Major depressive disorder (MDD) is the most common psychiatric illness worldwide [1]. Dysfunctional glutamatergic neurotransmission may underlie the pathophysiology of MDD [2]. The effects of Ketamine in MDD patients might be mediated through glutamatergic neurotransmission [1, 2]. The glutamatergic signaling pathway plays a key role in anti-depressant response to ketamine in patients with MDD [2]. A single intravenous infusion of the glutamatergic modulator ketamine elicits a fast-acting, robust, and relatively sustained anti-depressant [3]. The N-methyl-d-aspartate receptor antagonist, ketamine’s anti-depressant effects are mediated by a glutamate surge that leads to a cascade of events resulting in synaptogenesis and reversal of the adverse effects of chronic stress and depression [4]. Subjects with MDD are associated with a rise in the plasma of glutamate and low levels of glycine. There is a significant difference in the concentration of glutamate in the control group and the depressed subjects [5]. Serum glutamate levels are significantly higher in patients with depression than in healthy subjects [6]. Glutamine synthetase (GS) plays an important role in the turnover of glutamine and glutamate; a study of a depressed animal model has shown that GS activity has decreased. GS is an enzyme that plays an essential role in the metabolism of nitrogen by catalyzing the condensation of glutamate and ammonia to form glutamine. By inhibiting GS with sulfoximine, methionine reduces glutamate and glutamine levels but increases depression [7]. Glutamate dehydrogenase (GDH) is an enzyme observed in eukaryotic mitochondria.
Ketamine increases the level of factors involved in the mTOR signaling pathway and acts as a key regulator of the protein involved in glutamate metabolism, such as glutamine synthetase enzyme [8]. Ketamine normalizes the interaction between the default mode network and salience networks in subjects with MDD [9]. Marginally, more than 50% of patients diagnosed with MDD were treated using available anti-depressants and have shown complete remission [10]. Ketamine’s rapid-acting anti-depressant properties are related to its acute effects on prefrontal connectivity [11]. GS is involved in glutamate-related disturbances in major depression [12]. MDD patients were characterized by lower levels of L-asparagine and L-glutamine[13]. Asparagine synthetase (ASNS) catalyzes the synthesis of asparagine from aspartic acid [14].
Materials and Methods
Participants
The present study is descriptive-analytical and cross-sectional. Between March 2017 and November 2018, patients from Shaheed Yahyanezhad Hospital, affiliated with Babol University of Medical Sciences, were invited to take part in the current study. After participants had signed the informed consent, serum specimen samples of men (n=10) and women(n=19) enrolled in experience depression testing were obtained. The age group was considered as 42.27 ± 21.66 years old, and the weight group was 76.17 ±20.6 kg. Each participant in the study scored with the DSM-5 and Beck Scale at baseline and two months after the start of the trial. Twenty-nine un-medicated participants with MDD were selected to use a single intravenous infusion of ketamine hydrochloride (0.75 mg/kg over 20 minutes).
Inclusion criteria
Patients with MDD were considered to have major depression after clinical examination by a specialist physician. Based on DSM-5, there was no history of nicotine, alcohol, or substance use ever. Patients with MDD were treated with ketamine for at least two months.
Exclusion Criteria
Patients with other types of depression such as hyperactivity disorder, thyroid disorders such as hypothyroidism, fibroma, chronic fatigue, and bacterial and infectious diseases were excluded.
Experiments
Blood samples were taken at 3-5 ml before ketamine administration, and then 0.75 mg/kg of ketamine hydrochloride was administrated in these patients for 20 minutes by diffusion method. After two months, depression was tested again from the patients and samples were collected again from them. We gathered information on the participants’ age, weight, body mass index, and clinical characteristics. Serum levels of ASNS, GS and GDH
were measured using the enzyme-linked immunosorbent assay method according to the kit instructions. Kits are provided by Bioassay Technology Laboratory, China (Kit Code, E7394 HU, E4596HU and E1376HU, respectively).
Statistical analysis
The results were analyzed using SPSS version 24 software. The mean serum levels of biochemical variables in two groups were compared with variance analysis. Tukey’s two-to-two comparisons were performed; The Krimograv-Smirnov test was used to check the normal distribution of the variables studied. A one-way ANOVA test was used to compare the variables between the three groups. In addition, by correlation analysis and Pearson correlation coefficient, the association between biochemical parameters in three groups was separately evaluated. In all tests, the P value was less than 0.05.
Results
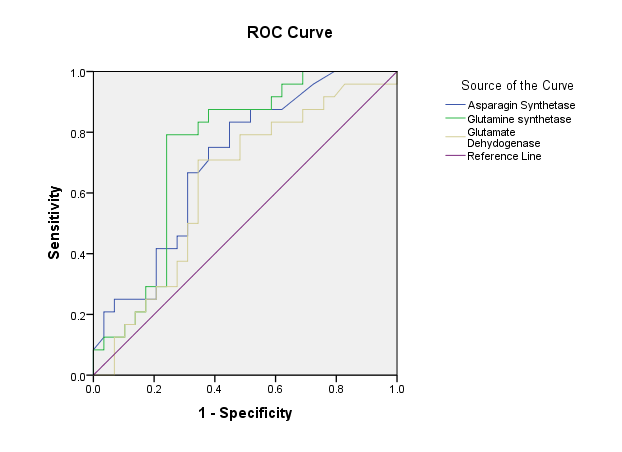
The major finding of our results revealed that after the administration of ketamine, the level of ASNS and GDH in patients with MDD reduced compared to pre-treatment status, and the level of GS in patients with MDD increased compared to pre-treatment status. The average level of ASNS, GS and GDH in the two groups before treatment and after with ketamine were shown in Table 1. In Table 2, the correlation between variables is shown using the Pearson statistical test. The results of Pearson correlation showed that there is a positive and significant correlation between the level of ASNS enzyme (R = 0.3, p = 0.03), GS (R = 0.3, p = 0.01) and glutamate dehydrogenase (R = 0.3, p = 0.03). The receiver operating characteristic (ROC) test was performed to evaluate the sensitivity and specificity of the measured factors in both the control and patient groups (Table 3). The correlation between ASNS and GDH is shown in Figure 1. The ROC curve for the sensitivity and specificity of ASNS, GS and GDH is shown in Figure 2. The results of the ROC test between the group of patients with MDD and the control group showed that ASNS had a sensitivity and specificity of 79% and 55%, respectively. It is also possible to differentiate between the patient group and the control group, with the area under the curve level of about 0.7, the cut-off point for which it is intended to be 3.2. A higher value indicates a healthy group, and a lower value than the group of patients with MDD.
The results of the Roc test showed that GS, with a sensitivity of 83% and a specificity of 64%, can distinguish between the healthy group and those with major depression. The area under the curve for the GS is 0.7, and the cut-off point is about 107.8, meaning higher values indicate that the healthy group and the smaller values represent the patient group.
The results of the Roc test showed that GDH with a sensitivity of 62% and a specificity of 75% can distinguish between the healthy group and those with major depression. The area under the curve for the GS is 0.6, and the cut-off point is about 175, meaning higher values indicate that the healthy group and the smaller values represent the patient group.
Our results showed that after two months of Ketamin administration, patients had good improvement, and their DSM-5 and Beck tests were normal. Additionally, we did not observe any side effects of the administration of ketamine.
Table 1. Comparison of serum levels of average, Asparagine synthetase, Glutamine synthetase, Glutamate dehydrogenase, 2 groups before treatment and after treatment
| Variables / Mean ±SE |
Before treatment |
After treatment |
p-value |
| Asparagine synthetase |
10.07±2.70 |
8.39 ±1.70 |
0.041 |
| Glutamine synthetase |
181.72±39.69 |
200.69±43.21 |
0.045 |
| Glutamate dehydrogenase |
10.44±3.33 |
8.9±2.02 |
0.65 |
Table 2. Pearson correlation to verify the existence of the relationship between the variables
| Variables |
Asparagine synthetase |
Glutamine synthetase |
Glutamate dehydrogenase |
|
R P |
R P |
R P |
| Asparagine synthetase |
- |
0.3 0.01 |
0.03 0.3 |
| Glutamine synthetase |
0.01 0.3 |
- |
0.5 0.05 |
| Glutamate dehydrogenase |
0.03 0.3 |
0.5 0.05 |
- |
Table 3. ROC test for sensitivity and specificity of variables in patient and healthy control groups
| Variables |
The area under the ROC curve |
Sensitivity |
Specificity |
Cut off |
p-value |
| Asparagine synthetase |
0.7 |
79% |
55% |
3.2 |
0.01 |
| Glutamine synthetase |
0.7 |
83% |
64% |
107.8 |
0.02 |
| Glutamate dehydrogenase |
0.6 |
62% |
75% |
175 |
0.1 |
Fig. 1. Correlation between asparagine synthetase and glutamate dehydrogenase
Fig. 2. ROC curve for sensitivity and specificity of asparagine synthetase, glutamine synthetase and glutamate dehydrogenase
Discussion
The results of our study for the first time showed that after the administration of ketamine, the level of AS and GDH in patients with MDD reduced compared to pre-treatment status. These results show that ketamine may act as a regulator of these enzymes, which are involved in glutamate, glutamine, α-ketoglutarate, asparagine and aspartate metabolism. GDH is an enzyme that converts glutamate to α-ketoglutarate, an enzyme that generates asparagine from aspartate. Reducing the level of AS and GDH causes changes in the metabolism of asparagine and glutamate in the nervous system. Following the decreased level of these enzymes, the asparagine and α-Ketoglutarate levels decrease. Asparagine is required for the function of the brain. α-Ketoglutarate plays a role in the detoxification of ammonia in the brain. However, after administration of ketamine, the level of GS in patients with MDD increased compared to pre-treatment status. This finding is comparable with the results of other investigators [5, 6]. Our results fit into the broader context of the existing literature on MDD and Ketamine so that changes in plasma concentrations of excitatory amino acids and serum glutamate are coordinated in depressed patients. GS is an enzyme that plays an essential role in producing glutamine. Besides, GS in the brain participates in the regulation of glutamate and termination of neurotransmitter signals. Glutamine plays a role as a precursor to the neurotransmitter glutamate. These results provide the first indication that ketamine’s acting may occur at the metabolism level in MDD patients. The overall result of this study shows that the amount of these enzymes can be used to change the amount of asparagine, glutamine and glutamate in the nervous system. Reducing the amount of As and GDH increases GS after the administration of ketamine, decreasing asparagine and increasing glutamine levels.
According to the results, the amount of AS and GDH decreased after ketamine administration, but GS content increased. GDH, As and GS levels can be used to diagnose patients with MDD. However, in view of the above mentioned with regard to the mechanism of action of the enzymes, there are many complications that must be solved, for example, the relationship between changes of these enzymes with the severity of this disease or clinical manifestation of MDD. Therefore, to explore the role of these enzymes, further studies are necessary. In our laboratory, new procedures will be conducted for exploration in a further study of the underlying mechanism. Our study has some limitations. First, a major limitation of the study is that the level of other enzymes involved in MDD was not measured; this fact does not allow us to derive conclusions about other enzyme changes in relation to MDD. The second limitation of our study was that the sample size was low.
Conclusion
Collectively, the current results highlight the promising role of GDH, ASNS and GS as enzymes due to their role in MDD. Ketamine may normalize the interaction between these enzymes and neurotransmitter levels in subjects with MDD. Plus, our results demonstrated that GDH, AS and GS levels can be measured to evaluate the ketamine effect in MDD patients. We suggest that ketamine’s rapid-acting anti-depressant properties might be related to its effects on GS, GDH and ASNS enzymes in MDD patients. However, further basic and clinical experimental investigations are needed to confirm its role and benefits. Consequently, the approach used in this study would be very important for understanding the role of these enzymes in MDD status and making another slice of the puzzle into a picture of this disease.
Ethical Considerations
All individuals voluntarily participated, and researchers noted that individual data remain confidential. Patients were informed of the implementation process, written consent was obtained, and ethical considerations were observed. All participants’ information is a secret plan and is not at the discretion of any real and legal person. This study was approved by the human subjects ethics board of Babol University of Medical Sciences and was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013. This study was reviewed and approved by the Ethics Committee of the Babol University of Medical Sciences (MU.Babol.HRI.REC1395.77).
Funding
This study was also supported by the MSc Thesis Project (No 3421, 950259) of Medical Sciences.
Conflict of Interest
The authors settled that there are no conflicts of interest concerning the publication of this article. Research funding played no role in the study design, in the collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the report for publication.
Acknowledgments
The authors would like to express their appreciation to all contributors who have achieved this study.
Authors’ Contributions
DQ designed the experiments. ET performed the experiments. KH-T analyzed the data. FKH contributed to the manuscript’s writing.
References
[1]. Ho MF, Correia C, Ingle JN, Kaddurah-Daouk R, Wang L, Kaufmann SH, et al. Ketamine and ketamine metabolites as novel estrogen receptor ligands: Induction of cytochrome P450 and AMPA glutamate receptor gene expression. Biochem Pharmacol. 2018: 152: 279-92.
[2]. Gilbert JR, Yarrington JS, Wills KE, Nugent AC, Zarate CA. Glutamatergic signaling drives ketamine-mediated response in depression: Evidence from dynamic causal modeling. Int J Neuropsychopharmacol. 2018: 21(8): 740-47.
[3]. Kadriu B, Musazzi L, Henter ID, Graves M, Popoli M, Zarate CA. Glutamatergic neurotransmission: Pathway to developing novel rapid-acting anti-depressant treatments. Int J Neuropsychopharmacol. 2019: 22(2): 119-35.
[ 4]. Abdallah CG, Adams TG, Kelmendi B, Esterlis I, Sanacora G, Krystal JH. Ketamine’s mechanism of action: A path to rapid-acting anti-depressants. Depress Anxiety. 2016; 33(8): 689-97.
[5]. Altamura C, Maes M, Dai J, Meltzer HY. Plasma concentrations of excitatory amino acids, serine, glycine, taurine and histidine in major depression. European Neuropsychopharmacology 1995; 5(1): 71-5.
[6]. Kim J, Schmid-Burgk W, Claus D, Kornhuber H. Increased serum glutamate in depressed patients. ArchivfürPsychiatrie und Nervenkrankheiten. 1982; 232(4): 299-304.
[7]. Son H, Baek JH, Go BS, Jung D, Sontakke SB, Chung HJ, et al. Glutamine has antidepressive effects through increments of glutamate and glutamine levels and glutamatergic activity in the medial prefrontal cortex. Neuropharmacology 2018; 143: 143-52.
[8]. Murrough JW. Ketamine as a novel anti-depressant: from synapse to behavior. Clinical Pharmacology & Therapeutics 2012; 91(2): 303-309.
[9].Evans JW, Szczepanik J, Brutsché N, Park LT, Nugent AC, Zarate CA. Default mode connectivity in major depressive disorder measured up to 10 days after ketamine administration. Biol Psychiatry 2018 15; 84(8): 582-90.
[10]. Filip M, Jasionowska J, Gałecki P. The importance of the ketamine in depressive disorders. Pol MerkurLekarski. 2018; 45(267): 134-36.
[11]. Abdallah CG, Dutta A, Averill CL, McKie S, Akiki TJ, Averill LA, et al. Ketamine, but Not the NMDAR Antagonist Lanicemine, Increases Prefrontal Global Connectivity in Depressed Patients. Chronic Stress (Thousand Oaks). 2018. Epub 2018: 21.
[12]. Bernstein HG, Meyer-Lotz G, Dobrowolny H, Bannier J, Steiner J, Walter M, et al. Reduced density of glutamine synthetase immunoreactive astrocytes in different cortical areas in major depression but not in bipolar I disorder Front Cell Neurosci. 2015; 10: 9: 273.
[13]. Juncai Pu, Yiyun Liu, Hanping Zhang, Lu Tian, Siwen Gui, Yue Yu, et al. An integrated meta-analysis of peripheral blood metabolites and biological functions in major depressive disorder. Mol Psychiatry. 2021; 26(8): 4265-276.
[14]. Staklinski SJ, Chang MC, Yu F, Collins Ruff K, Franz DN, Qian Z, et al. Cellular and molecular characterization of two novel asparagine synthetase gene mutations linked to asparagine synthetase deficiency. J Biol Chem. 2022; 298(9): 102385.