Sun, Feb 22, 2026
[Archive]
Volume 6, Issue 1 (February 2019)
IJML 2019, 6(1): 33-42 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Arbabi M, Soleimani Jevinani S, Nahrevanian H, Hooshyar H, Esmaeili Rastaghi A R, Delavari M et al . The Effects of Splenectomy on Pattern of Nitric Oxide Induction and Pathogenesis of Rodent Malaria Caused by Plasmodium berghei Infection
. IJML 2019; 6 (1) :33-42
URL: http://ijml.ssu.ac.ir/article-1-241-en.html
URL: http://ijml.ssu.ac.ir/article-1-241-en.html
Mohsen Arbabi 

 , Sara Soleimani Jevinani *
, Sara Soleimani Jevinani * 

 , Hossein Nahrevanian
, Hossein Nahrevanian 

 , Hossein Hooshyar
, Hossein Hooshyar 

 , Ahmad Reza Esmaeili Rastaghi
, Ahmad Reza Esmaeili Rastaghi 

 , Mahdi Delavari
, Mahdi Delavari 

 , Fatemeh Sadat Ghasemi
, Fatemeh Sadat Ghasemi 




 , Sara Soleimani Jevinani *
, Sara Soleimani Jevinani * 

 , Hossein Nahrevanian
, Hossein Nahrevanian 

 , Hossein Hooshyar
, Hossein Hooshyar 

 , Ahmad Reza Esmaeili Rastaghi
, Ahmad Reza Esmaeili Rastaghi 

 , Mahdi Delavari
, Mahdi Delavari 

 , Fatemeh Sadat Ghasemi
, Fatemeh Sadat Ghasemi 


Department of Medical Parasitology, Faculty of Medicine, Kashan University of Medical Sciences, Kashan, Iran.
Full-Text [PDF 554 kb]
(950 Downloads)
| Abstract (HTML) (2827 Views)
Discussion
References
Full-Text: (1355 Views)
Introduction
Malaria is a vector-borne infectious disease caused by parasitic protozoa of the genus Plasmodium. The parasites are spread through the bites of infected Anopheles mosquitoes. Approximately 3.2 billion people are at the risk of malaria. In 95 countries, there were about 214 million cases of malaria and an estimated 438,000 deaths in 2015. The majority of the cases occur in children under 15 years old [1, 2]. The disease is widespread in the tropical and subtropical areas including Sub-Saharan Africa, Asia, and Latin America [2, 3]. Malaria is commonly associated with poverty and has a major negative effect on economic development, including loss of budget due to heal thcare costs, loss of working ability, and some effects on tourism [4-6].
Rodent malaria parasites are applied extensively as the study models of human malaria [7, 8]. Four different species that infect African rodents have been adapted for laboratory use: Plasmodium berghei (P. berghei), P. yoelii, P. chabaudi and P. vinckei. Small differences exist in the biology of them in laboratory mice and this makes these species appealing models for investigating different aspects of human malaria, including antigenic variation, drug resistance and immune evasion [9, 10]. P. berghei preferably invades reticulocytes and usually produces infections in mice, hence inducing severe pathology [8].
The normal function of the spleen is to remove abnormal erythrocytes and intra-erythrocytic inclusions. Malaria infected red blood cells (RBCs) containing an increasingly large and rigid parasites [11, 12]. The parasitized RBCs (PRBC) adheres to the vascular endothelium and thereby avoid splenic removal. Damage to the malaria parasite as a result of either drug treatment or host defense mechanisms leads to parasite clearance. Splenectomy frequently delayed morbidity of the infection, suggesting an active role of the spleen in the generation of an immunopathological reaction during a primary infection in the intact animal [11, 13].
Nitric oxide (NO) has been identified as a major effector molecule during the majority of parasitic infections. It seems that NO is not the only antiparasitic agent for immunological activity against parasites [14]. There is evidence that the activated macrophages are able to eradicate intracellular Leishmania major, Toxoplasma gondii and extracellular Schistosoma sp. parasites by the release of NO [15]. The production of NO and its derivatives has been reported in the majority of parasitic infections, including Giardia lamblia, Entamoeba coli [16], Leishmania amazonensis [17], Toxoplasma gondii [18], Leishmania mexicana [19], Schistosoma mansoni [20], Opisthorchis viverrini [21] and Clonorchis sinensis [22].
Inducible NO (iNOS) is an antipathogen and tumoricidal agent. However, its production requires a tight control because of cytotoxic and immune modulation activity [23, 24]. Several parasites are highly sensitive to NO and their derivatives; however, some of the parasites represent a moderate sensitivity to the production of toxic molecules [25].
Interestingly, hemo-globin, myoglobin, and neuroglobin may protect intracellular protozoa from the antiparasitic effects of NO [26]. Despite the wide evidence about anti-protozoal effects of NO, little efforts have been made to develop NO-based drugs in human medicine and to avoid toxic effects against non-target host cells [27]. The main control of malaria is achieved by NO-mediated mechanisms. The protection from cerebral malaria in African children is linked with iNOS [24]. There is conflicting evidence regarding the role of NO in the process of resistance against malaria parasites. Schizonts treated in vitro with NO donors caused a delayed infection to mice in a dose and time-dependent manner, which suggests an inhibitory role for NO [28] with influence on parasitemia and survival of P. berghei in infected mice or rats [29, 30]. Moreover, severe malaria is associated with reduced NO production [31] and iNOS variants in regions of differing disease manifestation [32]. Low NO bioavailability might contribute to pathologic activation of the immune system [33] and to the experimental cerebral malaria [34]. The mechanism of NO action on malaria, explains the existence of its molecules in the food vacuole which is a critical parasitic compartment involved in hemoglobin degradation, heme detoxification and a target for antimalarial drug action [35, 36]; therefore hemoglobin protects Plasmodium parasites from oxygen radicals [37]. The aim of this study was to investigate the effect of splenectomy on NO induction and its role in the pathogenesis of rodent malaria caused by P. berghei infection in outbred NMRI mice.
Materials and Methods
Animals and groups
Thirty female outbred NMRI mice (supplied with the Karaj Laboratory Animal Unit, Pasteur Institute of Iran), 4 to 6 weeks old and weighed between 20-22 gr were used in this study. The mice were housed at room temperature (20-23°C) on a 12-h light and 12 h dark cycle, with unlimited access to food and tap water. Experiments with animals were conducted according to the ethical standards formulated in the Declaration of Helsinki, and measures were taken to protect animals from pain or discomfort. It was approved by the institutional ethical review board (Ethical Committee of the Pasteur Institute of Iran), where the antimalarial test was performed.
The animals were divided into four groups of five and the procedures were performed by each group as described below:
Group I: No intervention (healthy control)
Group II: with splenectomy (healthy test)
Group III: No intervention, Inoculation of contaminated blood (infected control)
Group IV: With splenectomy, inoculation of contaminated blood (infected test)
Surgical procedures
Twenty four hours after sub-inoculation of 106 PRBC into clean mice, groups II, and IV underwent surgery .Ether (diethyl ether; MERCK, Germany) induction was attained by placing the mouse in a bell jar containing cotton wool soaked in ether, and the maintenance of anesthesia during the operation was effected by Tarin & Sturdee's (1972( method. This was accomplished with anti-sepsis and sterilized, median laparotomy and spleen exposure. In order to increase mice survival animals during surgery, they were taken from the anesthesia after a few seconds. In animals, the ligation of the main pedicle and of the main spleen-gastric vessel was held and the organ was subject to total ablation. At the end of the surgery, the abdominal wall was closed. The mice received povidone iodine antiseptic and sterile dermal ointment tetracycline on the sutured area. The animals were then placed in the recovery room and incandescent surgical lamps were used to raise the temperature to around 30ºC during post-anesthetic recovery. Each operation lasted at least 10 minutes.
Malaria parasites
P. berghei NY, kindly donated by the School of Life Sciences, University of Manchester, UK. Malaria parasite was maintained by blood passage in NMRI mice when active parasites were required; otherwise it was stored at -70°C in Alserver’s solution (2.33 % glucose, 0.52 % NaCl and 1.00% sodium citrate in deionized water) and glycerol (9:1 parts by volume). The parasite was maintained at the Department of Parasitology, Pasteur Institute of Iran in Tehran.
Inoculation of malaria parasites
Mice were inoculated (0.2 ml) intravenously into a tail vein with blood from a donor mouse (41% parasitaemia P. berghei) diluted with 0.85% saline to contain 2×106 PRBC.
Assessment of pathology
Evaluation of the parasitemia level
The evolution of the parasitemia level in the mice was determined from thin smears made from the tail blood during 16 consecutive days. After taking the smear, it was fixed with 100% methanol and stained with Giemsa solution (Sigma Chemical Co., USA). The hemoscopic examination was performed using an optical microscope, with 1000×magnification. PRBCs were counted in five different fields, each having approximately 200 cells. Results are expressed as the mean percentage (%) of erythrocytes containing Giemsa positive bodies. Experiments were licensed under the Animals (Scientific Procedures) Act 1986. In compliance with the conditions of this license, infected animals were humanely killed at the onset of the terminal phase of malaria (P. berghei NY) infection.
Calculation of parameters
Assessment of degree of hepatosplenomegaly
Entire livers and spleens were removed post-mortem at the end of the experimental period from mice after induction of terminal general anesthesia by inhalation of diethyl ether (Sigma Co., Germany). Organs wet weight were measured and compared with controls as indices for the degrees of hepatomegaly and splenomegaly. Body weight was measured initially and at different times of experiments using a top pan balance (OHAUS Scale Corp., USA).
Preparation of tissue homogenates for Reactive Nitrogen Intermediates (RNI) assay
Mice were terminally anesthetized by inhalation of diethyl ether (BDH, UK) 1 h after determination of parasitemia and humanely sacrificed by cervical dislocation. After blood taking, the whole livers and spleens were removed post-mortem and weighed. Tissues were placed in separate 1.5 mL microfuge tubes and homogenized in ice-cold deionized water (d.H2O; 0.1 g wet tissue/mL) using an electrical homogenizer (Model RS541-242; RS Components, UK). Homogenates were centrifuged at 5500 g (Model 1-13 Micro-centrifuge; Sigma Co., UK) for 15 min.
Griess micro assay
NO were measured as the concentration of nitrites and nitrates using the Griess reaction adapted with modifications from Rockett et al. as described fully elsewhere [38]. Nitrate standard was prepared in experimental fluids and finally were values corrected for assay losses [35]. Briefly, 60 µL samples were treated with 200 µL Griess reagent (5% phosphoric acid, 1% sulfanilic acid and 0.1% N [1-naphthyl-1]-ethylenediamine dihydrochloride) [all from Sigma] dissolved in 100 mL d. H2O was added and proteins were subsequently precipitated by 200 µL trichloroacetic acid 10%. Tube contents were vortex mixed and then centrifuged (Eppendorf centrifuge 5415 C, Germany). Duplicate samples of supernatants were transferred to a 96-well flat-bottomed microplate (Costar, USA) and absorbances read at 520 nm using a microplate reader (Bio-TEK, power wave XS, USA). Values for the concentration of nitrate were calculated from standard calibration plot. Values are presented as mean±SEM μM/g wet weight for tissue and μM/ml for plasma.
Statistical analysis
Values are presented as the mean±standard error of mean (SEM) for groups of 5 samples. The significance of differences was determined by analysis of variances (ANOVA) and Student’s t-test using GraphPad Prism Software (GraphPad, San Diego, California, USA).
Results
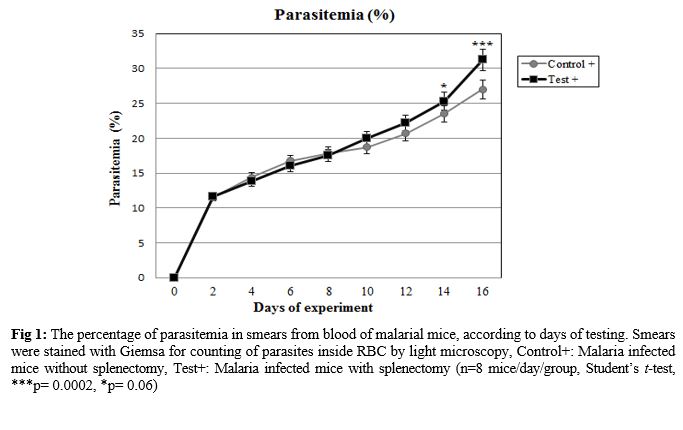
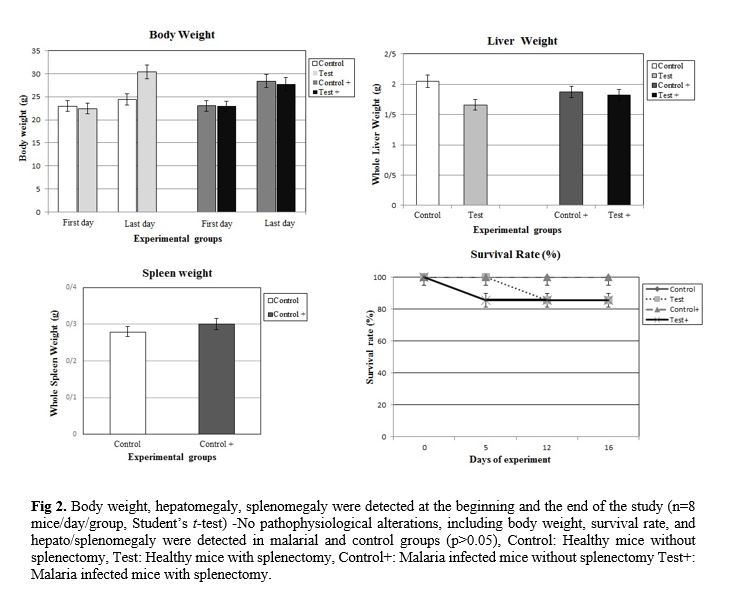
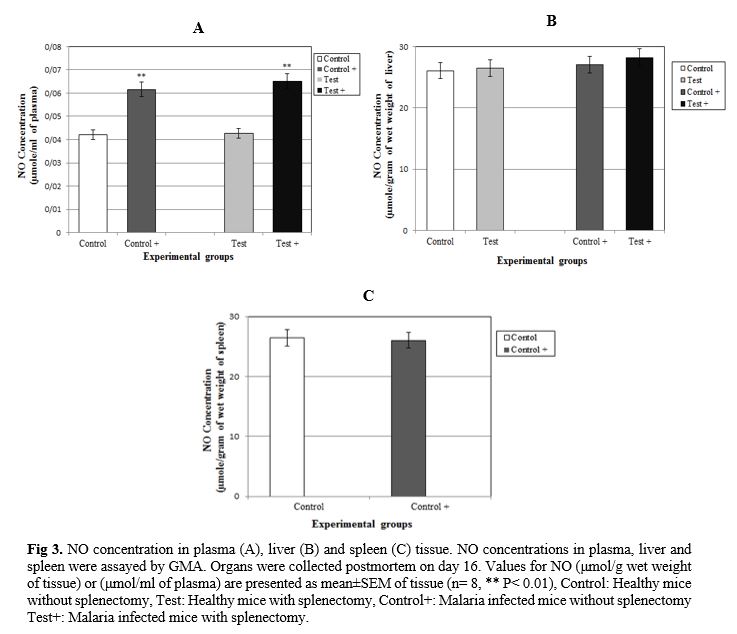
The effect of splenectomy on survival time of a primary infection was evaluated. The effect of splenectomy performed before or during primary infection on the survival period of mice was determined in several mouse strains in comparison with intact controls or sham-operated mice. Since no differences were observed between intact controls and sham-operated mice, the results of these groups were pooled. The parasitemia of animals was progressive in all groups inoculated by P. berghei (Fig. 1). At the end of the experiment (on day 16), the parasitemia was 26.99±0.46% among the group of non-splenectomized animals (Group III) in comparison with 31.25±0.72% among the group of splenectomized animals (Group IV). The average parasitemia among the groups at the end of the experiment was statistically significant (Group III, Group IV: p=0.0002) No pathophysiological changes, including body weight, hepatomegaly and splenomegaly were detected in the control and malarial groups (p>0.05). However, survival rate was significantly different (p<0.0001) (Fig. 2). NO concentrations in blood plasma, liver and spleen were assessed by Griess micro assay. The amount of plasma NO increased significantly in the infected groups (p= 0.0003). There was no difference among the titers of NO concentrations in both liver and spleen between control and test groups (p> 0.05) (Fig. 3).
Rodent malaria parasites are applied extensively as the study models of human malaria [7, 8]. Four different species that infect African rodents have been adapted for laboratory use: Plasmodium berghei (P. berghei), P. yoelii, P. chabaudi and P. vinckei. Small differences exist in the biology of them in laboratory mice and this makes these species appealing models for investigating different aspects of human malaria, including antigenic variation, drug resistance and immune evasion [9, 10]. P. berghei preferably invades reticulocytes and usually produces infections in mice, hence inducing severe pathology [8].
The normal function of the spleen is to remove abnormal erythrocytes and intra-erythrocytic inclusions. Malaria infected red blood cells (RBCs) containing an increasingly large and rigid parasites [11, 12]. The parasitized RBCs (PRBC) adheres to the vascular endothelium and thereby avoid splenic removal. Damage to the malaria parasite as a result of either drug treatment or host defense mechanisms leads to parasite clearance. Splenectomy frequently delayed morbidity of the infection, suggesting an active role of the spleen in the generation of an immunopathological reaction during a primary infection in the intact animal [11, 13].
Nitric oxide (NO) has been identified as a major effector molecule during the majority of parasitic infections. It seems that NO is not the only antiparasitic agent for immunological activity against parasites [14]. There is evidence that the activated macrophages are able to eradicate intracellular Leishmania major, Toxoplasma gondii and extracellular Schistosoma sp. parasites by the release of NO [15]. The production of NO and its derivatives has been reported in the majority of parasitic infections, including Giardia lamblia, Entamoeba coli [16], Leishmania amazonensis [17], Toxoplasma gondii [18], Leishmania mexicana [19], Schistosoma mansoni [20], Opisthorchis viverrini [21] and Clonorchis sinensis [22].
Inducible NO (iNOS) is an antipathogen and tumoricidal agent. However, its production requires a tight control because of cytotoxic and immune modulation activity [23, 24]. Several parasites are highly sensitive to NO and their derivatives; however, some of the parasites represent a moderate sensitivity to the production of toxic molecules [25].
Interestingly, hemo-globin, myoglobin, and neuroglobin may protect intracellular protozoa from the antiparasitic effects of NO [26]. Despite the wide evidence about anti-protozoal effects of NO, little efforts have been made to develop NO-based drugs in human medicine and to avoid toxic effects against non-target host cells [27]. The main control of malaria is achieved by NO-mediated mechanisms. The protection from cerebral malaria in African children is linked with iNOS [24]. There is conflicting evidence regarding the role of NO in the process of resistance against malaria parasites. Schizonts treated in vitro with NO donors caused a delayed infection to mice in a dose and time-dependent manner, which suggests an inhibitory role for NO [28] with influence on parasitemia and survival of P. berghei in infected mice or rats [29, 30]. Moreover, severe malaria is associated with reduced NO production [31] and iNOS variants in regions of differing disease manifestation [32]. Low NO bioavailability might contribute to pathologic activation of the immune system [33] and to the experimental cerebral malaria [34]. The mechanism of NO action on malaria, explains the existence of its molecules in the food vacuole which is a critical parasitic compartment involved in hemoglobin degradation, heme detoxification and a target for antimalarial drug action [35, 36]; therefore hemoglobin protects Plasmodium parasites from oxygen radicals [37]. The aim of this study was to investigate the effect of splenectomy on NO induction and its role in the pathogenesis of rodent malaria caused by P. berghei infection in outbred NMRI mice.
Materials and Methods
Animals and groups
Thirty female outbred NMRI mice (supplied with the Karaj Laboratory Animal Unit, Pasteur Institute of Iran), 4 to 6 weeks old and weighed between 20-22 gr were used in this study. The mice were housed at room temperature (20-23°C) on a 12-h light and 12 h dark cycle, with unlimited access to food and tap water. Experiments with animals were conducted according to the ethical standards formulated in the Declaration of Helsinki, and measures were taken to protect animals from pain or discomfort. It was approved by the institutional ethical review board (Ethical Committee of the Pasteur Institute of Iran), where the antimalarial test was performed.
The animals were divided into four groups of five and the procedures were performed by each group as described below:
Group I: No intervention (healthy control)
Group II: with splenectomy (healthy test)
Group III: No intervention, Inoculation of contaminated blood (infected control)
Group IV: With splenectomy, inoculation of contaminated blood (infected test)
Surgical procedures
Twenty four hours after sub-inoculation of 106 PRBC into clean mice, groups II, and IV underwent surgery .Ether (diethyl ether; MERCK, Germany) induction was attained by placing the mouse in a bell jar containing cotton wool soaked in ether, and the maintenance of anesthesia during the operation was effected by Tarin & Sturdee's (1972( method. This was accomplished with anti-sepsis and sterilized, median laparotomy and spleen exposure. In order to increase mice survival animals during surgery, they were taken from the anesthesia after a few seconds. In animals, the ligation of the main pedicle and of the main spleen-gastric vessel was held and the organ was subject to total ablation. At the end of the surgery, the abdominal wall was closed. The mice received povidone iodine antiseptic and sterile dermal ointment tetracycline on the sutured area. The animals were then placed in the recovery room and incandescent surgical lamps were used to raise the temperature to around 30ºC during post-anesthetic recovery. Each operation lasted at least 10 minutes.
Malaria parasites
P. berghei NY, kindly donated by the School of Life Sciences, University of Manchester, UK. Malaria parasite was maintained by blood passage in NMRI mice when active parasites were required; otherwise it was stored at -70°C in Alserver’s solution (2.33 % glucose, 0.52 % NaCl and 1.00% sodium citrate in deionized water) and glycerol (9:1 parts by volume). The parasite was maintained at the Department of Parasitology, Pasteur Institute of Iran in Tehran.
Inoculation of malaria parasites
Mice were inoculated (0.2 ml) intravenously into a tail vein with blood from a donor mouse (41% parasitaemia P. berghei) diluted with 0.85% saline to contain 2×106 PRBC.
Assessment of pathology
Evaluation of the parasitemia level
The evolution of the parasitemia level in the mice was determined from thin smears made from the tail blood during 16 consecutive days. After taking the smear, it was fixed with 100% methanol and stained with Giemsa solution (Sigma Chemical Co., USA). The hemoscopic examination was performed using an optical microscope, with 1000×magnification. PRBCs were counted in five different fields, each having approximately 200 cells. Results are expressed as the mean percentage (%) of erythrocytes containing Giemsa positive bodies. Experiments were licensed under the Animals (Scientific Procedures) Act 1986. In compliance with the conditions of this license, infected animals were humanely killed at the onset of the terminal phase of malaria (P. berghei NY) infection.
Calculation of parameters
Assessment of degree of hepatosplenomegaly
Entire livers and spleens were removed post-mortem at the end of the experimental period from mice after induction of terminal general anesthesia by inhalation of diethyl ether (Sigma Co., Germany). Organs wet weight were measured and compared with controls as indices for the degrees of hepatomegaly and splenomegaly. Body weight was measured initially and at different times of experiments using a top pan balance (OHAUS Scale Corp., USA).
Preparation of tissue homogenates for Reactive Nitrogen Intermediates (RNI) assay
Mice were terminally anesthetized by inhalation of diethyl ether (BDH, UK) 1 h after determination of parasitemia and humanely sacrificed by cervical dislocation. After blood taking, the whole livers and spleens were removed post-mortem and weighed. Tissues were placed in separate 1.5 mL microfuge tubes and homogenized in ice-cold deionized water (d.H2O; 0.1 g wet tissue/mL) using an electrical homogenizer (Model RS541-242; RS Components, UK). Homogenates were centrifuged at 5500 g (Model 1-13 Micro-centrifuge; Sigma Co., UK) for 15 min.
Griess micro assay
NO were measured as the concentration of nitrites and nitrates using the Griess reaction adapted with modifications from Rockett et al. as described fully elsewhere [38]. Nitrate standard was prepared in experimental fluids and finally were values corrected for assay losses [35]. Briefly, 60 µL samples were treated with 200 µL Griess reagent (5% phosphoric acid, 1% sulfanilic acid and 0.1% N [1-naphthyl-1]-ethylenediamine dihydrochloride) [all from Sigma] dissolved in 100 mL d. H2O was added and proteins were subsequently precipitated by 200 µL trichloroacetic acid 10%. Tube contents were vortex mixed and then centrifuged (Eppendorf centrifuge 5415 C, Germany). Duplicate samples of supernatants were transferred to a 96-well flat-bottomed microplate (Costar, USA) and absorbances read at 520 nm using a microplate reader (Bio-TEK, power wave XS, USA). Values for the concentration of nitrate were calculated from standard calibration plot. Values are presented as mean±SEM μM/g wet weight for tissue and μM/ml for plasma.
Statistical analysis
Values are presented as the mean±standard error of mean (SEM) for groups of 5 samples. The significance of differences was determined by analysis of variances (ANOVA) and Student’s t-test using GraphPad Prism Software (GraphPad, San Diego, California, USA).
Results
The effect of splenectomy on survival time of a primary infection was evaluated. The effect of splenectomy performed before or during primary infection on the survival period of mice was determined in several mouse strains in comparison with intact controls or sham-operated mice. Since no differences were observed between intact controls and sham-operated mice, the results of these groups were pooled. The parasitemia of animals was progressive in all groups inoculated by P. berghei (Fig. 1). At the end of the experiment (on day 16), the parasitemia was 26.99±0.46% among the group of non-splenectomized animals (Group III) in comparison with 31.25±0.72% among the group of splenectomized animals (Group IV). The average parasitemia among the groups at the end of the experiment was statistically significant (Group III, Group IV: p=0.0002) No pathophysiological changes, including body weight, hepatomegaly and splenomegaly were detected in the control and malarial groups (p>0.05). However, survival rate was significantly different (p<0.0001) (Fig. 2). NO concentrations in blood plasma, liver and spleen were assessed by Griess micro assay. The amount of plasma NO increased significantly in the infected groups (p= 0.0003). There was no difference among the titers of NO concentrations in both liver and spleen between control and test groups (p> 0.05) (Fig. 3).



Discussion
The spleen is a secondary lymphoid organ that perfectly induces innate and adaptive immune responses, and performs fundamental roles such as clearance and phagocytosis of both senescent and damaged RBCs and blood-borne pathogens including the malaria parasite, Plasmodium spp [37]. During the erythrocytic schizogony stage of malaria infection, the spleen contributes to innate resistance and is involved in the development of the induction and expression of acquired immunity [37, 38]. However, parasites retaliate by establishing chronic infections through evasion and modulation of immune responses and by remodeling the spleen thereby sometimes leading to immunopathology and severe disease [39]. In addition, several Plasmodium species escape spleen clearance through cytoadherence to endothelial cells in inner organs, causing end-organ dysfunction [40, 41]. Overall, while the spleen may not be essential for parasite clearance in a partially immune subject, it exerts a major protective role in non-immune subject via macrophage responses or mechanical retention/filtering of parasites. In addition to pathological complications, higher numbers of parasitemia and circulating mature forms were found in splenectomized subjects [42], prolonged waves of parasitemias and impaired parasite clearance have also been linked to splenectomy.
Usually splenectomy has an adverse effect on the host defense against the parasite, and infections become more severe in splenectomized humans and animals such as monkeys, birds, and rodents [43]. The animals without spleens also present deficiencies in the production of IgM due to sharp decrease of B lymphocytes, especially those in the area surrounding the spleen [44, 45]. Infection with the P. berghei parasite causes increasing levels of NO in infected groups. Conclusion reveals that splenectomy has minimal effects on levels of concentration of NO. It is possible that NO comes from several sources rather than spleen during rodent malaria and is released into circulation, which may replace NO shortage by splenic cells [46]. More studies are required to clarify the exact role of the spleen in the malaria infected host by using different malaria parasite strains and mouse species.
Conclusion
The use of conservative techniques aimed at full preservation of the spleen should always be sought given the importance of the spleen in clearing red blood cells parasitized by P. berghei. This could prove a key to prevent serious infections arising from malaria. However, the key role of NO and its metabolites in relation with other cytokines cannot be ignored in the immunoparasitology of malaria parasites.
Conflict of Interest
The authors confirm no conflict of interest.
Acknowledgements
This work was funded by the Department of Parasitology and Mycology, Kashan University of Medical Sciences and by collaboration with the Department of Parasitology, Pasteur Institute of Iran. Funding for this research was provided by the Kashan University of Medical Sciences, Iran (Grant No:93140).
Usually splenectomy has an adverse effect on the host defense against the parasite, and infections become more severe in splenectomized humans and animals such as monkeys, birds, and rodents [43]. The animals without spleens also present deficiencies in the production of IgM due to sharp decrease of B lymphocytes, especially those in the area surrounding the spleen [44, 45]. Infection with the P. berghei parasite causes increasing levels of NO in infected groups. Conclusion reveals that splenectomy has minimal effects on levels of concentration of NO. It is possible that NO comes from several sources rather than spleen during rodent malaria and is released into circulation, which may replace NO shortage by splenic cells [46]. More studies are required to clarify the exact role of the spleen in the malaria infected host by using different malaria parasite strains and mouse species.
Conclusion
The use of conservative techniques aimed at full preservation of the spleen should always be sought given the importance of the spleen in clearing red blood cells parasitized by P. berghei. This could prove a key to prevent serious infections arising from malaria. However, the key role of NO and its metabolites in relation with other cytokines cannot be ignored in the immunoparasitology of malaria parasites.
Conflict of Interest
The authors confirm no conflict of interest.
Acknowledgements
This work was funded by the Department of Parasitology and Mycology, Kashan University of Medical Sciences and by collaboration with the Department of Parasitology, Pasteur Institute of Iran. Funding for this research was provided by the Kashan University of Medical Sciences, Iran (Grant No:93140).
References
- World Health Organization. World Malaria Day 2016. Avaliable at: http://www.who.int/ campaigns/malaria-day/2016/event/en/
- World Health Organization. Malaria in children under five. Last update: 6 April 2016. Avaliable at: http://www.who.int/malaria/areas/high_risk_groups/children/en/
- Caraballo H, King K. Emergency department management of mosquito-borne illness: malaria, dengue, and West Nile virus. Emerg Med Pract. 2014; 16(5): 1-23.
- Snow RW. Global malaria eradication and the importance of Plasmodium falciparum epidemiology in Africa. BMC Med. 2015; 13(6): 23.
- David AF, Philip JR, Simon LC, Reto B, Solomon N. Antimalarial drug discovery: Efficacy models for compound screening. Nat Rev. 2004; 3(6): 509-20.
- Crawley J, Chu C, Mtove G, Nosten PF. Malaria in children. Lancet 2010; 375(9724): 1468-481.
- Otto TD, Böhme U, Jackson AP, Hunt M, Franke-Fayard B, Hoeijmakers WA, et al. A comprehensive evaluation of rodent malaria parasite genomes and geneexpression. BMC Biol. 2014; 12(86): 1-18.
- Craig AG, Grau GE, Janse C, Kazura JW, Milner D, Barnwell JW, et al; Participants of the hinxton retreat meeting on animal models for research on severe malaria. The role of animal models for research on severe malaria. PLoS Pathol. 2012; 8(2): e1002401.
- Lamb TJ, Brown DE, Potocnik AJ, Langhorne J. Insights into the immunopathogenesis of malaria using mouse models. Expert Rev Mol Med. 2006; 8(6): 1-22.
- Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008; 9(7): 725-32.
- Portillo ADH, Ferrer M, Brugat T, Martin-Jaular L, Langhorne J, Lacerda M. The role of the spleen in malaria. Cel Microbiol. 2012; 14(3): 343-55.
- Buffet PA, Safeukui I, Milon G, Mercereau-Puijalon O, David PH. Retention of erythrocytes in the spleen: a double-edged process in human malaria. Curr Opin Hematol. 2009; 16(3): 157-64.
- Tilley L, Dixon MW, Kirk K. The Plasmodium falciparum-infected red blood cell. Int J Biochem Cell Biol. 2011; 43(6): 839-42.
- Sharma JN, Al-Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases. Inflammo-pharmacol. 2007; 15(6): 252-59.
- Hafalla JC, Silvie O, Matuschewski K. Cell biology and immunology of malaria. Immunol Rev. 2011; 240(1): 297-316.
- Fernandes PD, Assreuy J. Role of nitric oxide and superoxide in Giardia lamblia killing. Braz J Med Biol Res. 1997; 30(1): 93-9.
- Augusto O, Linares E, Giorgio S. Possible roles of nitric oxide and peroxynitrite in murine leishmaniasis. Braz J Med Biol Res. 1996; 29(1): 853-62.
- Pohanka M. Role of oxidative stress in infectious diseases. A review. Folia Microbiol. 2013; 58(6): 503-13.
- Horta MF, Mendes BP, Roma EH, Noronha FM, Macêdo JP, Oliveira LS, et al. Reactive oxygen species and nitric oxide in cutaneous leishmaniasis. J Parasitol Res. 2012; 203818: 1-11.
- Oliveira SH, Fonseca SG, Romão PR, Ferreira SH, Cunha FQ. Nitric oxide mediates the microbicidal activity of eosinophils. Mem Inst Oswaldo Cruz. 1997; 92(2): 233-35.
- Satarug S, Haswell Elkins MR, Sithithaworn P, Bartsch H, Ohshima H, Tsuda M, et al. Relationships between the synthesis of N-nitrosodimethylamine and immune responses to chronic infection with the carcinogenic parasite, Opisthorchis viverrini, in men. Carcinogenesis 1998; 19(3): 485-91.
- James SL. Role of nitric oxide in parasitic infections. Microbiol Rev. 1995; 59(4): 533-47.
- Wandurska-Nowak E.The role of nitric oxide (NO) in parasitic infections. Wiad Parazytol 2004; 50(4): 665-78.
- Gradoni L, Ascenzi P. Nitric oxide and anti-protozoan chemotherapy. Parassitologia 2004; 46: 101-103.
- Stempin CC, Cerban FM. Macrophages and arginase induction as a mechanism for parasite escape. Medicina (B Aires) 2007; 67(6): 737-46.
- Ascenzi P, Fasano M, Gradoni L. Do hemo-globin and hemocyanin impair schistosoma killing by no? IUBMB Life 2002; 53(6): 287-88.
- Nahrevanian H. Involvement of nitric oxide and its up/down stream molecules in the immunity against parasitic infections. Braz J Infect Dis. 2009; 13(6): 440-48.
- Wang QH, Liu YJ, Liu J, Chen G, Zheng W, Wang JC, et al. Plasmodium yoelii: assessment of production and role of nitric oxide during the early stages of infection in susceptible and resistant mice. Exp Parasitol. 2009; 121(3): 268-73.
- Koka S, Lang C, Niemoeller OM, Boini KM, Nicolay JP, Huber SM, Lang F. Influence of NO synthase inhibitor L-NAME on parasitemia and survival of Plasmodium berghei infected mice. Cell Physiol Biochem. 2008; 21(5): 481-88.
- Safeukui I, Vatan R, Dethoua M, Agbo H, Haumont G, Moynet D, et al. A role of IgE and CD23/NO immune pathway in age-related resistance of Lewis rats to Plasmodium berghei Anka? Microbes Infect. 2008; 10(12): 1411-416.
- Yeo TW, Rooslamiati I, Gitawati R, Tjitra E, Lampah DA, Kenangalem E, et al. Pharmaco-kinetics of L-arginine in adults with moderately severe malaria. Antimicrob Agents Chemother. 2008; 52(12): 4381-387.
- Cramer JP, Nüssler AK, Ehrhardt S, Burkhardt J, Otchwemah RN, Zanger P, et al. Age-dependent effect of plasma nitric oxide on parasite density in Ghanaian children with severe malaria. Trop Med Int Health 2005; 10(7): 672-80.
- Sobolewski P, Gramaglia I, Frangos J, Intaglietta M, van der Heyde HC. Nitric oxide bioavailability in malaria. Trends Parasitol. 2005; 21(9): 415-22.
- Gramaglia I, Sobolewski P, Meays D, Contreras R, Nolan JP, Frangos JA, et al. Low nitric oxide bioavailability contributes to the genesis of experimental cerebral malaria. Nat Med. 2006; 12(12): 1417-422.
- Ostera G, Tokumasu F, Oliveira F, Sa J, Furuya T, Teixeira C, et al. Plasmodium falciparum:food vacuole localization of nitric oxide-derived species in intraerythrocytic stages of the malaria parasite. Exp Parasitol. 2008; 120(1): 29-38.
- Hunt NH, Golenser J, Chan-Ling T, Parekh S, Rae C, Potter S, et al. Immunopathogenesis of cerebral malaria. Int J Parasitol. 2006; 36(5): 569-82.
- Sobolewski P, Gramaglia I, Frangos JA, Intaglietta M, van der Heyde H. Plasmodium berghei resists killing by reactive oxygen species. Infect Immun 2005; 73(10): 6704-710.
- Rockett KA, Awburn MM, Rockett EJ, Cowden WB, Clark IA. Possible role of nitric oxide in malarial immunosuppression. Parasite Immunol. 1994; 16(5): 243-49.
- Negreiros RMA, Makimoto FH, Santana LLo, Ferreira LCl, Nakajima Gs, Santos MC. Experimental splenectomies and malaria in mice. Acta Cirúrgica Brasileira 2009; 24(6): 437-41.
- Engwerda CR, Beattie L, Amante FH. The importance of the spleen in malaria. Trends Parasitol. 2005; 21(2): 75-80.
- Del Portillo HA, Ferrer M, Brugat T, Martin-Jaular L, Langhorne J, Lacerda MV. The role of the spleen in malaria. Cell Microbiol 2012; 14(3): 343-355.
- Buffet PA, Safeukui I, Milon G, Mercereau-Puijalon O, David PH. Retention of erythrocytes in the spleen: a double-edged process in human malaria. Curr Opin Hematol. 2009; 16(3): 157-64.
- Fonager J, Pasini EM, Braks JA, Klop O, Ramesar J, Remarque EJ, et al. Reduced CD36-dependent tissue sequestration of Plasmodium-infected erythrocytes is detrimental to malaria parasite growth in vivo. J Exp Med. 2012; 209(1): 93-107.
- Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature 2002; 415(6872): 673-79.
- Wardemann H, Boehm T, Dear N, Carsetti R. B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen. J Exp Med. 2002; 195(6): 771-80.
- Nahrevanian H, Dascombe MJ. Simultaneous increases in immune competent cells and nitric oxide in the spleen during Plasmodium berghei infection in mice. J Microbiol Immunol Infec. 2006; 39(1): 11-7.
Type of Study: Research |
Subject:
Parasitology
Received: 2018/05/28 | Accepted: 2018/12/3 | Published: 2019/03/10
Received: 2018/05/28 | Accepted: 2018/12/3 | Published: 2019/03/10
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




