Wed, Feb 4, 2026
[Archive]
Volume 11, Issue 4 (November 2024)
IJML 2024, 11(4): 304-317 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Saeedi S, Ashrafzadeh M, Kashani K, Peighambarzadeh S Z, Sahebi Ala M, Khoram H et al . Taurine can Protect Against Arsenic-Induced Reproductive Toxicity Through Autophagy. IJML 2024; 11 (4) :304-317
URL: http://ijml.ssu.ac.ir/article-1-544-en.html
URL: http://ijml.ssu.ac.ir/article-1-544-en.html
Sadaf Saeedi 
 , Mohammadamin Ashrafzadeh
, Mohammadamin Ashrafzadeh 
 , Kian Kashani
, Kian Kashani 
 , Seyedeh Zeinab Peighambarzadeh
, Seyedeh Zeinab Peighambarzadeh 
 , Maryam Sahebi Ala
, Maryam Sahebi Ala 
 , Hazhir Khoram
, Hazhir Khoram 
 , Ali Olfati *
, Ali Olfati * 


 , Mohammadamin Ashrafzadeh
, Mohammadamin Ashrafzadeh 
 , Kian Kashani
, Kian Kashani 
 , Seyedeh Zeinab Peighambarzadeh
, Seyedeh Zeinab Peighambarzadeh 
 , Maryam Sahebi Ala
, Maryam Sahebi Ala 
 , Hazhir Khoram
, Hazhir Khoram 
 , Ali Olfati *
, Ali Olfati * 

Clinical Research Development Center, Motazedi Hospital, Kermanshah University of Medical Science, Kermanshah, Iran
Full-Text [PDF 603 kb]
(227 Downloads)
| Abstract (HTML) (341 Views)


References
Full-Text: (78 Views)
Introduction
About 200 million people worldwide are exposed to heavy metals such as arsenic trioxide, mainly in developing countries, via contaminated drinking water, soil, and air in methylated arsenic species [1]. Arsenic poisoning is a global health concern and is well-known for impaired semen quality and male reproductive toxicity. However, the precise mechanisms underlying its toxicity in the induction of testicular histopathological lesions and autophagosome degradation were still unclear.
Arsenic material is caused by the overproduction of reactive oxygen species (ROS) [2] and discharge or reduction of cellular antioxidants, finally causing an imbalance of oxidative state, promoting testicular tissue lesions [3], which leads to idiopathic infertilities [4, 5].
Pharmacological studies of Taurine (C₂H₇NO₃S; TAU) have demonstrated that its active components exhibit antioxidant and anti-inflammatory activities [6, 7]. TAU has been shown to have beneficial effects such as reducing inflammation and oxidative damage in organisms exposed to stressors and toxic substances [8], by stabilizing biological membranes, scavenging free radicals, and inhibiting specific enzymes responsible for ROS generation [9]. TAU has been proven to be biosynthesized in the reproductive system of male animals. It can promote the endocrine function of the hypothalamus-pituitary-testis axis (HPT), Spermatogenesis and maturation, testicular tissue development, maintain the homeostasis of the testicular environment, and enhance sexual ability [10]. Adedara et al. [11] described related mechanisms that include increased antioxidant capacity, reduced oxidative stress, restored the secretory activity of the HPT axis, inhibited inflammation and apoptosis, enhanced sperm mitochondrial energy metabolism, cell membrane stabilization effect, etc.
Some reports have suggested that TAU is regarded as a cytoprotective molecule due to its ability to upregulate antioxidant responses, maintain glutathione stores, increase membrane stability, and reduce inflammation, which may be linked to the improvement of fertility [12]. Yahyavy et al. [13] showed that TAU induces autophagy and suppresses apoptosis in TM3 cells, which are the primary testosterone-producing cells in the testes. This increasing autophagy is accompanied by increasing testosterone levels and reducing oxidative stress.
Our investigation tested the hypothesis that TAU protects testes injured with arsenic toxicity through downregulation of the autophagic genes by inhibition of oxidative changes. Thus, this study provides a new scientific approach concerning arsenic spermatogenesis dysfunction, due to TAU antioxidant activity.
Materials and Methods
Chemicals
TAU was purchased from Sigma-Aldrich (Taufkirchen, Germany). The enzyme-linked immunosorbent assay kits to evaluate oxidative stress indices (ROS and malondialdehyde) and sex hormonal levels including follicle stimulating hormone (FSH), luteinizing hormone (LH), and testosterone were purchased from Cosmo Bio Co. (Japan).
Animals and treatments
Adult male Sprague Dawley rats (220-230 g; 8-week-old), which were purchased from the Pasteur Institute of Iran (Karaj, Iran), were housed in standard conditions of the animal room with free access to food and water. Then, 24 adult male rats were challenged as follows (n=6/group) for 35 consecutive days: control (distilled water; gavage); 3 mg/l/day sodium arsenite [14]; TAU (1000 mg/kg; [15]) during arsenic exposure; and TAU during the study period.
Sampling
The body weight and food/water consumption per animal were recorded weekly. 24 h after the last treatment, animals were euthanized (by anesthesia with 0.64 mg/kg xylazine and 20 mg/kg ketamine (Alfasan, Woerden, the Netherlands)), weighed on a 1-mg digital assay balance; serum samples were processed for assessing sex hormone levels and testes were processed for weight, oxidative stress indices, histopathology, and RNA extraction for expression levels of autophagic marker genes.
Sex, hormonal, and sperm analysis
Blood was obtained from the tail vein and collected in vacuum tubes (non-heparinized). The serum was separated by centrifugation (4000 g for 10 min), stored at −20 °C, and evaluated by radioimmunoassay (kit of Monobind Inc., RIA-1000, Technicon) as recommended by the manufacturer.
Initially, the whole epididymis and testis were separated, and the length, height, and width of the testis were measured using a caliper. The entire testis was then fixed in 10% buffered neutral formalin. After the left cauda epididymides were removed from the rat, they were cut into 2–3 pieces, placed in a cell-culture dish (diameter 35 mm), and supplemented with 1 mL of preheated saline solution at 37 °C. The sperm (spermatozoa) count, motility, and asthenozoospermia were measured as reported in the previous study from our laboratory [16].
Oxidative stress levels
Briefly, the ROS level was assessed using 2,7-dichlorofluorescin dictate using assay kits with a fluorescence spectrophotometer (NanoDrop 2000, Thermo Fisher Scientific, Waltham, MA, USA). 150 mg testis tissue was pre-incubated with DCFH-DA (70 min at 37 °C). The conversion of DCF (green fluorescence, λ emission=525 nm) to DCFH (λ excitation=485 nm) was evaluated using a fluorescence spectrophotometer. The level of malondialdehyde was measured using commercial kits (absorbance of each parameter was monitored at 532 nm).
Histological and stereological study
The testes were fixed in Bouin’s solution for 24 h, dehydrated in a series of graded ethanol, and embedded in paraffin, then they were cut into 5-μm sections using a Leica slicer (Leica, Inc., Germany) and stained with a hematoxylin and eosin kit according to the manufacturer’s instructions. An optical microscope was used to observe histopathological changes (Olympus, Tokyo, Japan). Ten visual fields per slide and six sections per group were selected randomly for analysis under 40× magnification.
The diameter of seminiferous tubules, sperm mass, cellular layers of seminiferous tubules, and Leydig cell necrosis in the testis were measured using the version 9 stereo-investigator system (MBF Bioscience, Micro Bright Field, Inc., Germany) using the method described by Olfati et al. [16].
Determination of autophagic vacuoles
We analyzed autophagic vacuoles by monodansylcadaverine staining (MDC, a selective fluorescent marker). After staining, sections are dehydrated and cleared, and then stained with a 50 mM/L MDC fluorescent dye (45 min at 37 °C) in the dark. The stained samples were washed with phosphate-buffered saline (5 times 5 min each) in the dark and then allowed to dry at an ambient temperature to remove unbound antibodies. To inhibit fluorescence fading, de-paraffinized tissue was treated with HistoChoice® clearing agent. Finally, the optical intensity of autophagic vacuoles was examined by fluorescence microscopy (IX-71 or IX70; Olympus, Tokyo, Japan).
Quantitative reverse transcription polymerase chain reaction (QRT-PCR)
Total RNA was isolated from testes (weighing 30 mg) using the Trizol reagent according to the manufacturer’s protocol (Dena Zist Asia, Mashhad, Iran). 2% agarose gel electrophoresis was used to assess the integrity of total RNA, and the A260/280 ratio was in the range of 1.8–2.0, as evaluated by NanoDrop 2000 (Thermo Fisher, USA) at 260 and 280 nm. RNA was reverse transcribed using a PrimeScriptTM RT Master Mix kit (Biofact™ Co, Yuseong GU, Daejeon, Korea). QRT-PCR was carried out using the QuantStudio 7 Flex qRT-PCR system (Applied Biosystems, Foster, CA, USA) and SYBR@ Premix Ex TaqTM II kit. Specific primers were provided by Invitrogen, USA (Table 1). The thermocycling conditions were as follows: After initial denaturation at 95 °C for 120s, 40 amplification cycles were performed at 95 °C for 15s, 60 °C for 30s; Finally, 95 °C for 1 min, 55 °C for 30s, and 95 °C for 30s. The 2-ΔΔCt method was used to analyze the data, calculating the relative quantity of target nucleic acids.
Statistical analysis
Data were analyzed using the MIXED procedure of SAS 9.4. Data were reported as least squares means, and the Tukey-Kramer adjustment was applied to account for multiple comparisons. The PDIFF procedure of SAS was used to separate treatment means. Data comparisons were conducted using a one-way analysis of variance (ANOVA) followed by the Tukey-Kramer multiple comparison test.
Results
The results related to food intake, body, and testis weights of rats are presented in Table 2. No differences between experimental groups were found in these measures between rats during the experiment (p > 0.05). Effects of orally administered TAU on the sperm quality and sex hormone levels are presented in Table 3. Animals exposed to arsenic showed lower sperm indices (such as spermatozoa count, viability, and motility) and sex hormone levels (LH and testosterone) (p < 0.05).
Data demonstrated that arsenic exposure significantly increased the production of oxidative stress markers (like ROS and malondialdehyde) in testis tissue (Table 4; p<0.05). The effects of TAU of the diet during the study period resulted in a recovery to levels equivalent to the non-arsenic group.
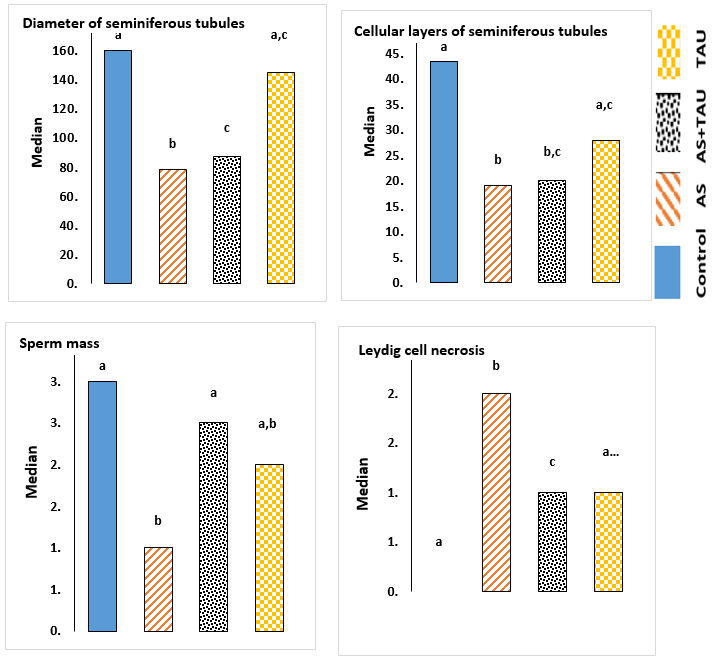
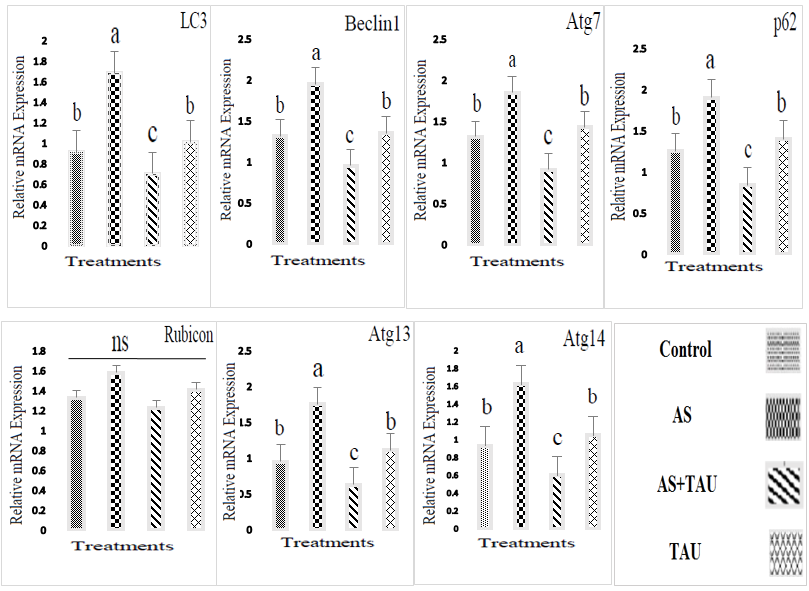
Arsenic causes defective pathologic effects, including a decrease in the diameter of seminiferous tubules and germinal epithelium height, a decrease in Sertoli cell numbers and Leydig cell area, and a decrease in sperm mass entering the channel (Fig.1; p < 0.05). Also, the mean Leydig cell necrosis in the arsenic-treated group was higher than in the other groups (p < 0.05). The administration of TAU significantly improved histopathology changes in testis tissue (p < 0.05). When the cells were viewed, MDC-labeled autophagic vacuoles appeared as distinct dot-like structures localizing in the perinuclear regions or distributed within the cytoplasm (Fig. 2). An increase in the number of MDC-labeled vesicles following exposure to arsenic was observed. In the no-sodium arsenite groups, histopathological sections were normal (completely) in all groups (Fig. 3). Meanwhile, arsenic exposure caused testicular damage and the walls of seminiferous tubules, shrinkage of the basal lamina, and congested blood vessels. At the same time, gene expression analysis for assays related to programmed cell survival or autophagy was examined by QRT-PCR (Fig. 4). The results showed that autophagic expression of markers was upregulated in the testis of arsenic-exposed groups (p < 0.05).
In sum, a TAU supplementation (1000 mg/kg for 35 consecutive days) produced the best therapeutic effect, resulting in recovery of sex hormone levels, repair of testicular tissue, and a reduction in death gene levels, plus a considerable improvement in testis tissue gene expression.
Discussion
The prevalence of infertility is increasing worldwide. Meanwhile, toxic metals cause a wide range of adverse effects on health, with one of them being a decrease in fertility. Nowadays, autophagy assessments in rodents pose a significant challenge due to the increasing ecotoxicological data on the impact of toxic metals on the male reproductive system. Environmental exposures to heavy toxic metals are implicated in infertility development. In 2024, some studies have been conducted to evaluate the potential adverse effects of sodium arsenite on reproductive health [3, 17, 18], specifically by increasing adrenocortical activity, particularly in its inorganic form (or “man-made”). Laboratory analysis of testis tissue has demonstrated that malondialdehyde and ROS are predictors of arsenic damage risk after adjustment for potential baseline blood factors. Oxidative stress, through binding to the thiol groups of biomolecules [19], metabolic inhibition, genotoxicity, and epigenetic alterations, such as microRNA-dependent regulation, are some of the underlying mechanisms of arsenic toxicity [1].
Arsenic material is caused by the overproduction of reactive oxygen species (ROS) [2] and discharge or reduction of cellular antioxidants, finally causing an imbalance of oxidative state, promoting testicular tissue lesions [3], which leads to idiopathic infertilities [4, 5].
Pharmacological studies of Taurine (C₂H₇NO₃S; TAU) have demonstrated that its active components exhibit antioxidant and anti-inflammatory activities [6, 7]. TAU has been shown to have beneficial effects such as reducing inflammation and oxidative damage in organisms exposed to stressors and toxic substances [8], by stabilizing biological membranes, scavenging free radicals, and inhibiting specific enzymes responsible for ROS generation [9]. TAU has been proven to be biosynthesized in the reproductive system of male animals. It can promote the endocrine function of the hypothalamus-pituitary-testis axis (HPT), Spermatogenesis and maturation, testicular tissue development, maintain the homeostasis of the testicular environment, and enhance sexual ability [10]. Adedara et al. [11] described related mechanisms that include increased antioxidant capacity, reduced oxidative stress, restored the secretory activity of the HPT axis, inhibited inflammation and apoptosis, enhanced sperm mitochondrial energy metabolism, cell membrane stabilization effect, etc.
Some reports have suggested that TAU is regarded as a cytoprotective molecule due to its ability to upregulate antioxidant responses, maintain glutathione stores, increase membrane stability, and reduce inflammation, which may be linked to the improvement of fertility [12]. Yahyavy et al. [13] showed that TAU induces autophagy and suppresses apoptosis in TM3 cells, which are the primary testosterone-producing cells in the testes. This increasing autophagy is accompanied by increasing testosterone levels and reducing oxidative stress.
Our investigation tested the hypothesis that TAU protects testes injured with arsenic toxicity through downregulation of the autophagic genes by inhibition of oxidative changes. Thus, this study provides a new scientific approach concerning arsenic spermatogenesis dysfunction, due to TAU antioxidant activity.
Materials and Methods
Chemicals
TAU was purchased from Sigma-Aldrich (Taufkirchen, Germany). The enzyme-linked immunosorbent assay kits to evaluate oxidative stress indices (ROS and malondialdehyde) and sex hormonal levels including follicle stimulating hormone (FSH), luteinizing hormone (LH), and testosterone were purchased from Cosmo Bio Co. (Japan).
Animals and treatments
Adult male Sprague Dawley rats (220-230 g; 8-week-old), which were purchased from the Pasteur Institute of Iran (Karaj, Iran), were housed in standard conditions of the animal room with free access to food and water. Then, 24 adult male rats were challenged as follows (n=6/group) for 35 consecutive days: control (distilled water; gavage); 3 mg/l/day sodium arsenite [14]; TAU (1000 mg/kg; [15]) during arsenic exposure; and TAU during the study period.
Sampling
The body weight and food/water consumption per animal were recorded weekly. 24 h after the last treatment, animals were euthanized (by anesthesia with 0.64 mg/kg xylazine and 20 mg/kg ketamine (Alfasan, Woerden, the Netherlands)), weighed on a 1-mg digital assay balance; serum samples were processed for assessing sex hormone levels and testes were processed for weight, oxidative stress indices, histopathology, and RNA extraction for expression levels of autophagic marker genes.
Sex, hormonal, and sperm analysis
Blood was obtained from the tail vein and collected in vacuum tubes (non-heparinized). The serum was separated by centrifugation (4000 g for 10 min), stored at −20 °C, and evaluated by radioimmunoassay (kit of Monobind Inc., RIA-1000, Technicon) as recommended by the manufacturer.
Initially, the whole epididymis and testis were separated, and the length, height, and width of the testis were measured using a caliper. The entire testis was then fixed in 10% buffered neutral formalin. After the left cauda epididymides were removed from the rat, they were cut into 2–3 pieces, placed in a cell-culture dish (diameter 35 mm), and supplemented with 1 mL of preheated saline solution at 37 °C. The sperm (spermatozoa) count, motility, and asthenozoospermia were measured as reported in the previous study from our laboratory [16].
Oxidative stress levels
Briefly, the ROS level was assessed using 2,7-dichlorofluorescin dictate using assay kits with a fluorescence spectrophotometer (NanoDrop 2000, Thermo Fisher Scientific, Waltham, MA, USA). 150 mg testis tissue was pre-incubated with DCFH-DA (70 min at 37 °C). The conversion of DCF (green fluorescence, λ emission=525 nm) to DCFH (λ excitation=485 nm) was evaluated using a fluorescence spectrophotometer. The level of malondialdehyde was measured using commercial kits (absorbance of each parameter was monitored at 532 nm).
Histological and stereological study
The testes were fixed in Bouin’s solution for 24 h, dehydrated in a series of graded ethanol, and embedded in paraffin, then they were cut into 5-μm sections using a Leica slicer (Leica, Inc., Germany) and stained with a hematoxylin and eosin kit according to the manufacturer’s instructions. An optical microscope was used to observe histopathological changes (Olympus, Tokyo, Japan). Ten visual fields per slide and six sections per group were selected randomly for analysis under 40× magnification.
The diameter of seminiferous tubules, sperm mass, cellular layers of seminiferous tubules, and Leydig cell necrosis in the testis were measured using the version 9 stereo-investigator system (MBF Bioscience, Micro Bright Field, Inc., Germany) using the method described by Olfati et al. [16].
Determination of autophagic vacuoles
We analyzed autophagic vacuoles by monodansylcadaverine staining (MDC, a selective fluorescent marker). After staining, sections are dehydrated and cleared, and then stained with a 50 mM/L MDC fluorescent dye (45 min at 37 °C) in the dark. The stained samples were washed with phosphate-buffered saline (5 times 5 min each) in the dark and then allowed to dry at an ambient temperature to remove unbound antibodies. To inhibit fluorescence fading, de-paraffinized tissue was treated with HistoChoice® clearing agent. Finally, the optical intensity of autophagic vacuoles was examined by fluorescence microscopy (IX-71 or IX70; Olympus, Tokyo, Japan).
Quantitative reverse transcription polymerase chain reaction (QRT-PCR)
Total RNA was isolated from testes (weighing 30 mg) using the Trizol reagent according to the manufacturer’s protocol (Dena Zist Asia, Mashhad, Iran). 2% agarose gel electrophoresis was used to assess the integrity of total RNA, and the A260/280 ratio was in the range of 1.8–2.0, as evaluated by NanoDrop 2000 (Thermo Fisher, USA) at 260 and 280 nm. RNA was reverse transcribed using a PrimeScriptTM RT Master Mix kit (Biofact™ Co, Yuseong GU, Daejeon, Korea). QRT-PCR was carried out using the QuantStudio 7 Flex qRT-PCR system (Applied Biosystems, Foster, CA, USA) and SYBR@ Premix Ex TaqTM II kit. Specific primers were provided by Invitrogen, USA (Table 1). The thermocycling conditions were as follows: After initial denaturation at 95 °C for 120s, 40 amplification cycles were performed at 95 °C for 15s, 60 °C for 30s; Finally, 95 °C for 1 min, 55 °C for 30s, and 95 °C for 30s. The 2-ΔΔCt method was used to analyze the data, calculating the relative quantity of target nucleic acids.
Statistical analysis
Data were analyzed using the MIXED procedure of SAS 9.4. Data were reported as least squares means, and the Tukey-Kramer adjustment was applied to account for multiple comparisons. The PDIFF procedure of SAS was used to separate treatment means. Data comparisons were conducted using a one-way analysis of variance (ANOVA) followed by the Tukey-Kramer multiple comparison test.
Results
The results related to food intake, body, and testis weights of rats are presented in Table 2. No differences between experimental groups were found in these measures between rats during the experiment (p > 0.05). Effects of orally administered TAU on the sperm quality and sex hormone levels are presented in Table 3. Animals exposed to arsenic showed lower sperm indices (such as spermatozoa count, viability, and motility) and sex hormone levels (LH and testosterone) (p < 0.05).
Data demonstrated that arsenic exposure significantly increased the production of oxidative stress markers (like ROS and malondialdehyde) in testis tissue (Table 4; p<0.05). The effects of TAU of the diet during the study period resulted in a recovery to levels equivalent to the non-arsenic group.
Arsenic causes defective pathologic effects, including a decrease in the diameter of seminiferous tubules and germinal epithelium height, a decrease in Sertoli cell numbers and Leydig cell area, and a decrease in sperm mass entering the channel (Fig.1; p < 0.05). Also, the mean Leydig cell necrosis in the arsenic-treated group was higher than in the other groups (p < 0.05). The administration of TAU significantly improved histopathology changes in testis tissue (p < 0.05). When the cells were viewed, MDC-labeled autophagic vacuoles appeared as distinct dot-like structures localizing in the perinuclear regions or distributed within the cytoplasm (Fig. 2). An increase in the number of MDC-labeled vesicles following exposure to arsenic was observed. In the no-sodium arsenite groups, histopathological sections were normal (completely) in all groups (Fig. 3). Meanwhile, arsenic exposure caused testicular damage and the walls of seminiferous tubules, shrinkage of the basal lamina, and congested blood vessels. At the same time, gene expression analysis for assays related to programmed cell survival or autophagy was examined by QRT-PCR (Fig. 4). The results showed that autophagic expression of markers was upregulated in the testis of arsenic-exposed groups (p < 0.05).
In sum, a TAU supplementation (1000 mg/kg for 35 consecutive days) produced the best therapeutic effect, resulting in recovery of sex hormone levels, repair of testicular tissue, and a reduction in death gene levels, plus a considerable improvement in testis tissue gene expression.
Discussion
The prevalence of infertility is increasing worldwide. Meanwhile, toxic metals cause a wide range of adverse effects on health, with one of them being a decrease in fertility. Nowadays, autophagy assessments in rodents pose a significant challenge due to the increasing ecotoxicological data on the impact of toxic metals on the male reproductive system. Environmental exposures to heavy toxic metals are implicated in infertility development. In 2024, some studies have been conducted to evaluate the potential adverse effects of sodium arsenite on reproductive health [3, 17, 18], specifically by increasing adrenocortical activity, particularly in its inorganic form (or “man-made”). Laboratory analysis of testis tissue has demonstrated that malondialdehyde and ROS are predictors of arsenic damage risk after adjustment for potential baseline blood factors. Oxidative stress, through binding to the thiol groups of biomolecules [19], metabolic inhibition, genotoxicity, and epigenetic alterations, such as microRNA-dependent regulation, are some of the underlying mechanisms of arsenic toxicity [1].
Table 1. Primer sequences and product size of target genes for quantitative reverse transcription polymerase chain reaction
| Genes | GenBank access No. | Primer sequence (5’-3’) | Product size (bp) |
| β-actin (housekeeping gene) | NM_007393.5 | F: AGACTTCGAGCAGGAGATGG | 233 |
| R: GCACTGTGTTGGCATAGAGG | |||
| LC3 | NM_026160.4 | F: GATAATCAGACGGCGCTTGC | 99 |
| R: ACTTCGGAGATGGGAGTGGA | |||
| Atg7 | NM_001253717.1 | F: TGTGGCTAGGACACTGATGG | 83 |
| R: TCACGGGATTGGAGTAGGAG | |||
| Atg13 | NM_145528.3 | F: TGGCGGAAGATTTGGACTCC | 84 |
| R: GGGTTTCCACAAAGGCATCG | |||
| Atg14 | NM_172599.4 | F: CTGGCCTTGTCACCATATCC | 120 |
| R: GCAGGCAAAGTACCAACCAC | |||
| p62 | NM_001290769.1 | F: ACAGCCAGAGGAACAGATGG | 240 |
| R: GGAGGGTGCTTCGAATACTG | |||
| Beclin1 | NM_019584.3 | F: CTCTGAAACTGGACACGAGC | 124 |
| R: CCTGAGTTAGCCTCTTCCTCC | |||
| Rubicon | NM_001200038.1 | F: TGTGGAAGGTTTGGTGTCAG | 108 |
| R: TGGATGAGCCCATGATACAG |
Table 2. Effect of sodium arsenite and taurine on food intake, body, and testis weight in an arsenic-treated rat model (means±S.D. of 6 rats)
| Groups | Food intake (g) | Body weight (g) | Testis weight (mg) | |||||
| Day | Right | Left | ||||||
| 0 | 15 | 35 | ||||||
| Control | 6.73±0.89 | 198.83±10.57 | 245.83±12.3 | 278.55±11.7 | 1344.17±34.62 | 1380.17±51.4 | ||
| Sodium arsenite | 5.51±0.65 | 208.33±3.55 | 245.17±5 | 263.18±4.7 | 1353.33±39.7 | 1358.33±44.31 | ||
| Sodium arsenite+Taurine | 6.98±0.78 | 206.67±9.22 | 240.33±7.13 | 270.63±6.9 | 1331.67±29.75 | 1384.33±34.08 | ||
| Taurine | 6.05±0.59 | 204.17±10.9 | 254.33±10.46 | 287.6±6.35 | 1358.17±32.3 | 1394.17±26.76 | ||
Sodium arsenite (3 mg/l/day, gavage); Sodium arsenite + Taurine (taurine 1000 mg/kg of the diet during arsenic exposure); Taurine (1000 mg/kg of the diet during the study period).
Table 3. Effect of sodium arsenite and taurine on sperm quality and sex hormones level in an arsenic-treated rat model (means±S.D. of 6 rats)
| Groups | Sperm | Hormone | |||||
| Spermatozoa (×106) | Viability (%) | Motility (%) | Testosterone (nmol/L) | FSH (IU/mL) | LH (IU/mL) | ||
| Control | 38.22±0.89 a | 76.16±0.52 a | 70.73±0.43 a | 1.33±0.15 a | 1.44±0.19 | 1.85±0.12 a | |
| Sodium arsenite | 20.65±0.38 b | 35.44±0.13 b | 29.30±0.33 b | 0.31±0.06 b | 1.26±0.04 | 1.12±0.07 d | |
| Sodium arsenite+Taurine | 41.19±0.38 a | 78.00±0.27 a | 74.14±0.38 a | 1.45±0.08 a | 1.53±0.11 | 1.80±0.21 ab | |
| Taurine | 33.12±0.18 a | 67.05±0.61 a | 65.20±0.64 a | 1.23±0.17 a | 1.43±0.08 | 1.46±0.05 bc | |
Sodium arsenite (3 mg/l/day, gavage); Sodium arsenite + Taurine (taurine 1000 mg/kg of the diet during arsenic exposure); Taurine (1000 mg/kg of the diet during the study period). The same superscripts (a-c) are not significantly different from each other in each column (p<0.05).
Table 4. Effect of sodium arsenite and taurine in the diet on oxidative stress levels in an arsenic-treated rat model (means±S.D. of 6 rats)
| Groups | Reactive oxygen species (×105) | Malondialdehyde (nmol/mg protein) |
| Control | 1.13±0.25 b | 2.16±0.58 b |
| Sodium arsenite | 2.39±0.83 a | 10.9±1.27 a |
| Sodium arsenite+Taurine | 1.01±0.48 b | 2.22±0.13 b |
| Taurine | 1.24±0.19 b | 1.99±0.17 b |
Sodium arsenite (3 mg/l/day, gavage); Sodium arsenite + Taurine (taurine 1000 mg/kg of the diet during arsenic exposure); Taurine (1000 mg/kg of the diet during the study period). The same superscripts are not significantly different from each other in each column (p<0.05).

Fig. 1. Stereology of the testicular components after arsenic and taurine treatments for 35 days. Graphs show mean± S.D.; Letters indicate that treatments differ p<0.05 within each group. Control; sodium arsenite (AS; 3 mg/l/day, gavage); AS + TAU (taurine 1000 mg/kg of the diet during arsenic exposure); TAU: 1000 mg/kg of the diet during the study period).

Fig. 2. Qualitative monodansylcadaverine staining (MDC)-labeled autophagic vacuoles in the rat testis (400× magnification, bar = 100 µm)

Fig. 3. Testicular histological structure after arsenic and taurine treatments for 35 days (×60)

Fig. 4. mRNA expression levels as detected by quantitative reverse transcription polymerase chain reaction. Graphs show mean±SEM (n = 6); Letters indicate that treatments differ by p<0.05 within each group. Sodium arsenite (AS; 3 mg/l/day, gavage); AS+TAU (taurine 1000 mg/kg of the diet during arsenic exposure); TAU: 1000 mg/kg of the diet during the study period.
The sperm quality analysis revealed that the testis is a target organ of arsenic. Our findings showed that rats exposed to arsenic have statistically lowered semen quality, including spermatozoa, viability, and motility, and reduced testosterone levels. In all animals, sperm production passes through four stages: 1) establishment of the germline, 2) proliferation of germ cells, 3) production of spermatids by meiosis, and 4) differentiation of mature sperm. At an appropriate time, spermatids undergo spermiogenesis [20]. At any stage of cell differentiation, disruption of Spermatogenesis may result in a decreased total spermatogenic cell count [21]. Our data showed that chemotherapeutic drugs can penetrate through the rat blood-testis barriers into reproductive tissue and can, at least, have an impact on sperm vitality, and finally cause disruption and reduction of Spermatogenesis. Results close to the results of this study have also been obtained in various previous studies. Krzastek et al. [22] demonstrated that arsenic inhibits 3β-hydroxysteroid dehydrogenase, which plays a crucial role in steroidogenesis. In the current study, this may be reversible with taurine antioxidant properties that may be protective against ROS. Rachamalla et al. [23] showed that arsenic exposure negatively affected male reproductive systems by lowering accessory organ and testicular weights, and sperm counts, increasing sperm abnormalities, and causing apoptotic cell death in Leydig and Sertoli cells, which resulted in decreased testosterone synthesis. Recently, Akhigbe et al. [24] found out that this may be due to its ability to bioaccumulate, evidenced by the observed rise in urinary levels of total arsenic. Spermatogenesis and steroidogenesis are regulated by a master switch (GnRH pulse generator), under the control of which the two separate feedback systems provide independent control of androgen (LH–testosterone) and sperm production (FSH–inhibin) [25]. Interestingly, a hormonal analysis revealed that testosterone and LH (both playing evolutionary roles in maintaining Spermatogenesis) were significantly decreased in the arsenic-injured groups as compared to the vehicle group (p < 0.05). Spermatogenesis is a fundamental process of proliferation and differentiation of germ cells into spermatozoa, which is dependent on androgens produced locally in response to LH. Testosterone, as the major androgen in the testis, is essential for the regulation of the development, growth, and metabolism of the male reproductive system [15]. However, the serum FSH produced by the adenohypophysis did not show significant changes. A likely explanation is that the hormones acted by stimulating spermatogonia proliferation and Sertoli cell function, thereby restoring tubular activity. The decreases in circulating testosterone and LH after tamoxifen citrate exposure may also be related to the main mechanisms: chemotherapeutic drugs can affect the HPT axis, especially the anterior pituitary, by altering sex hormone secretion and cellular activity.
In the development and regulation of the male reproductive system, the HPT axis plays a critical role [26]. In conclusion, the reproductive toxicity of arsenic is associated with disturbances in the spermatogenesis rate, changes in the gonadal internal environment (including hormone synthesis rates), and testicular damage in reproductive target tissues. TAU is the most abundant free amino acid in the human body. Many physiological roles are attributed to this amino acid. For some biological preparations, the use of TAU therapy has achieved better efficacy on human health and potential fertility issues, possibly by minimizing oxidative stress [27]. Recently, Davari Zanjani et al. [28] reported that TAU can be proposed as a potential treatment drug for acrylamide-induced infertility through regulating autophagy-related genes in oocytes.
TAU supplementation may be favorable due to its cytoprotective properties and potential systemic antioxidant effects, as suggested by previous studies [29, 30]. A line linking the current study to TAU has shown that TAU decreases ROS production, suggesting that it might influence risk factors of oxidative stress by protecting mitochondria against oxidative-nitrosative stress [31].
In agreement with current data, some studies have shown that TAU supplements improve Spermatogenesis, and testicular tissue development, and maintain the homeostasis of the testicular environment through regular monitoring of endocrine hormones [11, 12]. Following these results, some researchers have speculated that the proposed mechanism of action is that TAU is present in mitochondria, which is considered the accountable organelle for sperm movement [32, 33].
TAU may act by increasing the presence of specific peptides or amino acids in the blood. Indeed, at the current study, TAU at 1000 mg/kg of the diet during the study period showed a trend, but it was not enough to show significant improvements, maybe because TAU absorption through the digestive system is low. Autophagy is essential for male infertility, as it controls the development of germ cells, maintains cellular balance, and protects reproductive function by regulating the mTORC1-mTORC2 balance and promoting AKT/mTOR-mediated autophagy [34]. In the current study, a new approach was investigated, in which the upregulation of p62 expression increases the autophagy rate through the upregulation of autophagy-related genes.
Autophagy begins with the formation of autophagosomes, which envelop a part of the cytoplasm and deliver cytoplasmic components to the degradative organelle for subsequent recycling and breakdown [35-38]. As a result, all these consequences suggest that arsenic exposure induces the formation of autophagosomes in testicular cells, indicating that arsenic can enhance the formation of autophagosomes in the cytoplasm. The arsenic-induced impairment of the autophagic flux may be a kind of embodiment of arsenic toxicity. Apart from the formation of autophagosomes, another reason for autophagosome accumulation may be associated with the blockage of autophagosome degradation due to p62 aggregation. TAU treatments blocked arsenic-induced testis cell death. During TAU treatments, the diffused distribution of MDC across the cytoplasm transformed into a dot-like. Meanwhile, based on MDC and histopathology results, TAU therapy can elevate the autophagy pathway activity to survive stress and facilitate aggressiveness by suppressing stress responses and promoting metabolism and survival. In sum, arsenic exposure changes autophagy-related gene expression, and the effect of arsenic on autophagy-related genes can influence autophagosome degradation. Hence, it is recommended that future studies focus on the exact relationships between signaling pathways mediating autophagy in the male reproductive system. TAU can facilitate the fusion of autophagosomes with lysosomes by removing autophagic obstruction, thereby protecting against oxidative stress and potentially accelerating autophagy [28]. Apart from the results derived from research findings, key autophagic genes related to arsenic stress resistance were predicted, named p62. Results on stress resistance and comprehensive stress resistance opened a new window for future studies. In the current study, TAU administration significantly mitigated testis injury and increased blood serum sex hormone levels and relative expression of genes related to autophagy in arsenic-treated rats. From the above results, it was predicted that TAU administration has reversible and rapid antifertility effects on male reproductive function by interfering with the oxidative and steroidogenesis pathways to affect sperm production. Promising early results suggest that TAU supplementation exhibited a protective effect on the spermatogenic activity in an arsenic-treated damaged rat model, showing promise as a potential therapeutic agent. Limitations of this study included sample size, lack of prior research studies on the topic, and the measure used to collect the data. Since high arsenic levels can hurt both female and male fertility, more human studies are needed to support TAU as a viable treatment method for the improvement of reproductive damage. The information obtained in the rat model could stimulate the investigation of these non-proteinogenic amino acids, not only on their effects on male reproduction but also as a potential antioxidant with systemic activity. This could help to develop novel treatments and biotechnological applications both in human medicine and animal production. In a nutshell, arsenic exposure changes autophagy-related gene expression, and the effect of arsenic on the autophagy-related genes can influence autophagosome degradation. Hence, it is recommended that future experiments should focus on the exact relationships between signaling pathways mediating autophagy in the male reproductive system.
Conclusion
In fact, arsenic-induced testis cell death was blocked by TAU treatments. Meanwhile, based on histopathology results, TAU therapy can elevate the autophagy pathway activity to survive stress caused by heavy metals and to facilitate aggressiveness by suppressing stress responses (ROS production) and promoting metabolism and survival. The current study revealed that arsenic exposure induced the formation of autophagosomes. And, data have indicated a new role of p62 in reducing chemosensitivity during arsenic toxicity. Thus, this study provides a new scientific approach concerning arsenic spermatogenesis dysfunction, due to TAU antioxidant activity.
Ethical Considerations
Animals were handled according to the animal treatment protocol approved by Kermanshah University of Medical Sciences ethics committee (Ethic code IR. KUMS.REC.02514.1.71/3). The AR-RIVE guidelines for laboratory animal care and use were also followed.
Funding Statement
This research received no external funding.
Conflict of Interest
The authors declared no conflict of interest.
Acknowledgments
The authors gratefully acknowledge Prof. Dr. Mehrdad Payandeh (Kermanshah University of Medical Sciences, Kermanshah, Iran), Prof. Dr. Parisa Azimi (Neuroscience Research Center, Shahid Beheshti University of Medical Sciences, Iran), and My wife Mrs. Gila Olfati for their helpful discussions of this study and advice during the clinical trial.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Authors’ Contributions
S. S, M. A, K. K, SZ. P, M. SA, and H. KH: Review & Editing, Methodology, Investigation, Data curation. A.O: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. All authors conceived the responsibility for the initial design and main idea of the manuscript.
In the development and regulation of the male reproductive system, the HPT axis plays a critical role [26]. In conclusion, the reproductive toxicity of arsenic is associated with disturbances in the spermatogenesis rate, changes in the gonadal internal environment (including hormone synthesis rates), and testicular damage in reproductive target tissues. TAU is the most abundant free amino acid in the human body. Many physiological roles are attributed to this amino acid. For some biological preparations, the use of TAU therapy has achieved better efficacy on human health and potential fertility issues, possibly by minimizing oxidative stress [27]. Recently, Davari Zanjani et al. [28] reported that TAU can be proposed as a potential treatment drug for acrylamide-induced infertility through regulating autophagy-related genes in oocytes.
TAU supplementation may be favorable due to its cytoprotective properties and potential systemic antioxidant effects, as suggested by previous studies [29, 30]. A line linking the current study to TAU has shown that TAU decreases ROS production, suggesting that it might influence risk factors of oxidative stress by protecting mitochondria against oxidative-nitrosative stress [31].
In agreement with current data, some studies have shown that TAU supplements improve Spermatogenesis, and testicular tissue development, and maintain the homeostasis of the testicular environment through regular monitoring of endocrine hormones [11, 12]. Following these results, some researchers have speculated that the proposed mechanism of action is that TAU is present in mitochondria, which is considered the accountable organelle for sperm movement [32, 33].
TAU may act by increasing the presence of specific peptides or amino acids in the blood. Indeed, at the current study, TAU at 1000 mg/kg of the diet during the study period showed a trend, but it was not enough to show significant improvements, maybe because TAU absorption through the digestive system is low. Autophagy is essential for male infertility, as it controls the development of germ cells, maintains cellular balance, and protects reproductive function by regulating the mTORC1-mTORC2 balance and promoting AKT/mTOR-mediated autophagy [34]. In the current study, a new approach was investigated, in which the upregulation of p62 expression increases the autophagy rate through the upregulation of autophagy-related genes.
Autophagy begins with the formation of autophagosomes, which envelop a part of the cytoplasm and deliver cytoplasmic components to the degradative organelle for subsequent recycling and breakdown [35-38]. As a result, all these consequences suggest that arsenic exposure induces the formation of autophagosomes in testicular cells, indicating that arsenic can enhance the formation of autophagosomes in the cytoplasm. The arsenic-induced impairment of the autophagic flux may be a kind of embodiment of arsenic toxicity. Apart from the formation of autophagosomes, another reason for autophagosome accumulation may be associated with the blockage of autophagosome degradation due to p62 aggregation. TAU treatments blocked arsenic-induced testis cell death. During TAU treatments, the diffused distribution of MDC across the cytoplasm transformed into a dot-like. Meanwhile, based on MDC and histopathology results, TAU therapy can elevate the autophagy pathway activity to survive stress and facilitate aggressiveness by suppressing stress responses and promoting metabolism and survival. In sum, arsenic exposure changes autophagy-related gene expression, and the effect of arsenic on autophagy-related genes can influence autophagosome degradation. Hence, it is recommended that future studies focus on the exact relationships between signaling pathways mediating autophagy in the male reproductive system. TAU can facilitate the fusion of autophagosomes with lysosomes by removing autophagic obstruction, thereby protecting against oxidative stress and potentially accelerating autophagy [28]. Apart from the results derived from research findings, key autophagic genes related to arsenic stress resistance were predicted, named p62. Results on stress resistance and comprehensive stress resistance opened a new window for future studies. In the current study, TAU administration significantly mitigated testis injury and increased blood serum sex hormone levels and relative expression of genes related to autophagy in arsenic-treated rats. From the above results, it was predicted that TAU administration has reversible and rapid antifertility effects on male reproductive function by interfering with the oxidative and steroidogenesis pathways to affect sperm production. Promising early results suggest that TAU supplementation exhibited a protective effect on the spermatogenic activity in an arsenic-treated damaged rat model, showing promise as a potential therapeutic agent. Limitations of this study included sample size, lack of prior research studies on the topic, and the measure used to collect the data. Since high arsenic levels can hurt both female and male fertility, more human studies are needed to support TAU as a viable treatment method for the improvement of reproductive damage. The information obtained in the rat model could stimulate the investigation of these non-proteinogenic amino acids, not only on their effects on male reproduction but also as a potential antioxidant with systemic activity. This could help to develop novel treatments and biotechnological applications both in human medicine and animal production. In a nutshell, arsenic exposure changes autophagy-related gene expression, and the effect of arsenic on the autophagy-related genes can influence autophagosome degradation. Hence, it is recommended that future experiments should focus on the exact relationships between signaling pathways mediating autophagy in the male reproductive system.
Conclusion
In fact, arsenic-induced testis cell death was blocked by TAU treatments. Meanwhile, based on histopathology results, TAU therapy can elevate the autophagy pathway activity to survive stress caused by heavy metals and to facilitate aggressiveness by suppressing stress responses (ROS production) and promoting metabolism and survival. The current study revealed that arsenic exposure induced the formation of autophagosomes. And, data have indicated a new role of p62 in reducing chemosensitivity during arsenic toxicity. Thus, this study provides a new scientific approach concerning arsenic spermatogenesis dysfunction, due to TAU antioxidant activity.
Ethical Considerations
Animals were handled according to the animal treatment protocol approved by Kermanshah University of Medical Sciences ethics committee (Ethic code IR. KUMS.REC.02514.1.71/3). The AR-RIVE guidelines for laboratory animal care and use were also followed.
Funding Statement
This research received no external funding.
Conflict of Interest
The authors declared no conflict of interest.
Acknowledgments
The authors gratefully acknowledge Prof. Dr. Mehrdad Payandeh (Kermanshah University of Medical Sciences, Kermanshah, Iran), Prof. Dr. Parisa Azimi (Neuroscience Research Center, Shahid Beheshti University of Medical Sciences, Iran), and My wife Mrs. Gila Olfati for their helpful discussions of this study and advice during the clinical trial.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Authors’ Contributions
S. S, M. A, K. K, SZ. P, M. SA, and H. KH: Review & Editing, Methodology, Investigation, Data curation. A.O: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. All authors conceived the responsibility for the initial design and main idea of the manuscript.
References
- Muzaffar S, Khan J, Srivastava R, Gorbatyuk MS, Athar M. Mechanistic understanding of the toxic effects of arsenic and warfare arsenicals on human health and environment. Cell Biology Toxicology 2023; 39(1): 85-110.
- Zhang R, Tu L, Yang Y, Sun J, Liang T, Li Y, et al. Altered generation pattern of reactive oxygen species triggering DNA and plasma membrane damages to human liver cells treated with arsenite. Science of the Total Environment 2023; 900: 165821.
- Yang Y, Li Y, Li R, Wang Z. Research progress on arsenic, arsenic-containing medicinal materials, and arsenic-containing preparations: clinical application, pharmaco-logical effects, and toxicity. Frontiers Pharmacology 2024; 15: 1338725.
- Martinez V, Yen IH, Alvarez C, Williams AD, Ha S. Exposure to environmental chemicals and infertility among US reproductive-aged women. The International Journal of Environmental Research and Public Health 2024; 21(12): 1541.
- Baszyński J, Kamiński P, Mroczkowski S, Szymański M, Wasilow K, Stuczyński T, et al. Does aluminum, boron, arsenic, cadmium, lipoperoxidation, and genetic polymorphism determine male fertility? Ecotoxicology and Environmental Safety 2024; 284: 116919.
- Ouyang G, Wang N, Tong J, Sun W, Yang J, Wu G. Alleviation of taurine on liver injury of type 2 diabetic rats by improving antioxidant and anti-inflammatory capacity. Heliyon 2024; 10(7): 28400.
- Calabrese EJ, Pressman P, Hayes AW, Kapoor R, Dhawan G, Agathokleous E, Calabrese V. Taurine induces hormesis in multiple biological models: May have transformative implications for overall societal health. Chemico-Biological Interactions 2024; 392: 110930.
- Teles A, Guzmán-Villanueva L, Hernández-de Dios MA, Corona-Rojas DA, Maldonado-García M, Tovar-Ramírez D. Taurine enhances antioxidant enzyme activity and immune response in seriola rivoliana juveniles after lipopolysaccharide injection. Fishes 2025; 10(5): 225.
- Torres TM, Almeida-Monteiro PS, Nascimento RVD, Cândido-Sobrinho SA, Sousa CTN, Ferreira YM, et al. Effects of taurine, cysteine and melatonin as antioxidant supplements to the freezing medium of Prochilodus brevis sperm. Cryobiology 2024; 114: 104858.
- PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004-. PubChem Compound Summary for CID 1123, Taurine; [cited 2025 Oct. 7]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Taurine
- Adedara IA, Alake SE, Adeyemo MO, Olajide LO, Ajibade TO, Farombi EO. Taurine enhances spermatogenic function and antioxidant defense mechanisms in testes and epididymis of L-NAME-induced hypertensive rats. Biomedicine & Pharmacotherapy 2018; 97: 181-89.
- Du Y, Liu H, Zhang M, Zhang S, Hu J, Wu G, Yang J. Taurine increases spermatozoa quality and function in asthenospermia rats impaired by ornidazole. Advances in Experimental Medicine and Biology 2019; 1155: 507-520.
- Yahyavy S, Valizadeh A, Saki G, Khorsandi L. Taurine induces autophagy and inhibits oxidative stress in mice Leydig cells. JBRA Assisted Reproduction 2020; 24(3): 250-56.
- Li Y, Peng Q, Shang J, Dong W, Wu S, Guo X, et al. The role of taurine in male reproduction: Physiology, pathology and toxicology. Front Endocrinology (Lausanne) 2023; 14: 1017886.
- Baliou S, Adamaki M, Ioannou P, Pappa A, Panayiotidis MI, Spandidos DA, et al. Protective role of taurine against oxidative stress (Review). Molecular Medicine Reports 2021; 24(2): 605.
- Olfati A, Tvrda E. Riboflavin recovery of spermatogenic dysfunction via a dual inhibition of oxidative changes and regulation of the PINK1-mediated pathway in arsenic-injured rat model. Physiological Research 2021; 70(4): 591-603.
- Ommati MM, Sabouri S, Retana-Marquez S, Nategh Ahmadi H, Arjmand A, Alidaee S, et al. Taurine Improves Sperm Mitochondrial Indices, Blunts Oxidative Stress Parameters, and Enhances Steroidogenesis and Kinematics of Sperm in Lead-Exposed Mice. Reproduction 2023; 30(6): 1891-910.
- Olfati A, Moghaddam GH, Baradaran B, Hamidian G. The effect of estradiol benzoate and FSH on hormonal levels and stereology structure of testis in Ghezel lambs treated with Tamoxifen citrate. Revue De Médecine Vétérinaire 2018; 16(1/3): 58-64.
- Adeogun AE, Ogunleye OD, Akhigbe TM, Oyedokun PA, Adegbola CA, Saka WA, et al. Impact of arsenic on male and female reproductive function: a review of the pathophysiology and potential therapeutic strategies. Naunyn Schmiedebergs Archives Pharmacology 2024; 398(2): 1283-297.
- White-Cooper H, Doggett K, Ellis RE. The evolution of spermatogenesis. Sperm Biology 2009; 151-83.
- Forgacs Z, Massanyi P, Lukac N, Somosy Z. Reproductive toxicology of nickel-review. Journal of Environmental Science and Health, Part A: Toxic/Hazardous Substances and Environmental Engineering 2012; 47(9): 1249-260.
- Krzastek SC, Farhi J, Gray M, Smith RP. Impact of environmental toxin exposure on male fertility potential. Translation Andrology and Urology 2020; 9(6): 2797-813.
- Rachamalla M, Chinthada J, Kushwaha S, Putnala SK, Sahu C, Jena G, et al. Contemporary comprehensive review on arsenic-induced male reproductive toxicity and mechanisms of phytonutrient intervention. Toxics 2022; 10(12): 744.
- Akhigbe RE, Akhigbe TM, Adegbola CA, Oyedokun PA, Adesoye OB, Adeogun AE. Toxic impacts of arsenic bioaccumulation on urinary arsenic metabolites and semen quality: A systematic and meta-analysis. Ecotoxicology and Environmental Safety 2024; 281: 116645.
- Stefan S, Jens E. Regulation of spermatogenesis: An evolutionary biologist’s perspective. Seminars in Cell and Developmental Biology 2014; 29: 2-16.
- Yüce A, Ateşşahin A, Ceribaşi AO. Amelioration of cyclosporine A-induced renal, hepatic and cardiac damages by ellagic acid in rats. Basic & Clinical Pharmacology & Toxicology 2008; 103(2): 186-91.
- Baba SP, Bhatnagar A. Role of thiols in oxidative stress. Current Opinion in Toxicology 2018; 7: 133-39.
- Davari Zanjani M, Khodabandeh Z, Edalatmanesh MA. The protective effect of taurine and curcumin on autophagy-related genes in the oocytes of the mouse treated with acrylamide. Iran J Med Sci. 2025; 50(4): 260-69.
- Jangra A, Gola P, Singh J, Gond P, Ghosh S, Rachamalla M, et al. Emergence of taurine as a therapeutic agent for neurological disorders. Neural Regeneration Research 2024; 19(1): 62-8.
- Mihaiescu T, Turti S, Souca M, Muresan R, Achim L, Prifti E, et al. Caffeine and taurine from energy drinks-A review. Cosmetics 2024; 11(1): 12-20.
- Seneff S, Kyriakopoulos AM. Taurine prevents mitochondrial dysfunction and protects mitochondria from reactive oxygen species and deuterium toxicity. Amino Acids 2025; 57(1): 6-12.
- Folgerø T, Bertheussen K, Lindal S, Torbergsen T, Oian P. Mitochondrial disease and reduced sperm motility. Human Reproduction 1993; 8(11): 1863-868.
- Pribilova M, Skalickova S, Urbankova L, Baholet D, Nevrkla P, Kopec T, et al. Monitoring of taurine dietary supplementation effect on parameters of Duroc boar ejaculate in summer season. PLoS One 2024; 19(1): 288317.
- Chang Y, Deng H, He Y, Zhou B, Yuan D, Wu J, et al. Wuzi Yanzong administration alleviates sertoli cell injury by recovering AKT/mTOR-mediated autophagy and the mTORC1-mTROC2 balance in aging-induced testicular dysfunction. Journal of Ethnopharmacology 2024; 318: 116865.
- Marshall RS, Vierstra RD. Autophagy: the master of bulk and selective recycling. Annual Review of Plant Biology 2018; 69: 173-208.
- Kankuan W, Wanichanon C, Morani F, Thongrod S, Titone R, Siangcham T, et al. Starvation promotes autophagy-associated maturation of the testis in the giant freshwater prawn, macrobrachium rosenbergii. Frontiers Physiology 2019; 27(10): 1219.
- Hamali H, Masoumi F, Baradaran B, Hamidian G. Plasma rich-platelet (PRP) potentially attenuates tamoxifen spermatogenic dysfunction in rat’s model: sperm quality, histomorphometric, and apoptosis assessment. Asia Pacific Journal of Medical Toxicology 2025; 14: 7-12.
- Saeedi S, Olfati A, Sadeghi T, Veisi F, Zanganeh M, Jalilian N, et al. Japanese sake yeast potentially attenuates arsenic neurotoxicity in male rats model: behavioral, oxidative stress, and immunogenetics assessment. Asia Pacific Journal of Medical Toxicology 2024; 13(3): 84-89.
Type of Study: Research |
Subject:
Pathology
Received: 2025/04/10 | Accepted: 2024/08/31 | Published: 2024/10/31
Received: 2025/04/10 | Accepted: 2024/08/31 | Published: 2024/10/31
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




