Sat, Feb 7, 2026
[Archive]
Volume 11, Issue 4 (November 2024)
IJML 2024, 11(4): 283-291 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Sabrina B, Amrani A, Idrissi A, Sebbar E, Choukri M. Verification of Analytical Performance of the Jaffé Kinetic Creatinine Assay on the Architect ci-2800 According to ISO 15189 Guidelines. IJML 2024; 11 (4) :283-291
URL: http://ijml.ssu.ac.ir/article-1-553-en.html
URL: http://ijml.ssu.ac.ir/article-1-553-en.html
Faculty of Medicine and Pharmacy of Oujda, Mohammed First University, Morocco & Central Laboratory, Mohammed VI University Hospital of Oujda, Morocco
Keywords: Architect ci 2800, Creatinine, ISO 15189, Jaffé kinetic method, Repeatability, Reproducibility
Full-Text [PDF 303 kb]
(115 Downloads)
| Abstract (HTML) (218 Views)
Full-Text: (51 Views)
Introduction
Results
A total of three levels were analyzed to cover a representative range of creatinine concentrations commonly encountered in clinical practice. Each level underwent tests for repeatability (intra-series) and reproducibility (inter-series), supplemented by an assessment of measurement uncertainty and a method comparison between two Architect ci-2800 systems.
Repeatability
Repeatability was assessed by performing 30 consecutive measurements for each of the three levels (low, medium, high) in one series, without interruption or changes in analytical conditions. The results show coefficient of variations (CVs) of 1.13% at the low level, 1.05% at the medium level, and 0.50% at the high level. Such low CVs indicate excellent intra-series precision, fully meeting SFBC requirements and international guidelines (RICOS). For instance, at the high level, the observed CV (0.50%) is well below the SFBC threshold of 1.8%, demonstrating remarkable short-term measurement stability. Figures 1, 2, and 3 effectively illustrate the method’s precision and accuracy across the tested concentration ranges.
Reproducibility
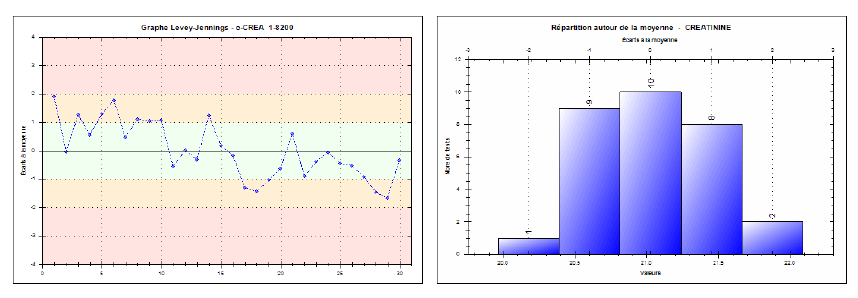
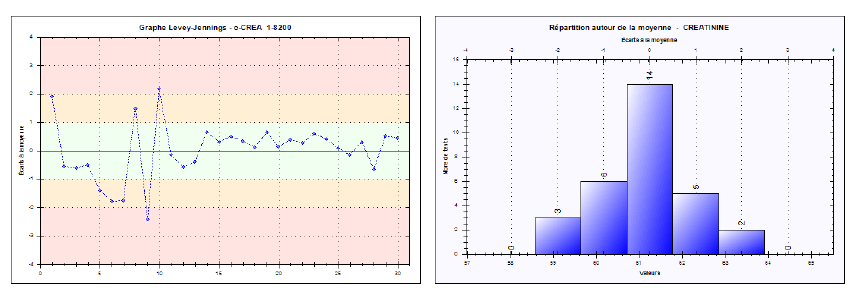
Reproducibility was evaluated by examining the spread of results over 30 consecutive days, including variations in reagent lots, calibrations, or operators. The internal controls at low (~6.94 mg/L), medium (~21.03 mg/L), and high (~61.24 mg/L) levels were measured daily, yielding inter-series CVs of 2.91%, 2.02%, and 1.75%, respectively. These results are also below the SFBC thresholds (e.g., 6% for low, 4.5% for medium, and 2.4% for high), underscoring the reliability and robustness of the Jaffé kinetic method over time. Corresponding Levey-Jennings charts (Figures 4, 5, and 6) confirm the strong reproducibility of this method.
Measurement uncertainty
Measurement uncertainty was estimated using the “CIQ + EEQ” approach, combining the laboratory’s internal dispersion (intermediate fidelity) with any difference (bias) compared to the external quality control target values. For the low level (6.94 mg/L), the expanded uncertainty U (k=2) is about 6.57%, which remains below the 8.2% RICOS criterion for total error. Likewise, the medium and high levels- at around 7.2% and 6.38%, respectively- are deemed acceptable.
Method comparison
Lastly, a method comparison between two Architect ci-2800 systems was performed using 30 patient samples spanning a broad range of creatinine values. Bland-Altman plots show that linear regression produces Y= 1.005×X – 0.365, with a correlation coefficient (r)=1.0 and an average bias of about 2.15% (Figures 7a, 7b). These values demonstrate excellent agreement between the two analyzers, confirming the method’s reliability across various operational contexts or when multiple instruments coexist in a single laboratory.

Discussion
References
Measuring creatinine is critical for evaluating renal function and diagnosing acute or chronic renal failure [1]. The choice and reliability of the method are among the decisive factors affecting the quality of laboratory test results. For strong performance, evaluations must be carried out according to regulations. Under NF EN ISO 15189 and 22870 standards, each method adopted by a clinical biology laboratory must be verified to ensure it is used within its intended scope, is well-controlled, and meets patient/prescriber needs. This is considered a “Scope A method verification” [2, 3].
The Jaffé kinetic method is based on the reaction between creatinine and picric acid in an alkaline medium, which forms a chromogenic complex. The rate of complex formation is proportional to the creatinine concentration [4]. Although the technique is cost-effective and rapid, it can be sensitive to certain interferences (bilirubin, ascorbic acid, ketones, etc.) [5].
Serum creatinine is one of the ten most frequently requested biochemical tests in our hospital, with more than 150 determinations performed each day. Because our laboratory is adopting ISO 15189 and will soon undergo an accreditation audit, a local verification of analytical performance is mandatory. Accurate creatinine results underpin the staging of chronic kidney disease, the dosage adjustment of nephrotoxic drugs such as aminoglycosides and vancomycin, and the follow-up of kidney‑transplant recipients [6, 7]. Yet, external quality‑assessment surveys still report inter-laboratory coefficients of variation greater than 8 % for kinetic Jaffé methods [8], a degree of imprecision that can lead to clinically meaningful discrepancies [9]. For these reasons, we deemed it essential to verify the performance of the new ARCHITECT ci‑2800 analyser.
The objective of this study is to verify the analytical performance (repeatability, reproducibility, measurement uncertainty, and inter-instrument comparison) of creatinine measurement on the Architect ci-2800, following recommended protocols (SFBC, RICOS, COFRAC) [3,10,11], as part of implementing a quality management system in our laboratory [12].
Materials and Methods
Instrument and Reagents
Creatinine measurement is performed on the Abbott Architect ci-2800 analyzer. The Architect ci8200 is an automated system that can run both clinical chemistry and immunoassays on a single integrated platform. It can process up to 1,400 tests per hour (1,200 in clinical chemistry and 200 in immunoassays). With a capacity for 365 samples, including 35 priority slots, the ARCHITECT ci8200 has up to 146 refrigerated reagent positions and a miniaturized Integrated Chip Technology module for electrolyte measurement (Na+, K+, Cl–). Specimens are loaded in open primary tubes (for samples) or hemolysis tubes (for calibrators, controls, or low volumes). Jaffé reagents, calibrators, and internal controls (low, medium, and high levels) are provided by the same manufacturer.
Samples and controls
The Jaffé kinetic method is based on the reaction between creatinine and picric acid in an alkaline medium, which forms a chromogenic complex. The rate of complex formation is proportional to the creatinine concentration [4]. Although the technique is cost-effective and rapid, it can be sensitive to certain interferences (bilirubin, ascorbic acid, ketones, etc.) [5].
Serum creatinine is one of the ten most frequently requested biochemical tests in our hospital, with more than 150 determinations performed each day. Because our laboratory is adopting ISO 15189 and will soon undergo an accreditation audit, a local verification of analytical performance is mandatory. Accurate creatinine results underpin the staging of chronic kidney disease, the dosage adjustment of nephrotoxic drugs such as aminoglycosides and vancomycin, and the follow-up of kidney‑transplant recipients [6, 7]. Yet, external quality‑assessment surveys still report inter-laboratory coefficients of variation greater than 8 % for kinetic Jaffé methods [8], a degree of imprecision that can lead to clinically meaningful discrepancies [9]. For these reasons, we deemed it essential to verify the performance of the new ARCHITECT ci‑2800 analyser.
The objective of this study is to verify the analytical performance (repeatability, reproducibility, measurement uncertainty, and inter-instrument comparison) of creatinine measurement on the Architect ci-2800, following recommended protocols (SFBC, RICOS, COFRAC) [3,10,11], as part of implementing a quality management system in our laboratory [12].
Materials and Methods
Instrument and Reagents
Creatinine measurement is performed on the Abbott Architect ci-2800 analyzer. The Architect ci8200 is an automated system that can run both clinical chemistry and immunoassays on a single integrated platform. It can process up to 1,400 tests per hour (1,200 in clinical chemistry and 200 in immunoassays). With a capacity for 365 samples, including 35 priority slots, the ARCHITECT ci8200 has up to 146 refrigerated reagent positions and a miniaturized Integrated Chip Technology module for electrolyte measurement (Na+, K+, Cl–). Specimens are loaded in open primary tubes (for samples) or hemolysis tubes (for calibrators, controls, or low volumes). Jaffé reagents, calibrators, and internal controls (low, medium, and high levels) are provided by the same manufacturer.
Samples and controls
- Patient samples: Ninety fresh serum samples were randomly selected to cover three clinically relevant levels between 5.63-8.71 mg/L (n = 30), 17-25,5 mg/L (n = 30), and – 49.7-74,6 mg/L (n = 30). Specimens showing visible haemolysis, icterus, or lipaemia were excluded.
- Internal quality controls (IQC): Three levels (low, medium, high) tested for reproducibility over 30 consecutive days.
- External evaluation of quality (EEQ): Laboratory results are compared with target values to estimate bias.
Measurement Protocols
- Repeatability (Intra-Series CV): 30 consecutive measurements of the same sample for each level, on the same day, with the same operator and under identical conditions.
- Intermediate Fidelity (Inter-Series CV): A given control is measured over 30 consecutive days (or 30 series), taking into account variations in reagent lots, calibrations, operators, etc.
- Measurement uncertainty: A “IQC + EEQ” approach (Cofrac SH-GTA 14) combines intra-laboratory variability (control CV) with the bias against EEQ, to derive the expanded uncertainty U (k=2).
- Method comparison: Thirty samples are simultaneously tested on a second Architect ci-2800. Linear regression (slope, intercept), correlation coefficient (r), and average bias are computed.
Method verification and validation software
The BYG4Lab verification and validation software automatically retrieves a large volume of data and generates reports and charts for repeatability, reproducibility, method comparison, linearity limits, and uncertainty analysis. Analytical results were exported automatically from both ARCHITECT ci‑2800 instruments to BYG4Lab. Once captured, the platform performs statistical processing, generates publication-quality graphs, and delivers automated clinical interpretations. It can also produce fully customised reports, tailored to the specific requirements of clinicians, quality managers, and accreditation bodies.
No generative artificial‑intelligence tool or machine‑learning algorithm was used to clean, process or interpret the raw analytical data; all computations relied exclusively on deterministic middleware routines and classical statistical methods.
Acceptance criteria
Results are compared to the SFBC (1999 version), RICOS (total error/TE and reference CV), and manufacturer specifications. For reproducibility, for instance:
The BYG4Lab verification and validation software automatically retrieves a large volume of data and generates reports and charts for repeatability, reproducibility, method comparison, linearity limits, and uncertainty analysis. Analytical results were exported automatically from both ARCHITECT ci‑2800 instruments to BYG4Lab. Once captured, the platform performs statistical processing, generates publication-quality graphs, and delivers automated clinical interpretations. It can also produce fully customised reports, tailored to the specific requirements of clinicians, quality managers, and accreditation bodies.
No generative artificial‑intelligence tool or machine‑learning algorithm was used to clean, process or interpret the raw analytical data; all computations relied exclusively on deterministic middleware routines and classical statistical methods.
Acceptance criteria
Results are compared to the SFBC (1999 version), RICOS (total error/TE and reference CV), and manufacturer specifications. For reproducibility, for instance:
- SFBC: CV < 6% (low), 4.5% (medium), 2.4% (high)
- RICOS: TE < 8.2% (depending on concentration), CV < ~2.7%
- Manufacturer: CV < 5% (generally stated)
Results
A total of three levels were analyzed to cover a representative range of creatinine concentrations commonly encountered in clinical practice. Each level underwent tests for repeatability (intra-series) and reproducibility (inter-series), supplemented by an assessment of measurement uncertainty and a method comparison between two Architect ci-2800 systems.
Repeatability
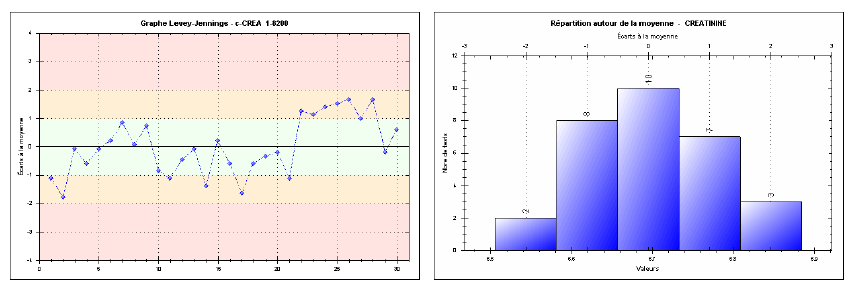
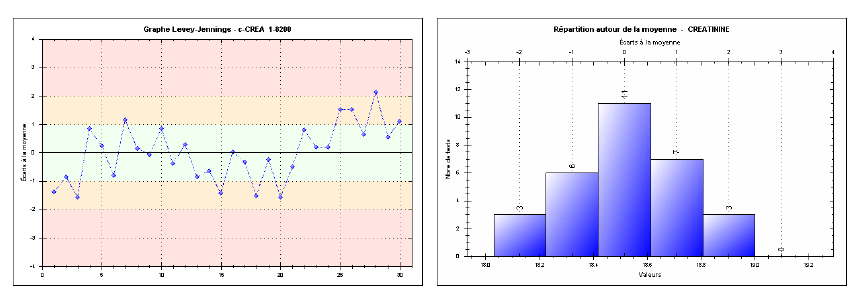
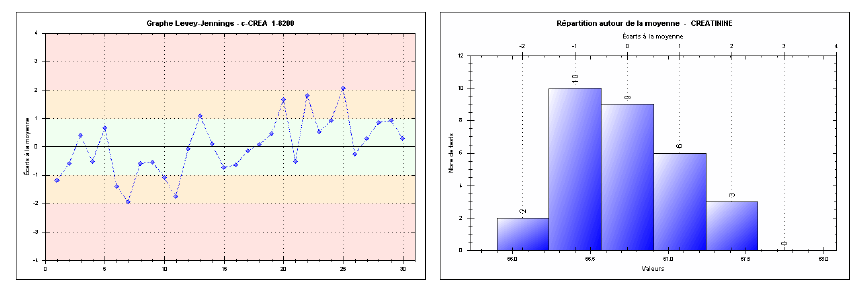
Repeatability was assessed by performing 30 consecutive measurements for each of the three levels (low, medium, high) in one series, without interruption or changes in analytical conditions. The results show coefficient of variations (CVs) of 1.13% at the low level, 1.05% at the medium level, and 0.50% at the high level. Such low CVs indicate excellent intra-series precision, fully meeting SFBC requirements and international guidelines (RICOS). For instance, at the high level, the observed CV (0.50%) is well below the SFBC threshold of 1.8%, demonstrating remarkable short-term measurement stability. Figures 1, 2, and 3 effectively illustrate the method’s precision and accuracy across the tested concentration ranges.
Reproducibility
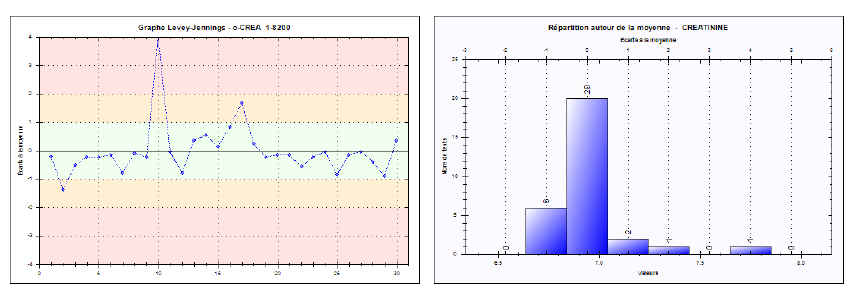
Reproducibility was evaluated by examining the spread of results over 30 consecutive days, including variations in reagent lots, calibrations, or operators. The internal controls at low (~6.94 mg/L), medium (~21.03 mg/L), and high (~61.24 mg/L) levels were measured daily, yielding inter-series CVs of 2.91%, 2.02%, and 1.75%, respectively. These results are also below the SFBC thresholds (e.g., 6% for low, 4.5% for medium, and 2.4% for high), underscoring the reliability and robustness of the Jaffé kinetic method over time. Corresponding Levey-Jennings charts (Figures 4, 5, and 6) confirm the strong reproducibility of this method.
Measurement uncertainty
Measurement uncertainty was estimated using the “CIQ + EEQ” approach, combining the laboratory’s internal dispersion (intermediate fidelity) with any difference (bias) compared to the external quality control target values. For the low level (6.94 mg/L), the expanded uncertainty U (k=2) is about 6.57%, which remains below the 8.2% RICOS criterion for total error. Likewise, the medium and high levels- at around 7.2% and 6.38%, respectively- are deemed acceptable.
Method comparison
Lastly, a method comparison between two Architect ci-2800 systems was performed using 30 patient samples spanning a broad range of creatinine values. Bland-Altman plots show that linear regression produces Y= 1.005×X – 0.365, with a correlation coefficient (r)=1.0 and an average bias of about 2.15% (Figures 7a, 7b). These values demonstrate excellent agreement between the two analyzers, confirming the method’s reliability across various operational contexts or when multiple instruments coexist in a single laboratory.

Fig. 1. Repeatability chart for the low-level creatinine control (Intra-series)

Fig. 2. Repeatability chart for the medium-level creatinine control (Intra-series)

Fig. 3. Repeatability chart for the high-level creatinine control (Intra-series)

Fig. 4. Levey-Jennings chart for the low-level creatinine control (Inter-series reproducibility)

Fig. 5. Levey-Jennings chart for the medium-level creatinine control (Inter-series reproducibility)

Fig. 6. Levey-Jennings chart for the high-level creatinine control (Inter-series reproducibility)

Fig. 7. A) Method comparison: Linear regression between two architect ci-2800 analyzers. B) Method comparison: Bland-Altman plot for creatinine results on two Architect ci-2800 analyzers
Discussion
The results highlight the robustness and reliability of the Jaffé kinetic method for creatinine measurement on the Architect ci-2800, both in terms of precision (repeatability and reproducibility) and inter-instrument comparability. The panel size (n = 90, 30 per concentration level) exceeds the minimum required by CLSI EP15‑A3 for precision and bias verification, while providing robust coverage of low, intermediate, and high values. During the repeatability evaluation, the CVs below 1.2% at all levels are particularly low, confirming outstanding short-term stability—a crucial factor in a busy routine testing environment where large numbers of samples are analyzed promptly [10, 11, 12]. Moreover, reproducibility over multiple days, factoring in a range of variations (reagent lot, calibrations, operators), consistently falls well beneath SFBC thresholds. This medium-term robustness guarantees consistent results, essential for long-term monitoring in patients with chronic renal insufficiency [10, 11, 13].
Today, measurement uncertainty is a growing concern in view of ISO 15189 requirements and the drive for better clinical interpretation. In this study, the observed measurement uncertainty remains moderate and complies with RICOS standards (≤ 8.2%). The three levels (low, medium, high) lie within a narrow range, reinforcing confidence in clinical interpretation, especially where creatinine fluctuations may be minor (early-stage renal disease, medication dose adjustments, etc.). A moderate bias compared to EEQ shows that the system’s calibration and systematic error control are well-managed [2, 14]. Regarding method comparison, the near-perfect correlation (r = 1.0) and modest average bias (~2.15%) underscore the solid agreement of results between two Architect ci-2800 instruments. This is particularly important for laboratories running multiple analyzers in parallel or seeking to standardize their equipment, as inter-system reproducibility facilitates trouble-free exchange of creatinine data—be it within the same facility or shared externally [15, 16]. Historically, the Jaffé kinetic method is recognized for its simplicity and cost-effectiveness, while also being potentially sensitive to various interferences (bilirubin, exogenous substances, etc.). However, the notably good precision recorded here suggests that any such sources of error are either well-controlled or minimally present in the patient population tested. Even so, continuous monitoring and regular review of internal and external quality control data are necessary to anticipate any drift [2, 5, 17, 18]. This verification study has several constraints. First, it was conducted in a single centre with one brand of instrument and reagent; the results, therefore, cannot be generalised to other Jaffé formulations or enzymatic assays. Second, although the panel of 90 sera covered the full medical range, we did not stratify samples by clinical diagnosis, so matrix effects linked to severe icterus, haemolysis, or paraproteinaemia may have been overlooked. Third, pass-through comparisons were limited to a second ci‑2800 analyser.
Conclusion
Overall, the data collected in this study demonstrate that Jaffé kinetic creatinine measurement on the Architect ci-2800 meets clinical requirements for monitoring patients with renal insufficiency or detecting acute renal disorders. It offers excellent precision (repeatability and reproducibility), a moderate measurement uncertainty aligning with RICOS standards, and near-perfect correlation with a second analyzer of the same model. These advantages make it a reliable solution for managing patients with renal conditions in compliance with quality guidelines (SFBC, ISO 15189). However, the laboratory must maintain continuous quality control and regularly analyze internal control and EEQ data to preempt any drift.
Ethical Considerations
According to the policy of our institution and national regulations, research that uses fully anonymised, residual specimens collected for routine care does not require prior review by an ethics committee or written informed consent.
Funding Statement
This research received no external funding.
Conflict of Interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank all the staff, medical and paramedical, for technical assistance with the ARCHITECT ci‑2800 instruments and for helpful discussions.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Authors' Contributions
SB conceived and designed the study, collected the samples, performed the laboratory analyses, and drafted the first manuscript. AA, AI, and ON collected data, and all authors critically reviewed, edited, and approved the final version. ES Supervised; CM supervised and approved the final version. All authors agree to be accountable for the work.
Today, measurement uncertainty is a growing concern in view of ISO 15189 requirements and the drive for better clinical interpretation. In this study, the observed measurement uncertainty remains moderate and complies with RICOS standards (≤ 8.2%). The three levels (low, medium, high) lie within a narrow range, reinforcing confidence in clinical interpretation, especially where creatinine fluctuations may be minor (early-stage renal disease, medication dose adjustments, etc.). A moderate bias compared to EEQ shows that the system’s calibration and systematic error control are well-managed [2, 14]. Regarding method comparison, the near-perfect correlation (r = 1.0) and modest average bias (~2.15%) underscore the solid agreement of results between two Architect ci-2800 instruments. This is particularly important for laboratories running multiple analyzers in parallel or seeking to standardize their equipment, as inter-system reproducibility facilitates trouble-free exchange of creatinine data—be it within the same facility or shared externally [15, 16]. Historically, the Jaffé kinetic method is recognized for its simplicity and cost-effectiveness, while also being potentially sensitive to various interferences (bilirubin, exogenous substances, etc.). However, the notably good precision recorded here suggests that any such sources of error are either well-controlled or minimally present in the patient population tested. Even so, continuous monitoring and regular review of internal and external quality control data are necessary to anticipate any drift [2, 5, 17, 18]. This verification study has several constraints. First, it was conducted in a single centre with one brand of instrument and reagent; the results, therefore, cannot be generalised to other Jaffé formulations or enzymatic assays. Second, although the panel of 90 sera covered the full medical range, we did not stratify samples by clinical diagnosis, so matrix effects linked to severe icterus, haemolysis, or paraproteinaemia may have been overlooked. Third, pass-through comparisons were limited to a second ci‑2800 analyser.
Conclusion
Overall, the data collected in this study demonstrate that Jaffé kinetic creatinine measurement on the Architect ci-2800 meets clinical requirements for monitoring patients with renal insufficiency or detecting acute renal disorders. It offers excellent precision (repeatability and reproducibility), a moderate measurement uncertainty aligning with RICOS standards, and near-perfect correlation with a second analyzer of the same model. These advantages make it a reliable solution for managing patients with renal conditions in compliance with quality guidelines (SFBC, ISO 15189). However, the laboratory must maintain continuous quality control and regularly analyze internal control and EEQ data to preempt any drift.
Ethical Considerations
According to the policy of our institution and national regulations, research that uses fully anonymised, residual specimens collected for routine care does not require prior review by an ethics committee or written informed consent.
Funding Statement
This research received no external funding.
Conflict of Interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank all the staff, medical and paramedical, for technical assistance with the ARCHITECT ci‑2800 instruments and for helpful discussions.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Authors' Contributions
SB conceived and designed the study, collected the samples, performed the laboratory analyses, and drafted the first manuscript. AA, AI, and ON collected data, and all authors critically reviewed, edited, and approved the final version. ES Supervised; CM supervised and approved the final version. All authors agree to be accountable for the work.
References
- Shahbaz H, Rout P, Gupta M. Creatinine clearance. [Updated 2024 Jul 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan‑. [cited 2025 Mar 5]. Available from: https://www.ncbi.nlm.nih.gov/ books/NBK544228/
- International Organization for Standardization. ISO 15189: 2022 Medical laboratories—requirements for quality and competence. Geneva: ISO; 2022.
- Comité Français d’Accréditation (COFRAC). Guide technique SH‑GTA (révision 04): Vérification et validation des méthodes en biologie médicale. Paris: Cofrac; 2024.
- Choosongsang P, Bhornsrivathanyou N, Aiadsakun P, Choosongsang P, Bodhikul A, Yamsuwan Y, et al. Glucose interference in serum and urine samples with various creatinine concentrations measured by the Jaffé kinetic method. EJIFCC 2023 8; 34(1): 57-65.
- Chaabouni Y, Miled A. Recommandations pour le dosage de la créatinine selon la méthode de Jaffé. Rev Tunis Biol Clin. 2017; 24(1): 33-6.
- Vassault A, Grafmeyer D, de Graeve J, Cohen R, Beaudonnet A, Bienvenu J. Quality specifications and allowable standards for validation of methods used in clinical biochemistry. Ann Biol Clin (Paris). 1999; 57(6): 685‑95.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024; 105(S 4S): 117‑314.
- Rybak MJ, Le J, Lodise TP. Therapeutic monitoring of vancomycin for serious Staphylococcus aureus infections: executive summary of a revised consensus guideline. Clin Infect Dis. 2020 ; 71(6): 1361‑364.
- Jeong TD, Cho EJ, Lee K, Lee W, Yun YM, Chun S, et al. Recent trends in creatinine assays in Korea: long‑term accuracy‑based proficiency testing survey data by the Korean Association of External Quality Assessment Service (2011‑2019). Ann Lab Med. 2021; 41(4): 372‑79.
- Marrington R, MacKenzie F. Understanding the limitations of your assay using EQA data with serum creatinine as an example. Clin Chem Lab Med. 2024; 62(9): 1824-834.
- Ricos C, Alvarez V, Cava F. The biological variation database [Internet]. Barcelona: Westgard QC; 2019. [cited 2025 Mar 5]. Available from: https://www.westgard.com/ biodatabase.htm
- Belmahi S, Nassiri O, Moujtahid D. Implementation of a quality control management system according to ISO 15189 in the biochemistry laboratory of Mohammed VI university hospital of Oujda. Clin Chim Acta. 2024; 558(S 1): 118688.
- Grari O, Himri A, Douzi N, Beyyoudh S, Elkhamlichi IE, Benaissa K, et al. Precision matters: repeatability and reproducibility of total PSA and homocysteine measurements on the Alinity i‑system. J Med Biochem. 2024; 43(4): 605‑609.
- Abdel Ghafar MT, El‑Masry MI. Verification of quantitative analytical methods in medical laboratories. J Med Biochem. 2021; 40(3): 225‑36.
- Comité Français d’Accréditation (COFRAC). SH‑GTA 14: Guide technique d’accréditation pour l’évaluation des incertitudes de mesure en biologie médicale. Paris: Cofrac; 2011.
- Kajeiou Z, Yacoubi L, Mokhtari I, Dahmani S, Sebbar EH, Choukri M. Verification of the analytical performance of the serum total cholesterol assay on Abbott ARCHITECT ci‑8200. World J Biol Pharm Health Sci. 2023; 15(2): 104‑13.
- Pum J. A practical guide to validation and verification of analytical methods in the clinical laboratory. Adv Clin Chem. 2019; 90: 215‑281.
- Giannoli JM, Taverna M, Ducoroy P. Recommendations for the implementation and monitoring of quality controls in medical laboratories. Ann Biol Clin (Paris). 2019; 77(5): 577‑97.
Type of Study: Research |
Subject:
Biochemistry
Received: 2025/06/6 | Accepted: 2025/08/16 | Published: 2024/10/31
Received: 2025/06/6 | Accepted: 2025/08/16 | Published: 2024/10/31
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






