Journal of Alzheimer’s Disease 2019; 69(2): 311-38.
[8]. Malandish A, Ghadamyari N, Karimi A, Naderi M. The role of exercise training on cardiovascular peptides in patients with heart failure: a systematic review and meta-analysis. Current Research in Physiology 2022; 5: 270-86.
[9]. Núñez-Marín G, Iraola D, Lorenzo M, De La Espriella R, Villar S, Santas E, et al. An update on utilising brain natriuretic peptide for risk stratification, monitoring and guiding therapy in heart failure. Expert Review of Molecular Diagnostics 2023; 23(6): 521-33.
[10]. Mirdar S, Arab A, Hedayati M, Hajizade A. The effect of pregnant rat swimming on hypoxia-inducible factor-1α levels of neonatal lung. Tehran University Medical Journal 2012; 69(12): 754.
[11]. Talaei Zavareh SA, Davari S, Gholami M, Salami M. Change in visual experience impairs rat’s spatial learning in morris water maze. Journal of Isfahan Medical School 2010; 28(111): 551-62.
[12]. Najizadeh MH, Mokhber Dezfouli MR, Nikhbakht Borujeni G, Vajhi A, Jabbari Fakhr M, Mehrara M, et al. Evaluation of plasma concentration of atrial natriuretic peptide (ANP) in horses with pulmonic valve regurgitation. Veterinary Clinical Pathology 2020; 14(55): 251-61.
[13]. Ostovaneh MR, Moazzami K, Yoneyama KA, Venkatesh B, Heckbert SR, Wu CO, et al. Change in NT-proBNP (N-terminal pro-B-type natriuretic peptide) level and risk of dementia in multi-ethnic study of atherosclerosis (MESA). Hypertension 2020; 75(2): 316-23.
[14]. Di Daniele N, Celotto R, Alunni Fegatelli D, Gabriele M, Rovella V, Scuteri A. Common carotid artery calcification impacts on cognitive function in older patients. High Blood Pressure & Cardiovascular Prevention 2019; 26: 127-34.
[15]. Rizzoni D, Rizzoni M, Nardin M, Chiarini G, Agabiti-Rosei C, Aggiusti C, et al. Vascular aging and disease of the small vessels. High Blood Pressure & Cardiovascular Prevention 2019; 26: 183-89.
[16]. Hilal S, Chai YL, Van Veluw S, Shaik MA, Ikram MK, Venketasubramanian N et al. Association between subclinical cardiac biomarkers and clinically manifest cardiac diseases with cortical cerebral microinfarcts. JAMA Neurology 2017; 74(4): 403-10.
[17]. Kondziella D, Göthlin M, Fu M, Zetterberg H, Wallin A. B-type natriuretic peptide plasma levels are elevated in subcortical vascular dementia. Neuroreport 2009; 20(9): 825-27.
[18]. Hu WT. Erratum: Plasma multianalyte profiling in mild cognitive impairment and Alzheimer disease. Neurology 2012; 79(18): 1935.
[19]. Tynkkynen J, Hernesniemi JA, Laatikainen T, Havulinna AS, Salo P, Blankenberg S, et al. High-sensitivity cardiac troponin I and NT-proBNP as predictors of incident dementia and Alzheimer’s disease: the FINRISK Study. Journal of Neurology 2017; 264: 503-11.
[20]. Ferguson IT, Elbejjani M, Sabayan B, Jacobs Jr DR, Meirelles O, Sanchez OA, et al. N-terminal pro-brain natriuretic peptide and associations with brain magnetic resonance imaging (MRI) features in middle age: The CARDIA brain MRI study. Frontiers in Neurology 2018; 9: 307.
[21]. Sabayan B, van Buchem MA, Sigurdsson S, Zhang Q, Harris TB, Gudnason V, et al. Cardiac hemodynamics are linked with structural and functional features of brain aging: the age, gene/ environment susceptibility (AGES)‐ Reykjavik Study. Journal of the American Heart Association 2015; 4(1): 1294.
[22]. Mirza SS, de Bruijn RF, Koudstaal PJ, van den Meiracker AH, Franco OH, Hofman A, et al. The N-terminal pro B-type natriuretic peptide, and risk of dementia and cognitive decline: a 10-year follow-up study in the general population. Journal of Neurology, Neurosurgery & Psychiatry 2016; 87(4): 356-62.
[23]. Ohba H, Takada H, Musha H, Nagashima J, Mori N, Awaya T, et al. Effects of prolonged strenuous exercise on plasma levels of atrial natriuretic peptide and brain natriuretic peptide in healthy men. American Heart Journal 2001; 141(5): 751-58.
[24]. Aengevaeren VL, Hopman MT, Thijssen DH, van Kimmenade RR, de Boer MJ, Eijsvogels TM. Endurance exercise-induced changes in BNP concentrations in cardiovascular patients versus healthy controls. International Journal of Cardiology 2017; 227: 430-35.
Type of Study: Research |
Subject:
Genetics/ Biotechnology
Received: 2024/01/28 | Accepted: 2024/05/13 | Published: 2024/10/1
Received: 2024/01/28 | Accepted: 2024/05/13 | Published: 2024/10/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






