Introduction
Kolanut is the fruit of the Cola species and belongs to the family of Sterculiacaea [1]. In Nigeria, varieties of kola nut are Cola acuminata (C. acuminata), Cola nitida, and Garcinia cola [2]. C. acuminata has high ceremonial values among the Igbo’s and Yoruba’s in Nigeria. Kolanut has a bitter taste when chewed and contains caffeine [2]. However, kola nut possesses medicinal and pharmacological values [3]. It is used for the treatment of whooping cough, malaria, fever, asthma, and acts as a bronchodilator because of caffeine [4, 5]. Also, kola nuts have antimicrobial [1], analeptic and lipolytic properties and can stimulate gastric juice secretion as well [6]. Traditionally, the leaves, tugs, bark, fruit follicles, and flowers were used in the treatment of dysentery, diarrhea, coughs, vomiting, and chest complaints [7]. Kola nuts have been linked with natural fertility regulation [8].Infertility is defined as the inability to achieve pregnancy after 12 months or more of unprotected sexual intercourse [9], which is a primary reproductive health concern in Nigeria [10], and women usually bear the blame. Most cases of infertility in Nigeria and Africa are due to infection [11, 12]. In males, causes of infertility are namely obstruction of the seminal tract, sexual disorder, inflammation, nutritional deficiencies, prolonged exposure to chemical, excessive heat, heavy metals, hormonal imbalance, lifestyle, abnormal sperm count or quality, and interference with spermatogenesis [13, 14]. Low levels of plasma testosterone, gonadotropins, or high levels of plasma estrogen
are among hormonal disturbances that may lead to infertility [15, 16]. Environmental estrogenic agents such as phytoestrogen diets may reduce male fertility by disrupting the normal hormonal balance in the body [17, 18, 19]. Some studies have validated the use of plants either to enhance sexual desire or to improve spermatogenesis [20, 21, 22]. Many herbs used in the traditional remedies to treat male infertility include Alframomum melegueta, Boerhaavia diffusa, Momordica charantia, Sphenocetum jolyanum, Telferia occidentalis, Phyllanthus amarus, Irvingia gabonensis, Landolphia dulci, Alliumm cepa, and Cissus populnea [23, 24].
Materials and Methods
Experimental animals
A total of 36 adult male Albino Wistar rats weighing between 200-250 g were used in this study. These animals were procured from the animal house of the department of physiology, University of Nigeria, Enugu Campus, and housed in the animal house of the college of medicine, university of Nigeria, Enugu Campus. They were allowed to acclimatize for two weeks and fed with commercially available rat feed. Ethical approval of this study was obtained from the Ethics Committee of the University of Nigeria, Nsukka.
Plant material collection
The pods of kola nut were obtained from Ukehe town, Igbo-Etiti Local Government, Enugu State, and authenticated by the Botany Department, University of Nigeria, Nsukka.
Plant aqueous extraction
2000 g of air-dried C. acuminata pods were macerated in 400 ml of distilled water, sieved with a muslin cloth, and kept in a refrigerator until required. The extractive value of the extract was determined and was given a value of 180 mg/ml.
Phytochemical screening
The phytochemical constituents of C. acuminata were determined with the method described by Trease and Evans [25] in the department of pharmacognosy, faculty of pharmaceutical sciences, university of Nigeria, Nsukka.
Proximate analysis
The method described by Pearson [26] was used to determine the percent of crude protein, moisture, crude fiber, fat, carbohydrate, and ash content in the pods of C. acuminata. The method of AOAC [27] was used to determine the mineral contents in the plant material. Vitamin E was also estimated using the technique of Emmerie and Engel [28].
Preliminary acute toxicity testing
The acute toxicity testing (LD50 determination) was conducted using the variation method of Lorke [29] with slight modification. Graded doses of 10, 100, 1000, 2000, 5000 mg/kg body weight (b.wt.) were administered to groups A, B, C, D, and E, respectively of 4 rats. Each animal was given a single oral dose of Cola acuminata pod extract (CAPE) after 24 hours quickly in the respective groups. After the drug administration, clinical observations were done hourly for 24 hours to record mortality or clinical signs of toxicity.
Experimental design
The animals were divided into groups (I, II, and III), including 12 rats. Each group comprised three batches (A, B, and C) with the following respective duration of treatment 4, 6, and 8 weeks of 4 rats each. The choice of up to 8 weeks of administration of the plant material intended to cover the spermatogenic cycle in rats.
The treatments were as follows:
Group I: This group served as the control group and would receive water by oral gavage.
Group II: Rats were treated with 400 mg/kg b.wt. of CAPE.
Group III: Rats were treated with 800 mg/kg b.wt. of CAPE.
All treatments were administered by oral gavage via an oral cannula daily for 4, 6, and 8 weeks for the individual batches (A, B, and C) in the three groups. The animals were at the end of each treatment period when sacrificed under chloroform anesthesia and one of the testes and its associated cauda epididymis, seminal vesicle and vas deferens were excised and preserved in 10% formal saline for further histological processing with light microscopy. The other cauda epididymis was removed for further sperm parameter analyses.
Epididymal sperm count and motility
Several incisions (1 mm) were made on other excised cauda epididymis, which was suspended in 1 ml of Ham’s F-10 solution (Sigma Aldrich Chemical Co., U.S.A.). After 10 minutes of incubation at 37°C, sperm concentration and motility were determined by hemocytometer method [30].
Histological processing
The tissues were cut up into smaller pieces (about 3 mm thick), then they were processed with the automatic tissue processor and sectioned at 5µm using the Rotary Microtome (Heitz 150 Rotary Microtome, Cambridge model). Sections were stained according to Hematoxylin and Eosin technique by Baker et al. [31].
Microscopy and photomicrography
The sections were examined using an Olympus Binocular Microscope with an in-built lighting system. The sections were photographed using a digital microscope camera (Hewlett Packard® attached to an eyepiece of an Olympus Binocular Microscope.
Testosterone assay
Blood samples were obtained from the retro-orbital sinus of the rats in each study group before the sacrifice. They were spun at 2500 revolution per minute for 10 minutes in an angle-head desktop centrifuge at room temperature. Subsequently, serum samples obtained were assayed for testosterone in batches with the control sera at both physiological and pathological levels by standard quantitative enzyme-linked immunosorbent assay technique with microwell kits from Syntro Bioresearch Inc. California, U.S.A.
Statistical analysis
Data obtained were analyzed using the SPSS software. All data were expressed, where appropriate, as Mean±Standard error of the mean. The difference between the mean scores was determined with the student’s t-test and one-way analysis of variance (ANOVA). Finally, the results were considered significant at p<0.05.
Results
Acute toxicity testing
No toxic symptoms or mortality were observed in the treated animals, which lived up to 14 days after administering CAPE at a single dose level of 5000 mg/kg of body weight. The behavioral patterns of animals were observed first for 2 hours, followed by 6 hours and then 14 hours after the administration and the animals in all treated groups were healthy and did not display significant changes in behavior, skin effects, breathing, impairment in food intake and water consumption, postural abnormalities and hair loss.
Phytochemical screening
Table 1 shows the result of the phytochemical screening of CAPE. The plant material contains an abundant amount of saponins, a moderate amount of proteins, the presence of terpenoids, tannins, and resins, trace amounts of flavonoids, alkaloids, reducing sugars, fats, and oil, steroids and glycosides.
Proximate analysis
The result of the proximate composition of the dried C. acuminata pods is displayed in Table 2. It showed lower amounts of protein (2.14%) and fat (1.84%) but a larger amount of carbohydrates (24.54%). The crude fiber content was found to be 35.18% and an ash content of 20.20%.
Vitamin E determination
A value of 0.511 mg/L was obtained from Vitamin E determination of the pods.
Cauda epididymal sperm count and motility
Daily oral administration of the CAPE for 4 and 6 weeks slightly increased the epididymal sperm counts in CAPE-treated groups (Fig. 1), but this change was not statistically significant (p>0.05) when compared with the values obtained in the control group (Table 3). However, after 8 weeks of CAPE treatment, a significant reduction (p<0.05) was observed in the treatment groups compared with their corresponding control group. The results of the sperm motility analysis revealed no significant change after 4 and 6 weeks of treatment with CAPE (Fig. 2). Nevertheless, there was a significant reduction (p<0.05) in the treated groups when compared with the corresponding control group after 8 weeks, as shown in Table 3.
Serum testosterone levels
A significant decrease (p<0.05) in the mean testosterone levels were observed in rats treated with 400 mg/kg CAPE (groups II) and 800 mg/kg CAPE (group III) at 6 weeks and 8 weeks, respectively when compared with the corresponding control group (Table 4 and Fig. 3).
Histological findings
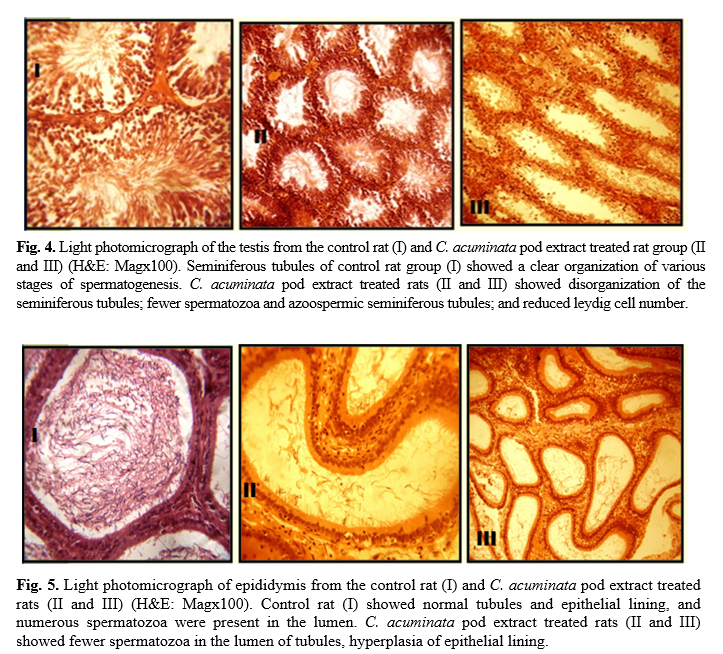
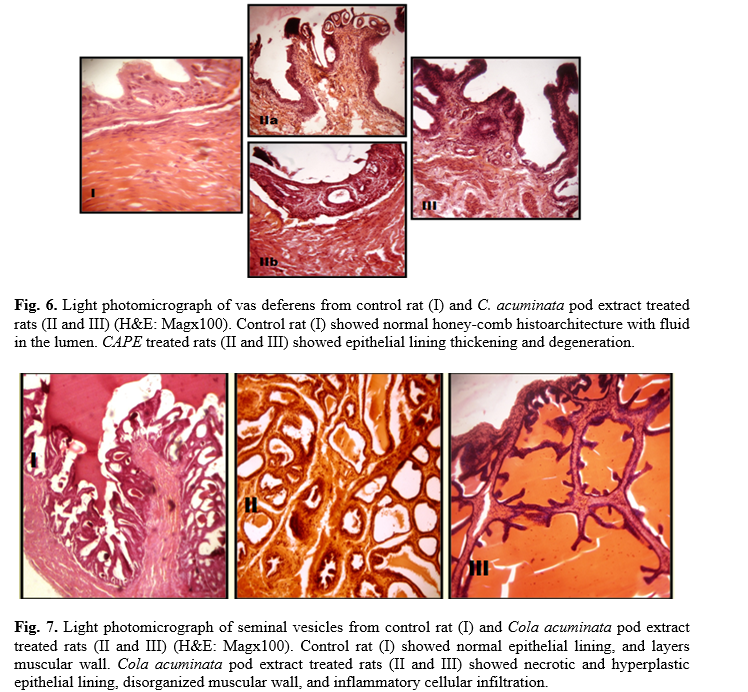
Administration of 400 mg/kg and 800 mg/kg CAPE for 6 and 8 weeks caused visible lesions upon microscopical evaluation within the seminiferous tubules (Fig. 4), epididymis (Fig. 5), vas deferens (Fig. 6) and seminal vesicles (Fig. 7) of treated rats. A summary of the histological findings in these tissues is provided in table 5.
Table 1. Phystochemical constituents of C. acuminata pods
| Constituents |
Inference |
| Flavonoids |
Trace amounts (-) |
| Alkaloids |
Trace amounts (-) |
| Saponins |
Abundant amounts (+++) |
| Tannins |
Present (+) |
| Resins |
Present (+) |
| Proteins |
Moderate amounts (++) |
| Reducing Sugars |
Trace amounts (-) |
| Fats and oil |
Trace amounts (-) |
| Steroids |
Trace amounts (-) |
| Terpenoids |
Present (+) |
| Glycosides |
Trace amounts (-) |
| Acidic compounds |
Neutral |
Table 2. Proximate composition of dried C. acuminata pods
| Sample Identification |
Parameters (%) |
| Kola Nut Pod |
Moisture |
Ash |
Crude Fibre |
Protein |
Fat |
Carbohy-Drate |
| 16.10 |
20.20 |
35.18 |
2.14 |
1.84 |
24.54 |
| Group |
Dose of CAPE mg/kg body weight |
4 Weeks |
6 Weeks |
8 Weeks |
|
| Motility (%) |
Counts (x106/ml) |
Motility (%) |
Counts (x106/ml) |
Motility (%) |
Counts (x106/ml) |
|
| I (Control) |
0 |
78.25±2.32 |
64.25±6.26 |
80.00±2.32 |
61.50±23.29 |
78.25±1.55 |
71.75±4.09 |
| II |
400 |
74.75±2.95 |
98.20±14.07 |
75.75±2.17 |
81.50±15.13 |
48.75±1.75* |
18.50±3.12* |
| III |
800 |
74.50±3.30 |
76.00±9.11 |
71.75±3.35 |
106.25±16.75 |
28.25±6.43* |
20.25±7.15* |
|
|
|
|
|
|
|
|
|
|
|
* P<0.05; CAPE=
C. acuminata pod extract
Table 4. Effects of CAPE extract on serum testosterone levels in male albino rats
| Group |
Dose of CAPE mg/kg
body weight |
Serum testosterone levels (ng/ml) |
| |
|
4 weeks |
6 weeks |
8 weeks |
| I (Control) |
0 |
1.95±0.34 |
3.52±1.56 |
1.10±0.12 |
| II |
400 |
5.75±3.18 |
0.95±0.17b |
0.75±0.10 |
| III |
800 |
2.92±0.90 |
2.27±0.31 |
0.60±0.04a |
|
|
|
|
|
|
a: Statistically significant; and b: Significant when compared with the control group (I) and high dose group (III), respectively; CAPE=
C. acuminata pod extract
Discussion
This study demonstrates the effects of daily oral administration of CAPE for 8 weeks on the reproductive system of male albino Wistar rats. Preliminary acute toxicity testing of CAPE in this study revealed an oral LD50 of >5000 mg/kg body weight in rats. Hence, CAPE would be considered safe, according to Organisation for Economic Cooperation and Development (OECD) standards [32].
Preliminary phytochemical screening of CAPE in this study demonstrated the abundant amount of saponins, the moderate amount of proteins, and the presence of terpenoids, tannins, and resins, and the trace amount of flavonoids, alkaloids, reducing sugars, fats, oil, steroids, and glycosides. The observed effects in this study can be a direct or indirect action of a single or combination of the phytochemical principles.
However, the extract in Nigerian folklore medicine is used to improve sperm count in sub-fertile males [33]. On the contrary, the present study has shown that oral delivery of CAPE over 8 weeks results in a significant decrease in cauda epididymis sperm count and motility. Testis and epididymis undergo histoarchitectural changes. Besides, microscopical evaluation reveals that spermatogenic cells and sperm production are reduced. Determination of sperm count, degree of motility, and viability are parameters used to assess sperm quality and quantity [30].
Caudal epididymal sperm count and motility were significantly reduced after treatment with CAPE and thus may have some implications for fertility, which correlates with the work of Aprioku and Clement, who reported a significant decrease in sperm count and motility [34]. The decline in sperm count may suggest that CAPE interferes with spermatogenesis and may also interferes with seminiferous epithelium and metabolic processes associated with sperm motility. So, it may result in a decrease in sperm motility [35]. Orisakwe et al. documented that drugs or chemicals that affect testicular function can impair the quantity and quality of spermatozoa [36]. The marginal increase in sperm counts in both treatment groups at 6 weeks may be attributed to increased output of spermatozoa from the testis to the epididymis for storage and maturation, rather than increased spermatogenesis. Its effects on spermatogenesis are observed between 53-60 days of post-treatment in an experiment, which is equivalent to one spermatogenic cycle in rats at which the testicular results would be interpreted to epididymal events [37]. Thus, the reduced sperm count at 8 weeks (56 days) can be the resultant effect of CAPE on spermatogenesis.
Vitamin E, a fat-soluble antioxidant, helps to improve the quality and quantity of spermatozoa [38]. Insufficient vitamins and low natural antioxidant intake can cause deleterious effects on spermatogenesis and producing healthy sperm, while sufficient consumption can protect sperm DNA from oxidative stress leading to the improvement of male fertility [39]. However, the amount of vitamin E in CAPE (0.511mg/l) can be considered low to exert the fertility benefits. Abbas and Luma reported that vitamin E had a protective effect on the reproductive system of male rats [40]. Also, Sukmawati et al. reported that vitamin E had an ameliorative effect on testicular histological features of allethrin treated rats [41].
The serum testosterone levels decreased marginally and significantly at 8 weeks in groups B and C when compared with their respective control groups. It may be inferred that CAPE exerts antiandrogenic effects in male rats, and this may be through the hypothalamic-pituitary –axis or by a direct effect on the testis. Thus, the observed testosterone deficiency in the present study may also explain the significantly reduced sperm characteristics. This finding is similar to the Aprioku, and Clement, who reported a reduction in serum testosterone concentration at 50 and 100 mg/kg extract exposed rats [34]. Similarly, Oyedeji et al. reported that aqueous extract of Cola nitida caused a significant decrease in testosterone levels of male albino rats [42].
The extent of spermatogenesis is determined by intratesticular testosterone concentration and not the serum peripheral levels [43]. It suggests that there may be changes in the rate of spermatogenesis when peripheral testosterone levels are unaffected, which may be via some mechanism that obstructs the availability of the hormone at the testicular level to impair spermatogenesis. Previous studies have shown that plant extracts can deplete testicular testosterone while sparing the peripheral testosterone levels to a large extent [44, 45].
Histologically, the study of the various reproductive organs (testis, epididymis, seminal vesicles, and vas deferens) revealed multiple degrees of derangement, a disturbance in spermatogenic cells and few or no spermatozoa in the seminiferous tubules of the testes. The reduced Leydig cells population observed would account for the reduced number of primary spermatocytes. Interstitial cells of the leydig are known to secrete the testicular testosterone necessary for the division of germ cells [46]. Thus, it suggests that CAPE contains antispermatogenic agents. Some plant extracts such as Mondia whitei, Eurycoma longifolia, Tinospora cordifolia, Leptadenia hastate have been observed to have a direct effect on spermatogenesis [47 -49].
The microscopical examination of the accessory organs showed some evidence of hyperplasia. These effects may be attributed to direct action of CAPE principles or by the depleted levels of androgens. Circulating androgens help to maintain a healthy reproductive accessory organ structure and its function [50]. The derangement in the histoarchitecture of these organs would alter their function. Besides, the impaired function of the reproductive organs has been shown to decrease sperm motility [51, 52]. These findings are also similar to the research carried out by Aprioku and Clement, who reported dose-dependent histological changes in the testis of rats treated with C. acuminata seed extract [34].
Conclusion
C. acuminata pod causes marked alterations in reproductive organs and has antispermatogenic and antiandrogenic effects when administered orally over 8 weeks in mature male rats, thus contradicting its use as a traditional remedy for low sperm count in males.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
Our gratitude goes to Barr. V.C Odo and all those who contributed to the success of this research and presentation of this manuscript.
References
- Sonibare MA, Soladoye MO, Esan OO, Sonibare OO. Phytochemical and antimicrobial studies of four species of Cola Schott and Endl. (Sterculiaceae). Afri J Tradit Complem Altern Med. 2009; 6(4): 518-25.
- Adeniyi A, Olufunmilayo AD, Akinnuoye AG. Comparative study of the proximate and fatty acid profiles of cola nitida, cola acuminata and garcinia kola. Am J Food Sci Nutr. 2017; 4(6): 80-4.
- Adebayo SA, Oladele OI. Medicinal values of kolanut in nigeria: implication for extension service delivery. Life Sci. 2012; 9(2): 887-91.
- Jayeola CO. Preliminary studies on the use of kolanuts (cola nitida) for soft drink production. J Food Technol Afri. 2001; 6 (1): 25-6.
- Kim K. Encyclopedia of Alternative Medicine. 2001; 22(8): 203-204.
- Germplasm resources information network (GRIN). Germplasm resources Information Network. Beltsville, Maryland. 2007.
- Bukola FT. Conversion of Kola nut waste into beneficial products for environmental protection. J Environ Sci Technol. 2018; 11(9): 233-37.
- Tachie-Obeng E, Brown K. Cola nitida and Cola acuminata. A state of knowledge report undertaken for the central African regional program for the environment. Supported by the biodiversity support program, a consortium of world wildlife fund, the nature conservancy and the world resources institute. 2001; p. 1-36.
- World Health Organization. Infertility definitions and terminology. 2019; https://www.who.int/ reproductivehealth/topics/infertility/definitions/en/ [Accessed 4/1/2020]
- Okonofua F. The case against new reproductive technologies in developing countries. Bri J Obstet Gynaecol. 1996; 103(10): 957-62.
- Akinloye O, Truter, EJ. A review of management of infertility in Nigeria: framing the ethics of a national health policy. Int J Women’s Health. 2011; 3(2): 265-75.
- Odunvbun W, Oziga D, Oyeye L, Ojeogwu C. Pattern of infertility among infertile couple in a secondary health facility in Delta State, South Nigeria. Trop J Obstet Gynaecol. 2018; 35: 244-248.
- Mahat RK, Arora M, Bhale DV, Holkar S, Kumar S, Yadav, T. Risk factors and causes of male infertility-a review. Biochem Anal Biochem. 2016; 5(2): 271-79.
- Sharma A. Male infertility; evidences, risk factors, causes, diagnosis and management in human. Ann Clin Lab Res. 2017; 5(3): 188.
- Wahab NA, Mokhtar NM, Halim, WN, Das S. The effect of eurycoma longifolia Jack on spermatogenesis in estrogen-treated rats. Clinics 2010; 65(1), 93-8.
- Duca Y, Aversa A, Condorelli RA, Calogero AE, La Vignera S. Substance abuse and male hypogonadism. J Clin Med. 2019; 8 (5): 732.
- Cederroth CR, Zimmermann C, Beny JL, Schaad O, Combepine C, Descombes P, et al. Potential detrimental effects of a phytoestrogen-rich diet on male fertility in mice. Mol Cell Endocrinol. 2010; 321(2): 152-60.
- Jefferson WN, Patisaul HB, Williams CJ. Reproductive consequences of developmental phytoestrogen exposure. Reprod. 2012; 143(3), 247-60.
- Yuan G, Liu Y, Liu G, Wei L, Wen Y, Huang S, et al. Associations between semen phytoestrogens concentrations and semen quality in Chinese men. Environ Int. 2019; 129(1): 136-44.
- Kotta S, Ansari SH, Ali J. Exploring scientifically proven herbal aphrodisiacs. Pharmacogn Rev. 2013; 7(13): 1–10.
- Chauhan NS, Sharma V, Dixit VK, Thakur M. A review on plants used for improvement of sexual performance and virility. Biomed Res Int. 2014; 2014(1): 1-19.
- Erhabor JO, Idu M. Aphrodisiac potentials of the ethanol extract of Aloe barbadensis Mill. root in male Wistar rats. BMC complement Altern Med. 2017; 17(360): 1-10.
- Kamatenesi-Mugisha M, Oryem-Origa H. Traditional herbal remedies used in the management of sexual impotence and erectile dysfunction in western Uganda. Afri Health Sci. 2005; 5(1): 40-9.
- Okoli RI, Aigbe O, Ohaji-Obodo JO, Mensah JK. Medicinal herbs used for managing some common ailments among Esan people of Edo State, Nigeria. Pak J Nutr. 2007; 6(5): 490-96.
- Trease, GE, Evans WC. Textbook of Pharmacognosy.12th ed, London: Bailliere Tindall and Company Publisher, 1989; p. 343-83.
- Pearson D. Chemical analysis of foods. 7th ed, London: Church Hill Livingstone, 1976; Pp. 72-73,138-143
- AOAC. Official Methods of Analysis, Association of Official Analytical Chemists 15th ed, Washington D.C. 1990; p. 123.
- Emmerie A, Engel C. Colorimetric determination of tocopherol (Vitamin E). Rec Trav Chim Pays Bas. 1939; 58(4): 283-89.
- Lork D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983; 54: 275-287.
- World Health Organization. WHO Laboratory Manual for Examination of Human Semen and Sperm-Cervical Mucus Interaction, 4th ed. New York: Cambridge Press. 1999.
- Baker FJ, Silverton RE, Pallister CJ. Introduction to medical laboratory technology, 7th ed, Butterworth Heinemann. 1985; p. 229-30.
- OECD. Guidelines for the testing of chemicals. Organization for economic cooperation and development, Paris. 1981.
- Sadiq D, Ezi-Ashi T, Onuaguluchi G. Checklist of medicinal plants of nigeria and their uses. Enugu, Nigeria: Jamoe and Trinity-Biz Publishers, 2015; p. 87-88.
- Aprioku JS, Clement EO. Subchronic Cola acuminata seed exposure: Effects on body weight and male reproductive parameters in rats. J Reprod Infertil. 2018; 9(1): 20-7
- Aprioku JS, Kari FE. Spermatic effects of short-term administration of aqueous cola acuminata seed extract in wistar albino rats Eur J Biomed Pharm Sci. 2018; 5(2): 998-1002.
- Orisakwe OE, Obi E, Udemezue OO. Effect of halofantrin on testicular architecture and testosterone level in guinea pigs. Eur Bull Drug Res. 2003; 11(4) :105-109
- Mishra RK, Singh SK. Reversible antifertility effect of aqueous rhizome extract of Curcuma longa L. in male laboratory mice. Contraception 2009; 79(1): 479-87.
- Saddein E, Haghpanah T, Nematollahi-Mahani SN, Seyedi F, Ezzatabadipou M. Preventative effects of vitamin e on testicular damage and sperm parameters in the first-generation mice pups due to pre- and postnatal mancozeb exposure. J Toxicol. 2019; 2019(1): 1-12.
- Hala MA. Protective effect of Nigella sativa, linseed and celery oils against testicular toxicity induced by sodium valproate in male rats. J Am Sci. 2011; 7(5): 687-693.
- Abbas SM, Luma WK. The protective impact of vitamin E against atenolol effect on reproductive efficiency in male rats. Adv Anim Vet Sci. 2017; 5(3): 133-38.
- Sukmawati Y, Arisanty D, Tofrizal A, Amir A. Vitamin E ameliorates testicular histological features and androgen binding protein levels in testicle of rats induced by allethrin. J Adv Vet Anim Res. 2019; 6(4):486-91.
- Oyedeji KO, Bolarinwa AF, Adedeji AA. Effect of aqueous extract of Cola nitida (Kola nut) on reproductive functions in male Albino rats. Res J Med Sci. 2012; 6(1): 281-85
- Murray R, Mayes P, Granner K, Rodwell V. Harper’s Biochemistry. 22nd ed, East Norwalk: A publishing Division of Prentice Hall. 1990; p. 516-29.
- Sarkar M, Gangopadhyay P, Basak B, Chakrabarty K, Banerji J, Adhikary P, et al. The reversible antifertility effect of Piper betle Linn. on Swiss albino male mice. Contraception 2000; 62(5): 271-74.
- Oze G, Nwanjo H, Oze R, Akubugwo E, Orisakwe E, Aka P. Reproductive impairment associated with the ethanolic extract of alstonia boonei (De-Wild) stem bark in male rats. Internet J Third World Med. 2006; 6(1): 1-6.
- Orlu EE, Ogbalu OK. Partial inhibitory effect of ethanol extract of Lepidagathis alopecuroides (Vahl) on spermatogenesis in Sprague-Dawley rats. J Anim Vet Adv. 2012; 4(3): 214-20.
- Watcho P, Zelefack F, Nguelefack TB, Ngouela S, Telefo PB, Kamtchouing P, et al. Effects of the aqueous and hexane extracts of mondia whitei on the sexual behaviour and some fertility parameters of sexually inexperienced male rats. Afri J Trad Complem Altern Med. 2007; 4(1): 37-46
- Gupta RS, Sharma A Antifertility effect of Tinospora cordifolia (Willd.) stem extract in male rats. Indian J Exp Biol. 2003; 41(8): 885-89.
- Bayala B, Telefo PB, Bassole IHN, Tamboura HH, Belemtougri RG, Sawadogo L, et al. Antispermatogenic activity of leptadenia hastata (Pers.) decne leaf stems aqueous extracts in male wistar rats. J Pharm Toxicol. 2011; 6(1): 391-99.
- Gurung P, Yetiskul E, Jialal I. Physiology, male reproductive system. [Updated 2020 May 29]. In: StatPearls [Internet]. Treasure Island (F.L.): StatPearls Publishing; 2020; https://www.ncbi. nlm.nih.gov/books/NBK538429/. [Cited 20/6/2020]
- Abarikwu SO. Causes and risk factors for male-factor infertility in Nigeria: a review. Afr J Reprod Health. 2013; 17(4): 150-66.
- Temidayo SO, Stefan SD. Diabetes mellitus and male infertility. Asian Pac J Reprod. 2018; 7(5):6-14.









































 , Nkiruka C Azubuike
, Nkiruka C Azubuike 
 , Peter U Achukwu
, Peter U Achukwu 
 , Michael C Ugwu
, Michael C Ugwu 
 , Chisom H Udeogu
, Chisom H Udeogu 
 , Ozoemena C Ike
, Ozoemena C Ike