Wed, Feb 4, 2026
[Archive]
Volume 11, Issue 3 (August 2024)
IJML 2024, 11(3): 244-250 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Charostad J, Abdollahi M, Akhavan Tafti F, Taghvai N, Javad Zadeh Shahshahani H, Astani A. Prevalence of Torque Teno Virus in Thalassemia Patients in Yazd City. IJML 2024; 11 (3) :244-250
URL: http://ijml.ssu.ac.ir/article-1-535-en.html
URL: http://ijml.ssu.ac.ir/article-1-535-en.html
Javad Charostad 
 , Mahdieh Abdollahi
, Mahdieh Abdollahi 
 , Fatemeh Akhavan Tafti
, Fatemeh Akhavan Tafti 
 , Naghi Taghvai
, Naghi Taghvai 
 , Hayedeh Javad Zadeh Shahshahani
, Hayedeh Javad Zadeh Shahshahani 
 , Akram Astani *
, Akram Astani * 


 , Mahdieh Abdollahi
, Mahdieh Abdollahi 
 , Fatemeh Akhavan Tafti
, Fatemeh Akhavan Tafti 
 , Naghi Taghvai
, Naghi Taghvai 
 , Hayedeh Javad Zadeh Shahshahani
, Hayedeh Javad Zadeh Shahshahani 
 , Akram Astani *
, Akram Astani * 

Department of Microbiology, Faculty of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran & Hematology and Oncology Research Center, Non-communicabe Diseases Research Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
Full-Text [PDF 235 kb]
(152 Downloads)
| Abstract (HTML) (380 Views)
References
Full-Text: (158 Views)
Introduction
The Torque Teno virus (TTV) was discovered by Nishizawa et al. (1997) in Japan among individuals suffering from non-A-E transfusion-induced hepatitis. Due to the extensive occurrence of TTV in blood donors, patients with thalassemia commonly contract different genotypes of this virus via therapeutic blood transfusions [1]. The TTV has a genome composed of single-stranded circular DNA. It is crucial to note that this virus belongs to the Anelloviridae family rather than the previously speculated Circoviridae family [2]. In terms of its composition, this virus possesses roughly 3900 nucleotides and a diameter ranging from 30 to 50 nm [3].
Although saliva and oral faecal droplets can transmit this virus, blood transfusion is the main method of transmission. The presence of the virus in the bloodstream has been reported in a range of 1-10 blood donors in America, 2% in England, 12-14% in Japan, 14% in Korea, 13% in Germany, 62% in Brazil, 11% in Spain, and 10% in Colombia. These statistics highlight the global prevalence of the virus [4]. The close relationship between the transmission of this virus through blood and blood products has made its role in blood transfusion-related diseases to be investigated [1].
The TTV has been linked to a variety of medical conditions, such as liver diseases, kidney transplantation, and allograft rejection. Scientific investigation has demonstrated that the TTV viral load holds significant significance in understanding TTV infection, particularly in the context of liver and kidney transplantation. In recipients of liver transplants, the TTV viral load demonstrates a positive correlation with the intensity of immunosuppression. It is connected to acute rejection and infection during the initial year after transplantation [5, 6].
Similarly, in kidney transplant recipients, TTV load kinetics predict allograft rejection, and there is an association between TTV and subclinical graft rejection [7]. Although TTV infection did not change the aspartate transaminase (AST) and alanine transaminase (ALT) enzyme levels in biliary tract patients, liver involvement may still exist in these patients. In summary, the investigation emphasizes the possible hazard of TTV contamination in individuals with thalassemia, specifically those receiving repeated blood transfusion therapy and haemodialysis [8].
Further research is needed to understand the clinical implications of TTV infection in thalassemia patients and to develop strategies for prevention and treatment.
Materials and Methods
Study population and patient information
In this cross-sectional study, the number of 99 thalassemia patients who visited the special disease center in Yazd from the beginning of August 2015 to the end of September 2015 was included based on the inclusion and exclusion criteria. The inclusion criteria were: 1) patients residing in Yazd city and 2) patients who provided written informed consent to participate in the study. Demographic information of all patients, including age, gender, duration of blood transfusion, and history of hepatitis B and C infections, was obtained from all patients through a questionnaire.
Collecting and preparing samples
Sampling was performed using standard blood transfusion methods and in special gel tubes (Austria-Greiner bio-one-Gel clot activator vacuumed tube). After transportation to the laboratory, the serum was separated from the blood samples using a centrifuge with a speed of 4000 g, and the separated serum was kept in a -70-degree freezer until the tests were performed.
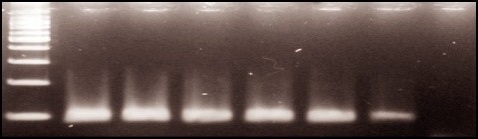
Genome extraction and DNA identification of TTV virus. DNA was extracted using a DNA extraction kit (Roche, Germany) according to the kit protocol, which was stored at -20 °C until the experiment. All samples underwent β-globin amplification (110 bp) using PCO3/PC04 primers (Table 1), to assess DNA quality, and those yielding positive results were subjected to subsequent analysis (Fig. 1).
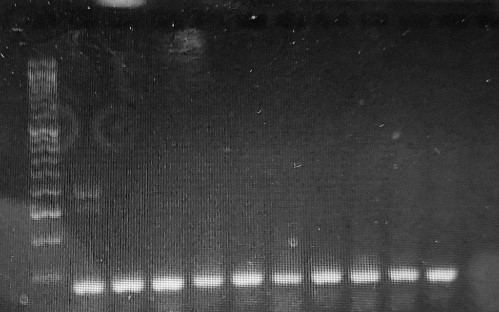
To identify the TTV genome in thalassemia patients, the Nested polymerase chain reaction (PCR) technique was employed to enhance the detection of low viral loads. In the first round, primers NG054 (sense, positions 3–22) and NG147 (antisense, positions 211–233) were used, followed by NG133 (sense, positions 91–115) and NG132 (antisense, positions 204–222) in the second round, based on previous studies [9] (Table 1). These nested primers amplify conserved region at TTV 5′UTR. Both positive and negative control samples were included in each PCR run. All PCR products were then run on 1.5% agarose gel at 90 V and 45 minutes. When a band of 60 bp was present, the TTV gene was considered present (Fig. 2).
Statistical analysis
Frequency and percentage were used to categorize qualitative variables. The Chi-Square Pearson test was used to examine the relationship between different parameters. SPSS software version 22 was used for data analysis. P-value < 0.05 was considered as statistically significant.
Results
Clinical parameters
Of the 99 patients participating in the study, 52 were men and 47 were women. The patients' age range was from 10 to 50 years, with the average age being 50 years, and most were less than 30 years old. The duration of the injection was divided into 5-year intervals, and most people were over 15 years old. The history of hepatitis B (HBV) and C (HCV) infections is briefly shown in Table 2.
Although saliva and oral faecal droplets can transmit this virus, blood transfusion is the main method of transmission. The presence of the virus in the bloodstream has been reported in a range of 1-10 blood donors in America, 2% in England, 12-14% in Japan, 14% in Korea, 13% in Germany, 62% in Brazil, 11% in Spain, and 10% in Colombia. These statistics highlight the global prevalence of the virus [4]. The close relationship between the transmission of this virus through blood and blood products has made its role in blood transfusion-related diseases to be investigated [1].
The TTV has been linked to a variety of medical conditions, such as liver diseases, kidney transplantation, and allograft rejection. Scientific investigation has demonstrated that the TTV viral load holds significant significance in understanding TTV infection, particularly in the context of liver and kidney transplantation. In recipients of liver transplants, the TTV viral load demonstrates a positive correlation with the intensity of immunosuppression. It is connected to acute rejection and infection during the initial year after transplantation [5, 6].
Similarly, in kidney transplant recipients, TTV load kinetics predict allograft rejection, and there is an association between TTV and subclinical graft rejection [7]. Although TTV infection did not change the aspartate transaminase (AST) and alanine transaminase (ALT) enzyme levels in biliary tract patients, liver involvement may still exist in these patients. In summary, the investigation emphasizes the possible hazard of TTV contamination in individuals with thalassemia, specifically those receiving repeated blood transfusion therapy and haemodialysis [8].
Further research is needed to understand the clinical implications of TTV infection in thalassemia patients and to develop strategies for prevention and treatment.
Materials and Methods
Study population and patient information
In this cross-sectional study, the number of 99 thalassemia patients who visited the special disease center in Yazd from the beginning of August 2015 to the end of September 2015 was included based on the inclusion and exclusion criteria. The inclusion criteria were: 1) patients residing in Yazd city and 2) patients who provided written informed consent to participate in the study. Demographic information of all patients, including age, gender, duration of blood transfusion, and history of hepatitis B and C infections, was obtained from all patients through a questionnaire.
Collecting and preparing samples
Sampling was performed using standard blood transfusion methods and in special gel tubes (Austria-Greiner bio-one-Gel clot activator vacuumed tube). After transportation to the laboratory, the serum was separated from the blood samples using a centrifuge with a speed of 4000 g, and the separated serum was kept in a -70-degree freezer until the tests were performed.
Genome extraction and DNA identification of TTV virus. DNA was extracted using a DNA extraction kit (Roche, Germany) according to the kit protocol, which was stored at -20 °C until the experiment. All samples underwent β-globin amplification (110 bp) using PCO3/PC04 primers (Table 1), to assess DNA quality, and those yielding positive results were subjected to subsequent analysis (Fig. 1).
To identify the TTV genome in thalassemia patients, the Nested polymerase chain reaction (PCR) technique was employed to enhance the detection of low viral loads. In the first round, primers NG054 (sense, positions 3–22) and NG147 (antisense, positions 211–233) were used, followed by NG133 (sense, positions 91–115) and NG132 (antisense, positions 204–222) in the second round, based on previous studies [9] (Table 1). These nested primers amplify conserved region at TTV 5′UTR. Both positive and negative control samples were included in each PCR run. All PCR products were then run on 1.5% agarose gel at 90 V and 45 minutes. When a band of 60 bp was present, the TTV gene was considered present (Fig. 2).
Statistical analysis
Frequency and percentage were used to categorize qualitative variables. The Chi-Square Pearson test was used to examine the relationship between different parameters. SPSS software version 22 was used for data analysis. P-value < 0.05 was considered as statistically significant.
Results
Clinical parameters
Of the 99 patients participating in the study, 52 were men and 47 were women. The patients' age range was from 10 to 50 years, with the average age being 50 years, and most were less than 30 years old. The duration of the injection was divided into 5-year intervals, and most people were over 15 years old. The history of hepatitis B (HBV) and C (HCV) infections is briefly shown in Table 2.
Table 1. Sequence primers used in Nested PCR
| Primer name | Primer Sequences (5’-3’) | Primer size (bp) | |
| β-globin | F: PCO3 | ACACAACTGTGTTCACTAGC | 110 |
| R: PC04 | CAACTTCATCCACGTTCACC | ||
| The first round of PCR | F: NG054 | 5′ TTTGCTACGTCACTAACCAC 3’ | 151 |
| R: NG147 | 5′GCGAGTCCCCGAGCCCGAATTGCC 3′ | ||
| The second round of PCR | F: NG133 | 5′GTAAGTGCACTTCCGAATGGCTGA3′ | 60 |
| R: NG132 | 5′AGCCCGAATTGCCCCTTGAC3′ |
PCR= Polymerase chain reaction
Clinical findings and molecular test results
The data obtained from the molecular test revealed that the serum of 43 people with thalassemia was infected with TTV, among which 22 cases were observed in women and 21 cases in men (p= 0.525). Comparing the relationship between age and the virus showed that most patients were significantly less than 30 years old, so 29 positive samples were observed among these people (p = 0.016).
Discussion
The impact of TTV virus infection on haematological diseases has been extensively studied worldwide. Significant differences have been observed in the prevalence of TTV among people with thalassemia in different communities. Therefore, this study aimed to investigate the frequency of TTV infection in thalassemia patients and its relationship with age, sex, injection duration, and history of hepatitis B and C viral infections in Yazd city.
The data obtained from the molecular test revealed that the serum of 43 people with thalassemia was infected with TTV, among which 22 cases were observed in women and 21 cases in men (p= 0.525). Comparing the relationship between age and the virus showed that most patients were significantly less than 30 years old, so 29 positive samples were observed among these people (p = 0.016).
Discussion
The impact of TTV virus infection on haematological diseases has been extensively studied worldwide. Significant differences have been observed in the prevalence of TTV among people with thalassemia in different communities. Therefore, this study aimed to investigate the frequency of TTV infection in thalassemia patients and its relationship with age, sex, injection duration, and history of hepatitis B and C viral infections in Yazd city.
Table 2. Clinical parameters of 99 patients according to age, Gender, duration of blood transfusion and history of HCV and HBV
| Parameters | TTV positive group N (%) |
TTV negative group N (%) |
P-value | |
| Age | ≤ 30 | 29 (67.4) | 49 (87.5) | 0.016 |
| > 30 | 14 (32.6) | 7 (12.5) | ||
| Gender | Male | 21 (49) | 31 (55) | 0.520 |
| Female | 22 (51) | 25 (45) | ||
| Duration of blood transfusion | 0-5 years | 4 (9.4) | 8 (14.2) | 0.656 |
| 5-10 years | 5 (11.6) | 10 (17.9) | ||
| 10-15 years | 8 (18.6) | 10 (17.9) | ||
| >15 years | 26 (60.4) | 28 (50) | ||
| History of HCV | 1 (2.3) | 3 (5.3) | 0.448 | |
| History of HBV | 0 | 0 | NA | |
HBV= Hepatitis B virus; HCV= Hepatitis C virus; TTV= Torque Teno virus

Fig. 1. Agarose gel electrophoresis of β- globin. Products: Lane 1-5: Positive results, Lane 6: Negative result

Fig. 2. Torque Teno virus detection by electrophoresis on agarose gel. Products: Lanes 1-10: Positive results, Lane 11: Negative result
The molecular test revealed that the TTV was present in 43% of the serum samples collected from these patients.
In the Bouzari study in Isfahan (2007), TTV-DNA was detected in 17.9% of HBsAg carriers, 14% of anti-HCV-positive patients, 22.2% of non-A–non-E hepatitis patients, 22.8% of intravenous drug users, and 57.1% of fulminant hepatitis patients. TTV-DNA was also found in 20.4% of healthy subjects. There was no statistical difference in the prevalence between the different subgroups [13]. In Masia's study in Italy (2001), TTV DNA was detected with the PCR method in 10 (11.1%) patients with autoimmune hepatitis versus four (4.4%) in the control group, and no significant difference was found between the two groups [14].
In our study, the TTV was detected in 2.3% of HCV-positive patients and in none of HBV patients. In a study conducted in Ahwaz, Zandieh showed that 57.2% of samples obtained from patients and 20% of blood donors (control group) were positive for TTV-DNA detected by PCR. The two groups had a statistically significant difference in TTV prevalence [15]. Studies from Turkey and Italy have reported that TTV frequency among thalassemia patients is 61% and 73%, respectively [16, 17]. Takács's study among Italian multiply transfused patients detected TTV DNA in 47 (69.1%) adult beta-thalassemia major patients. TTV DNA was detected by semi-nested PCR (using N22 primers) in 18.5% of persons and 50.4% of patients with hepatitis of unknown origin in Hungary [18]. In another study in Turkish multitransfused patients with thalassemia, TTV was detected in 63% of the thalassemia patients and 54% of the control patients [19]. Our results are consistent with those of other countries. The frequency of this virus in different regions may be due to geographical differences. Also, The significant variation in viremia rate and TTV frequency in different studies can be attributed to various factors, such as sample size, diagnostic tests used, and selection of different target primers.
Sequenced by next-generation sequencing, the virome of 30 patients with beta-thalassemia major was compared in Brazil. The transfusion profile of beta-thalassemic patients received an average of 34 blood units (the average volume of transfusion events for the group). TTV 11 (1145 reads) was the most representative of the thalassemic group [20]. The frequency of TTV infections in transfusion-dependent beta-thalassemia by real-time SYBR Green PCR (evaluated the expression of MiR-222 and MiR-15a) in Shiraz was 21.1% and infection was approximately less than the reported prevalence in other parts of the world [21].
These results and previous studies suggest that TTV-DNA has been transmitted to recipients through blood and blood products. Blood transfusion is one of the most effective ways to transmit TTV [8, 15]. Because immune responses resulting from the TTV virus have not been detected so far, and no method has been reported to identify viral antigens in plasma, no serological methods can be used to identify the TTV virus. The PCR method is commonly employed in studies to identify this virus in serum samples of thalassemia patients [10].
The extensive genetic diversity of TTV means that choosing the right primer significantly impacts PCR sensitivity. The N22 fragment of ORF1 has been widely used in PCR target studies. Although the designed primer fragments have become more advanced over time, they may still not be able to amplify the TTV isolate, especially when its amount is low in the sample, so the 5′-UTR of the TTV genome for Primer design is much better [11].
The current research confirms that TTV is transmitted through blood and blood products, based on its strong presence in the serum of thalassemia patients. The association between age and viral frequency suggests a potential link to increased infection risk. However, further studies are warranted to clarify this relationship due to limitations such as the relatively small sample size. Although the current study did not find a statistically significant relationship between viral frequency and other parameters, it was observed that individuals in the TTV-positive group generally had a longer history of injections [12].
Conclusion
In the present study, we demonstrated a high frequency of TTV infection among thalassemia patients in Yazd city. This notable prevalence may serve as an indicator of the epidemiological status of the virus in this population. The findings highlight the need for preventive measures and the development of appropriate health and treatment policies to ensure the provision of safe blood for patients in need.
Ethical Considerations
This study was approved by the Ethics Committee of Shahid Sadoughi University of Medical Sciences and Health Services, Yazd, Iran under the Ethic code (IR.SSU.MEDICINE.REC.1396.1).
Funding
This study was supported financially by Shahid Sadoughi University of medical science
Conflict of Interest
The authors declared no conflict of interest.
Acknowledgment
We would like to thank Dr Mohsen Nakhaei for
Cooperation in analyzing data.
Authors' Contributions
A. A, J. Ch: Study concept and design; J. Ch, M. A: Performing experiments; F. AT: Sample collection, N. T: Analysis; J. Ch, A. A: Interpretation of data; H. Sh: Drafting of the manuscript.
In the Bouzari study in Isfahan (2007), TTV-DNA was detected in 17.9% of HBsAg carriers, 14% of anti-HCV-positive patients, 22.2% of non-A–non-E hepatitis patients, 22.8% of intravenous drug users, and 57.1% of fulminant hepatitis patients. TTV-DNA was also found in 20.4% of healthy subjects. There was no statistical difference in the prevalence between the different subgroups [13]. In Masia's study in Italy (2001), TTV DNA was detected with the PCR method in 10 (11.1%) patients with autoimmune hepatitis versus four (4.4%) in the control group, and no significant difference was found between the two groups [14].
In our study, the TTV was detected in 2.3% of HCV-positive patients and in none of HBV patients. In a study conducted in Ahwaz, Zandieh showed that 57.2% of samples obtained from patients and 20% of blood donors (control group) were positive for TTV-DNA detected by PCR. The two groups had a statistically significant difference in TTV prevalence [15]. Studies from Turkey and Italy have reported that TTV frequency among thalassemia patients is 61% and 73%, respectively [16, 17]. Takács's study among Italian multiply transfused patients detected TTV DNA in 47 (69.1%) adult beta-thalassemia major patients. TTV DNA was detected by semi-nested PCR (using N22 primers) in 18.5% of persons and 50.4% of patients with hepatitis of unknown origin in Hungary [18]. In another study in Turkish multitransfused patients with thalassemia, TTV was detected in 63% of the thalassemia patients and 54% of the control patients [19]. Our results are consistent with those of other countries. The frequency of this virus in different regions may be due to geographical differences. Also, The significant variation in viremia rate and TTV frequency in different studies can be attributed to various factors, such as sample size, diagnostic tests used, and selection of different target primers.
Sequenced by next-generation sequencing, the virome of 30 patients with beta-thalassemia major was compared in Brazil. The transfusion profile of beta-thalassemic patients received an average of 34 blood units (the average volume of transfusion events for the group). TTV 11 (1145 reads) was the most representative of the thalassemic group [20]. The frequency of TTV infections in transfusion-dependent beta-thalassemia by real-time SYBR Green PCR (evaluated the expression of MiR-222 and MiR-15a) in Shiraz was 21.1% and infection was approximately less than the reported prevalence in other parts of the world [21].
These results and previous studies suggest that TTV-DNA has been transmitted to recipients through blood and blood products. Blood transfusion is one of the most effective ways to transmit TTV [8, 15]. Because immune responses resulting from the TTV virus have not been detected so far, and no method has been reported to identify viral antigens in plasma, no serological methods can be used to identify the TTV virus. The PCR method is commonly employed in studies to identify this virus in serum samples of thalassemia patients [10].
The extensive genetic diversity of TTV means that choosing the right primer significantly impacts PCR sensitivity. The N22 fragment of ORF1 has been widely used in PCR target studies. Although the designed primer fragments have become more advanced over time, they may still not be able to amplify the TTV isolate, especially when its amount is low in the sample, so the 5′-UTR of the TTV genome for Primer design is much better [11].
The current research confirms that TTV is transmitted through blood and blood products, based on its strong presence in the serum of thalassemia patients. The association between age and viral frequency suggests a potential link to increased infection risk. However, further studies are warranted to clarify this relationship due to limitations such as the relatively small sample size. Although the current study did not find a statistically significant relationship between viral frequency and other parameters, it was observed that individuals in the TTV-positive group generally had a longer history of injections [12].
Conclusion
In the present study, we demonstrated a high frequency of TTV infection among thalassemia patients in Yazd city. This notable prevalence may serve as an indicator of the epidemiological status of the virus in this population. The findings highlight the need for preventive measures and the development of appropriate health and treatment policies to ensure the provision of safe blood for patients in need.
Ethical Considerations
This study was approved by the Ethics Committee of Shahid Sadoughi University of Medical Sciences and Health Services, Yazd, Iran under the Ethic code (IR.SSU.MEDICINE.REC.1396.1).
Funding
This study was supported financially by Shahid Sadoughi University of medical science
Conflict of Interest
The authors declared no conflict of interest.
Acknowledgment
We would like to thank Dr Mohsen Nakhaei for
Cooperation in analyzing data.
Authors' Contributions
A. A, J. Ch: Study concept and design; J. Ch, M. A: Performing experiments; F. AT: Sample collection, N. T: Analysis; J. Ch, A. A: Interpretation of data; H. Sh: Drafting of the manuscript.
References
- Hu YW, Al‐Moslih MI, Al Ali MT, Uzicanin S, Perkins H, Yi QL, et al. Clinical outcome of frequent exposure to Torque Teno virus (TTV) through blood transfusion in thalassemia patients with or without hepatitis C virus (HCV) infection. Journal of Medical Virology 2008; 80(2): 365-71.
- Hino SJRimv. TTV, a new human virus with single stranded circular DNA genome. Reviews in Medical Virology 2002; 12(3): 151-58.
- Mushahwar I, Erker J, Dille B, Desai S, editors. Recently discovered blood-borne viruses. Forum (Genoa, Italy) 2001; 11(2): 98-122.
- Rezahosseini O, Drabe CH, Sørensen SS, Rasmussen A, Perch M, Ostrowski SR, et al. Torque-Teno virus viral load as a potential endogenous marker of immune function in solid organ transplantation. Transplantation Reviews 2019; 33(3): 137-44.
- Engel B, Görzer I, Campos-Murguia A, Hartleben B, Puchhammer-Stöckl E, Jaeckel E, et al. Association of torque teno virus viremia with liver fibrosis in the first year after liver transplantation. Frontiers in Immunology 2023; 14: 1215868.
- Mrzljak A, Vilibic-Cavlek TJ. Torque teno virus in liver diseases and after liver transplantation. World Journal of Transplantation 2020; 10(11): 291.
- van Rijn AL, Wunderink HF, Sidorov IA, de Brouwer CS, Kroes AC, Putter H, et al. Torque teno virus loads after kidney transplantation predict allograft rejection but not viral infection. Journal of Clinical Virology 2021; 140: 104871.
- Jalali H, Mahdavi MR, Zaeromali N. Torque Teno Virus (TTV) among β-thalassemia and haemodialysis patients in Mazandaran province (north of Iran). International Journal of Molecular and Cellular Medicine 2017; 6(1): 56-60.
- Ergünay K, Ustaçelebi S, Bayraktar Y, Günalp A. [Detection of TT virus DNA by nested-PCR method in non A-E hepatitis cases]. Mikrobiyoloji Bulteni 2005; 39(1): 53-62.
- Gore EJ, Gard L, Niesters HG, Van Leer Buter CC. Understanding torquetenovirus (TTV) as an immune marker. Frontiers in Medicine 2023; 10: 1168400.
- Kato T, Mizokami M, Mukaide M, Orito E, Ohno T, Nakano T, et al. Development of a TT virus DNA quantification system using real-time detection PCR. Journal of Clinical Microbiology 2000; 38(1): 94-8.
- Irshad M, Joshi Y, Sharma Y, Dhar I. Transfusion transmitted virus: A review on its molecular characteristics and role in medicine. World Journal of Gastroenterology 2006; 12(32): 5122.
- Bouzari M, Shaykh Baygloo N, Zandieh T. Prevalence of TT virus in general population of Isfahan. Hakim Research Journal 2007; 4(9): 52-8.
- Masia G, Ingianni A, Demelia L, Faa G, Manconi P, Pilleri G, et al. TT virus infection in Italy: prevalence and genotypes in healthy subjects, viral liver diseases and asymptomatic infections by parenterally transmitted viruses. Journal of Viral Hepatitis 2001; 8(5): 384-90.
- Zandieh T, Babaahmadi B, Pourfathollah A, Galedari H, Emam J, Jalalifar MA. Transfusion transmitted virus (TTV) infection in thalassemic patients. Iran J Public Health. 1; 34(4): 24-9.
- Sampietro M, Tavazzi D, Martinez di Montemuros F, Cerino M, Zatelli S, Lunghi G, et al. TT virus infection in adult beta-thalassemia major patients. Haematologica 2001; 86(1): 39-43.
- Erensoy S, Sayiner AA, Türkoğlu S, Canatan D, Akarca US, Sertöz R, Ozacar T, Batur Y, Badur S, Bilgiç A. TT virus infection and genotype distribution in blood donors and a group of patients from Turkey. Infection 2002; 30(5): 299-302.
- Takács M, Balog K, Tóth G, Balogh Z, Szomor KN, Brojnás J, et al. TT virus in Hungary: sequence heterogeneity and mixed infections. FEMS Immunology & Medical Microbiology 2003; 35(2): 153-57.
- Özyürek E, Ergünay K, Kuskonmaz B, Ünal S, Çetin M, Ustaçelebi S, et al. Transfusion-transmitted virus prevalance in Turkish patients with thalassemia. Pediatric Hematology and Oncology 2006; 23(4): 347-53.
- Valença IN, Rós FA, Zucherato VS, Silva-Pinto AC, Covas DT, Kashima S, Slavov SN. Comparative metavirome analysis in polytransfused patients. Brazilian Journal of Medical and Biological Research 2021; 54(12): 11610.
- Iravani Saadi M, Noshadi E, Ahmadyan M, Valandani FM, Kheradmand N, Karimi Z, et al. Expression of MiR-222 and MiR-15a in patients with transfusion-dependent thalassemia and its association with Torque Teno Virus and Cytomegalovirus infections. SSRN 2023. (Preprint)
Type of Study: Research |
Subject:
Hematology & Blood Banking
Received: 2024/10/27 | Accepted: 2025/03/4 | Published: 2024/10/1
Received: 2024/10/27 | Accepted: 2025/03/4 | Published: 2024/10/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




