Mon, Feb 16, 2026
[Archive]
Volume 9, Issue 1 (February 2022)
IJML 2022, 9(1): 6-16 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Rajabi A, Pourentezari M, Dortaj H, Shahedi A, Yadegari M, Izadi S, et al . In Vitro Study of Hyaluronic Acid Based Scaffolds and Its Effect on Cartilage Regeneration. IJML 2022; 9 (1) :6-16
URL: http://ijml.ssu.ac.ir/article-1-411-en.html
URL: http://ijml.ssu.ac.ir/article-1-411-en.html
Ali Rajabi 

 , Majid Pourentezari *
, Majid Pourentezari * 

 , Hengameh Dortaj
, Hengameh Dortaj 

 , Abbas Shahedi
, Abbas Shahedi 

 , Maryam Yadegari
, Maryam Yadegari 

 , Sepideh Izadi
, Sepideh Izadi 

 , Fatemeh Zakizadeh
, Fatemeh Zakizadeh 

 , Zeinolabedin Sharifian Dastjerdi
, Zeinolabedin Sharifian Dastjerdi 




 , Majid Pourentezari *
, Majid Pourentezari * 

 , Hengameh Dortaj
, Hengameh Dortaj 

 , Abbas Shahedi
, Abbas Shahedi 

 , Maryam Yadegari
, Maryam Yadegari 

 , Sepideh Izadi
, Sepideh Izadi 

 , Fatemeh Zakizadeh
, Fatemeh Zakizadeh 

 , Zeinolabedin Sharifian Dastjerdi
, Zeinolabedin Sharifian Dastjerdi 


Yazd Neuroendocrine Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
Full-Text [PDF 421 kb]
(703 Downloads)
| Abstract (HTML) (1725 Views)

Full-Text: (1184 Views)
Introduction
Articular cartilage has limited potential for spontaneous healing. This property often leads to osteoarthritis, pain, and dysfunction of the affected joint [1, 2]. Although many accepted treatments are accessible to repair damaged articular cartilage structures, including micro-fractures, cell implants, and tissue transplants, these methods often do not repair strong, well-repaired articular cartilage [3-5]. In recent years, cartilage tissue engineering (CTE) has prepared a prospective strategy for articular regeneration by combining cells with scaffolds.
Fakhari et al. developed this scaffold in tissue engineering (TE) applications by researching hyaluronic acid (HA) hydrogels. HA is a non-sulfated glycosamino-glycan (GAG) and is a significant component of the extracellular matrix of cartilage. HA provides a native microenvironment for mesenchymal stem cells and can increase functional cartilage formation compared to other synthetic hydrogels such as polyethylene glycol (PEG) [6, 7].
HA has several biomedical applications due to cellular interactions and its presence and role in the extracellular matrix of many tissues [8]. Among the applications mentioned for HA are drug delivery and tissue bulking [6, 9].
One of the significant goals of TE approaches with HA hydrogels is cartilage tissue repair [10]. Since HA is abundant in healthy cartilage (such as the matrix around cartilage cells) and is involved in cartilage homeostasis, it has been extensively studied as part of hydrogels and scaffolds for cartilage repair [11, 12]. Mesenchymal stem cells enclosed in HA-based hydrogels show higher expression of cartilage markers in both in vitro and in vivo than those compared to ineffective PEG hydrogels [13].
CTE is a promising way to repair cartilage tissue damage. The most common methods used in CTE include the proper combination of granule cells, biocompatible scaffolds, and biological agents that support the formation of new cartilage [14]. Success in cartilage tissue regeneration depends on individual or combination characteristics of cells, biological agents, and scaffolding [15]. We investigated appropriate cells and biological agents and a convenient scaffold for CTE. We also studied HA scaffolds and HA-based composite scaffolds in CTE and cells and growth factors used with this scaffold to induce and enhance chondrogenesis.
Cells in CTE
Chondrocytes are the most common cells used in CTE. Besides, they play an essential role in cartilage regeneration. On the other hand, stem cells can achieve self-renewal and differentiate into multiple lineages. They can be taken from donor cartilage such as the meniscus, the nose, and the trachea. They can construct, maintain, and regenerate cartilage tissue in vitro [16]. Autologous cartilage is difficult to access, and the cells collected from the patient's joints are relatively inactive. Chondrocyte proliferation in monolayer culture leads to disintegration and is presented as decreased proteoglycan synthesis and type II collagen expression and type I collagen overexpression [17]. Young donor chondrocytes are more metabolically active and have higher chondrogenic potential and fast expansion compared to cells taken from adult donors [18, 19]. To dominate and control the limited storage of primary cells, the application of multipotent stem cells is recommended, which are mainly isolated from bone marrow, adipose tissue, and before implantation [20, 21]. Adult mesenchymal stem cell (MSC) sources are available in various tissues, including trabecular bone, bone marrow, deciduous teeth, periosteum, articular cartilage, adipose tissue, muscle, and synovial membrane [21-23].
Growth factors in CTE
Growth factors and chemical stimuli such as transforming growth factor-β (TGF-β) conversion, insulin such as growth factor-1, and bone morphogenic protein-6 are required [24]. However, using the chemical inducers mentioned, the researchers found that neo-cartilage tissues were not similar to native hyaline cartilage due to having more type I collagen and type X collagen and less type II collagen. Therefore, researchers are trying to find an alternative to MSC induction to produce better quality cartilage tissue and lower costs [25].
Scaffolds in CTE
Scaffolds acting as the artificial extracellular matrix (ECM) also have pivotal roles in determining cartilage reconstruction. The scaffold is a three-dimensional construct in which cells can attach and migrate. Fibers, meshes, sponges, and hydrogels scaffolds have been administered as carriers for chondrocytes and stem cells in CTE. The ideal scaffold should be biocompatible, non-toxic, non-stimulatory for inflammatory cells and non-immunogenic [26]. It must also have specific characteristics that lead to cell adhesion, proliferation, differentiation into specific phenotypes such as mechanical support of CTE and porosity, leading to the release and exchange of nutrients and the excretion of waste products [26, 27]. In addition, scaffold components must be resistant to decay at physiological pH and body temperature, be biodegradable and allow new cartilage to regenerate and replace the original structure [28]. A suitable scaffold for CTE is a scaffold with high porosity and the ability to connect pores to pores. High porosity provides a good environment for cell adhesion, growth,
and regeneration. The interconnected porous organization facilitates cell migration, exchange of nutrients and physiological gases into the cell, and metabolic of cells [29]. Mechanical stimulation can certainly boost the mechanical features of CTE [30]. CTE studies have focused on two loading regimes: direct or unbound compression and hydrostatic pressure. Direct dynamic compression applied to cartilaginous scaffolds typically increases the production and/or proliferation of the ECM and improves the compressive properties of the engineered tissue [31].
The main goal of TE is to create implant-like structures that can replace damaged tissue. Scaffolds with good porosity provide a suitable environment for cell migration, cell proliferation, and other activities [32-34].
HA as scaffold for CTE
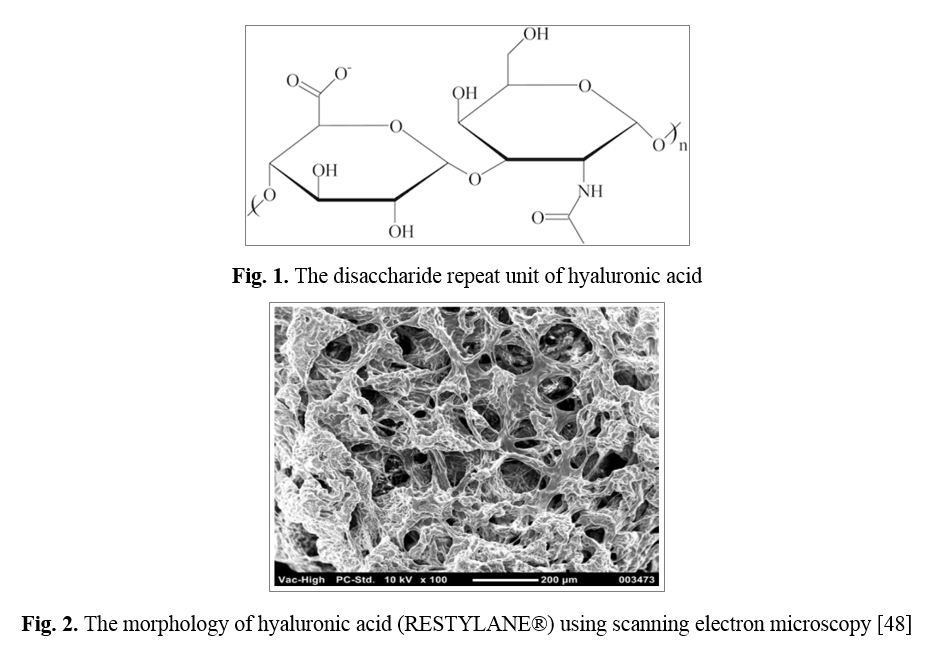
HA also known as hyaluronan, is a linear, anionic, non-sulfated GAG with a combination of saccharide gland units: β-1,4-D and β-1 glucuronic acid, 3 - N -acetyl- D-glucosamide [35]. It is a high molecular weight (105-107 kDa) natural biopolymer that can contain 5000-30000 sugar molecules in the backbone structure [36]. HA is one of the main components of ECM and cartilage tissue. HA is synthesized in the inner cell membrane by the synthesis of hyaluronan. After synthesis, it is transferred to the ECM through the membrane after 3-5 days, where it is destroyed by the family of hyaluronidase enzymes [37]. HA is found in the ECM of all living tissues, with varying concentrations and molecular weights, and is more prevalent in mechanically loaded tissues such as cartilage, dermis, and vocal folds [38, 39]. Due to many carboxyl and hydroxyl groups, HA is a highly hydrophilic compound that creates a gel-like structure as a result of the intermolecular interaction of macromolecules in the aqueous medium [40]. It acts as a protection against the penetration of microorganisms and toxic agents [41], lubricant due to its viscoelastic properties in the synovial liquid [42], and the transparent aqueous solution is a filler of eye structures [43]. HA is commonly known to play an essential role in cell division and migration, angiogenesis, wound healing, and tissue regeneration, and its effects are related to molecular weight [44]. Due to its biocompatibility, biodegradation, and chemical modification, HA is of potential interest in the TE field [45]. The use of cells, scaffolds, and growth factors promote tissue regeneration, which can overcome the development of autologous and allogeneic transplant-related immunological responses [21]. HA can interact with stem cell surface receptors, transmit signals within the cell, and affect cell activity, such as proliferation, survival, motility, and differentiation [45]. In various studies of HA and HA-based materials such as biological scaffolds and injectable hydrogels, in vitro and in vivo tests have been used that show positive results for tissue regeneration. HA does not promote cell adhesion, but it can be modified by motifs such as Arginine-Glycine-Aspartic Acid (RGD) to increase cell attachment [46]; HA is a polymer of disaccharides composed of repeating units of β‐1,4‐d‐glucuronic acid and β‐1,3‐N‐acetyl‐d‐glucosamine (Fig. 1) [47]. One of the unique properties of hyaluronic acid is its rheological property, which is also observed in low concentrations, characterizing the intertwined chains of this hydrogel (Fig. 2) [48]. The binding of HA can be divided into two incomplete and complete groups, which causes HA to form a polymer network by covalent bonding with the interfaces and is insoluble in water by forming a polymer which is due to the complete bonding. Incomplete bonding of part of the covalent bond of HA molecules is stimulated, and a small amount of water solution remains (Fig. 3) [49].
HA composite hydrogels consisting of two or more natural/synthetic biopolymers have the advantages of biopolymers, while they enhance some of the disadvantages through improved biodegradation and adjustable mechanical strength. Using HA and modified several methods and other covalent bonding materials, various hybrid hydrogels have been increased for administration in CTE [45].
The purpose of this study is to review the effect of HA hydrogels as a scaffold to help repair cartilaginous lesions. Scientific Information Database (SID), MEDLINE, PubMed, OVID, and Scopus databases from 2010 to 2021 were used for this study.
Fakhari et al. developed this scaffold in tissue engineering (TE) applications by researching hyaluronic acid (HA) hydrogels. HA is a non-sulfated glycosamino-glycan (GAG) and is a significant component of the extracellular matrix of cartilage. HA provides a native microenvironment for mesenchymal stem cells and can increase functional cartilage formation compared to other synthetic hydrogels such as polyethylene glycol (PEG) [6, 7].
HA has several biomedical applications due to cellular interactions and its presence and role in the extracellular matrix of many tissues [8]. Among the applications mentioned for HA are drug delivery and tissue bulking [6, 9].
One of the significant goals of TE approaches with HA hydrogels is cartilage tissue repair [10]. Since HA is abundant in healthy cartilage (such as the matrix around cartilage cells) and is involved in cartilage homeostasis, it has been extensively studied as part of hydrogels and scaffolds for cartilage repair [11, 12]. Mesenchymal stem cells enclosed in HA-based hydrogels show higher expression of cartilage markers in both in vitro and in vivo than those compared to ineffective PEG hydrogels [13].
CTE is a promising way to repair cartilage tissue damage. The most common methods used in CTE include the proper combination of granule cells, biocompatible scaffolds, and biological agents that support the formation of new cartilage [14]. Success in cartilage tissue regeneration depends on individual or combination characteristics of cells, biological agents, and scaffolding [15]. We investigated appropriate cells and biological agents and a convenient scaffold for CTE. We also studied HA scaffolds and HA-based composite scaffolds in CTE and cells and growth factors used with this scaffold to induce and enhance chondrogenesis.
Cells in CTE
Chondrocytes are the most common cells used in CTE. Besides, they play an essential role in cartilage regeneration. On the other hand, stem cells can achieve self-renewal and differentiate into multiple lineages. They can be taken from donor cartilage such as the meniscus, the nose, and the trachea. They can construct, maintain, and regenerate cartilage tissue in vitro [16]. Autologous cartilage is difficult to access, and the cells collected from the patient's joints are relatively inactive. Chondrocyte proliferation in monolayer culture leads to disintegration and is presented as decreased proteoglycan synthesis and type II collagen expression and type I collagen overexpression [17]. Young donor chondrocytes are more metabolically active and have higher chondrogenic potential and fast expansion compared to cells taken from adult donors [18, 19]. To dominate and control the limited storage of primary cells, the application of multipotent stem cells is recommended, which are mainly isolated from bone marrow, adipose tissue, and before implantation [20, 21]. Adult mesenchymal stem cell (MSC) sources are available in various tissues, including trabecular bone, bone marrow, deciduous teeth, periosteum, articular cartilage, adipose tissue, muscle, and synovial membrane [21-23].
Growth factors in CTE
Growth factors and chemical stimuli such as transforming growth factor-β (TGF-β) conversion, insulin such as growth factor-1, and bone morphogenic protein-6 are required [24]. However, using the chemical inducers mentioned, the researchers found that neo-cartilage tissues were not similar to native hyaline cartilage due to having more type I collagen and type X collagen and less type II collagen. Therefore, researchers are trying to find an alternative to MSC induction to produce better quality cartilage tissue and lower costs [25].
Scaffolds in CTE
Scaffolds acting as the artificial extracellular matrix (ECM) also have pivotal roles in determining cartilage reconstruction. The scaffold is a three-dimensional construct in which cells can attach and migrate. Fibers, meshes, sponges, and hydrogels scaffolds have been administered as carriers for chondrocytes and stem cells in CTE. The ideal scaffold should be biocompatible, non-toxic, non-stimulatory for inflammatory cells and non-immunogenic [26]. It must also have specific characteristics that lead to cell adhesion, proliferation, differentiation into specific phenotypes such as mechanical support of CTE and porosity, leading to the release and exchange of nutrients and the excretion of waste products [26, 27]. In addition, scaffold components must be resistant to decay at physiological pH and body temperature, be biodegradable and allow new cartilage to regenerate and replace the original structure [28]. A suitable scaffold for CTE is a scaffold with high porosity and the ability to connect pores to pores. High porosity provides a good environment for cell adhesion, growth,
and regeneration. The interconnected porous organization facilitates cell migration, exchange of nutrients and physiological gases into the cell, and metabolic of cells [29]. Mechanical stimulation can certainly boost the mechanical features of CTE [30]. CTE studies have focused on two loading regimes: direct or unbound compression and hydrostatic pressure. Direct dynamic compression applied to cartilaginous scaffolds typically increases the production and/or proliferation of the ECM and improves the compressive properties of the engineered tissue [31].
The main goal of TE is to create implant-like structures that can replace damaged tissue. Scaffolds with good porosity provide a suitable environment for cell migration, cell proliferation, and other activities [32-34].
HA as scaffold for CTE
HA also known as hyaluronan, is a linear, anionic, non-sulfated GAG with a combination of saccharide gland units: β-1,4-D and β-1 glucuronic acid, 3 - N -acetyl- D-glucosamide [35]. It is a high molecular weight (105-107 kDa) natural biopolymer that can contain 5000-30000 sugar molecules in the backbone structure [36]. HA is one of the main components of ECM and cartilage tissue. HA is synthesized in the inner cell membrane by the synthesis of hyaluronan. After synthesis, it is transferred to the ECM through the membrane after 3-5 days, where it is destroyed by the family of hyaluronidase enzymes [37]. HA is found in the ECM of all living tissues, with varying concentrations and molecular weights, and is more prevalent in mechanically loaded tissues such as cartilage, dermis, and vocal folds [38, 39]. Due to many carboxyl and hydroxyl groups, HA is a highly hydrophilic compound that creates a gel-like structure as a result of the intermolecular interaction of macromolecules in the aqueous medium [40]. It acts as a protection against the penetration of microorganisms and toxic agents [41], lubricant due to its viscoelastic properties in the synovial liquid [42], and the transparent aqueous solution is a filler of eye structures [43]. HA is commonly known to play an essential role in cell division and migration, angiogenesis, wound healing, and tissue regeneration, and its effects are related to molecular weight [44]. Due to its biocompatibility, biodegradation, and chemical modification, HA is of potential interest in the TE field [45]. The use of cells, scaffolds, and growth factors promote tissue regeneration, which can overcome the development of autologous and allogeneic transplant-related immunological responses [21]. HA can interact with stem cell surface receptors, transmit signals within the cell, and affect cell activity, such as proliferation, survival, motility, and differentiation [45]. In various studies of HA and HA-based materials such as biological scaffolds and injectable hydrogels, in vitro and in vivo tests have been used that show positive results for tissue regeneration. HA does not promote cell adhesion, but it can be modified by motifs such as Arginine-Glycine-Aspartic Acid (RGD) to increase cell attachment [46]; HA is a polymer of disaccharides composed of repeating units of β‐1,4‐d‐glucuronic acid and β‐1,3‐N‐acetyl‐d‐glucosamine (Fig. 1) [47]. One of the unique properties of hyaluronic acid is its rheological property, which is also observed in low concentrations, characterizing the intertwined chains of this hydrogel (Fig. 2) [48]. The binding of HA can be divided into two incomplete and complete groups, which causes HA to form a polymer network by covalent bonding with the interfaces and is insoluble in water by forming a polymer which is due to the complete bonding. Incomplete bonding of part of the covalent bond of HA molecules is stimulated, and a small amount of water solution remains (Fig. 3) [49].
HA composite hydrogels consisting of two or more natural/synthetic biopolymers have the advantages of biopolymers, while they enhance some of the disadvantages through improved biodegradation and adjustable mechanical strength. Using HA and modified several methods and other covalent bonding materials, various hybrid hydrogels have been increased for administration in CTE [45].
The purpose of this study is to review the effect of HA hydrogels as a scaffold to help repair cartilaginous lesions. Scientific Information Database (SID), MEDLINE, PubMed, OVID, and Scopus databases from 2010 to 2021 were used for this study.


Mechanical properties of HA
HA undergoes multiple degradation procedures because of hydrolysis and enzymatic hydrolysis by naturally arising hyaluronidase. Non-enzymatic reactions can degrade HA. These include acid and alkali hydrolysis, ultrasonic decomposition, thermal decomposition, and oxidant degradation [51]. Achieving mechanical properties is important in the design of HA-based hydrogels. In principle, they must have ECM-like mechanical properties in normal tissues, be sufficiently resistant to enzymatic and non-enzymatic degradation, and not deform against the compressive forces of the surrounding tissues. The mechanical properties of artificial substrates in vitro environments significantly influence some cell functions such as adhesion, proliferation, migration, and differentiation [52]. Due to disadvantages such as hydrophilic nature and lack of mechanical integrity, HA needs chemical modification and cross-linking to change it for CTE applications [53, 55]. In order to control the degree of degradation and improve its mechanical properties, in designing HA-based scaffolds for cartilage tissue, various strategies such as cross-linking or using a composite structure to create a stable material are used [53, 54].
The results of the studies are summarized in Table 1.
HA undergoes multiple degradation procedures because of hydrolysis and enzymatic hydrolysis by naturally arising hyaluronidase. Non-enzymatic reactions can degrade HA. These include acid and alkali hydrolysis, ultrasonic decomposition, thermal decomposition, and oxidant degradation [51]. Achieving mechanical properties is important in the design of HA-based hydrogels. In principle, they must have ECM-like mechanical properties in normal tissues, be sufficiently resistant to enzymatic and non-enzymatic degradation, and not deform against the compressive forces of the surrounding tissues. The mechanical properties of artificial substrates in vitro environments significantly influence some cell functions such as adhesion, proliferation, migration, and differentiation [52]. Due to disadvantages such as hydrophilic nature and lack of mechanical integrity, HA needs chemical modification and cross-linking to change it for CTE applications [53, 55]. In order to control the degree of degradation and improve its mechanical properties, in designing HA-based scaffolds for cartilage tissue, various strategies such as cross-linking or using a composite structure to create a stable material are used [53, 54].
The results of the studies are summarized in Table 1.
Table 1. Hyaluronic Acid scaffolds in cartilage tissue engineering
| Author | Year | Scaffold Type | Growth Factor | Cell | Outcome |
| Yuan et al. [55] |
2015 | HA+ collagen | chondrogenic medium + icariin | Rabbit chondrocytes | Expression of sox9, aggrecan, collagen type II genes from seed cartilage is increased. Production of glycosaminoglycans and collagen type II was much higher in HA-Ica/ collagen hydrogels. |
| Mondal et al. [56] |
2016 | HA+ divinyl sulfone | chondrogenic medium | Adipose-derived stem cells | Cytotoxicity analysis showed that all hydrogels are cytotoxic and can be used to deliver AMSCs. Hydrogels have been shown to aid in forcing various AMSC differentials, and Thoms may be potential support in repairing articular cartilage in osteoarthritis. |
| Chen et al. [57] |
2016 | Glucosamine in gelatin/HA cryogel | chondrogenic medium | Rabbit articular chondrocytes | Cryogel scaffolds containing 9% glucosamine showed better efficacy in maintaining cartilage phenotype by affecting cell proliferation increasing the secretion of GAGs and COL II. |
| Mahapatra et al. [58] |
2016 | Alginate + Hyaluronic acid + Collagen type I (Alg-HA-Col) | chondrogenic medium | Rat articular chondrocytes | The mRNA levels of chondrodite phenotypes, including SOX9, type II collagen, and aggregates, are significantly regulated when cells are cultured in Alg-HA-Col gel compared to those cultured in Alg-HA be. The secretion of glycosaminoglycan sulfate, a specific cartilage matrix molecule, was observed in collagen composite hydrogels. |
| Kim et al. [59] |
2017 | Oxidized hyaluronate + glycol chitosan | chondrogenic medium | ATDC5 cells | These hydrogels are well adapted to physiological conditions and can act as an injectable cell transport system in CTE. |
| Amann et al. [60] |
2017 | HA | chondrogenic medium | Human articular chondrocytes/hADSC | Hyaluronic acid stimulates the differentiation of collagen from collagen hydrogel supplementation in a dose-dependent manner. 1% HA showed the best results |
| La Gatta et al. [61] |
2017 | Hyaluronan+lysine Methyl-ester cross-linking |
chondrogenic medium | Human articular chondrocytes | Primary human chondrocytes cultured hydrogels are viable and maintained in their lineage. They also secrete cartilage-specific matrix proteins. These scaffolds are promising candidates for CTE. |
| Liu et al. [62] |
2018 | Glycol chitosan/oxidized hyaluronic acid And Glycol chitosan/oxidized hyaluronic acid+ ECM |
chondrogenic medium | BMSCs | To evaluate chondroinductivity induction of ECM in vitro, BMSCs were compared in S1 (G-CS/OHA) and S3 (G-CS/OHA/ECM 2-weight) hydrogels. Higher levels of glycosaminoglycans (GAG) and type II collagen (COL II) were accumulated in the S3 hydrogel. |
| Lin et al. [63] |
2019 | Methacrylate gelatin | chondrogenic medium | human BMSCs | mGL/mHA with a ratio of 9: 1 (٪, w/v) leads to the lowest hBMSC hypertrophy and the highest glycosaminoglycan production, with a slight increase in the total volume of the structure. |
| Sharifian et al. [45] |
2019 | HA + Fibrin + Polylactic acid-polyglycolic acid | chondrogenic medium | hADSCs | poly(lactide-co-glycolide)/fibrin/HA stimulates cartilage production in hADSCs. Decreased hypertrophic markers and increased characteristic markers of hyaline cartilage were observed in hydrogels. |
| Jooybar et al. [64] |
2019 | HA-tyramine (HA+TA) | chondrogenic medium + platelet lysate | Human mesenchymal stem cells | Platelet laser materials have a significant function in supporting human mesenchymal stem cells (hMSCs), acting like cell binding, viability, and proliferation in the three-dimensional hydrogel. When placed in a cartilaginous differentiation medium, hMSCs produce hyaline cartilage produced by HA-TA. |
| Wang et al. [65] |
2019 | polypeptides | chondrogenic medium | Rabbit BMSCs | Adhesion and proliferation were represented, and an experimental study of BMSC demonstrated that the PAP-3SF/6.5COL/0.5HA scaffold had good biocompatibility. |
| Ren et al. [66] |
2020 | Maleimide-modified hyaluronic acid+ collagen mimetic peptide (GPO)8-CG-RGDS | chondrogenic medium +matrix metalloproteinase | Bone mesenchymal stem cells | A combination of CMP with an MMP-sensitive peptide can have the possibility to differentiate mesenchymal stem cells into cartilage and prevent the hypertrophic phenotype throughout differentiation. |
| Tsanaktsidou et al. [67] |
2020 | Methacrylated hyaluronic acid (MeHA)+ chondroitin sulfate | chondrogenic medium + matrix metalloproteinase | human mesenchymal stem cells | Methacrylated hyaluronic acid and chondroitin sulfate hydrogels have been developed to create an environment conducive to the growth and proliferation of human mesenchymal stem cells and promote their differentiation from tubular phenotypes, even if grown in an expansion medium. |
HA= Hyaluronic acid; AMSCs= Adipose tissue-derived mesenchymal stem cells; hBMSCs= human bone marrow mesenchymal stem cells; CTE= Cartilage tissue engineering; ADSC= Adipose derived stem cells; BMSC= Bone marrow stem cell
Conclusion and future trends
Although an extensive range of surgical methods is accessible to treat cartilage injuries and be prosperous in short-term and long-term follow-up, none of them are qualified to fully return the activity and construction of damaged cartilage to its original state. HA is a promising bright spot to help reduce side effects. Its effectiveness is due to many practical methods, including lubrication, anti-inflammatory effects, and cartilage protection. HA treatment demonstrates great potential that we hope will be identified with further research. Further research is needed to obtain a specific HA molecular mass to achieve clinical efficacy and expand its applications to complete control of the disease and its complications.
Although the widespread use of HA hydrogels is important in biomedical applications, the effect of HA on cellular behavior, especially through cell surface receptors such as CD44, has been poorly studied. We reviewed articles that examined hyaluronic acid scaffolds for cell differentiation into cartilage and their effect on surface receptors.
HA adhesion has a large effect on the hMSC response, leading to increased cell proliferation, proliferation, and the formation of focal adhesions. HA parameters have been shown to affect hMSC cartilage formation, as seen through gene expression profiles, potentially by activating cytoskeletal organization and cell ability. HA fibrous hydrogels are a promising alternative to non-fibrous hydrogels for regenerative strategies that can be used in the future. HA can direct articular cartilage. Combining this material with other natural and synthetic scaffolds has been shown to have cartilage induction capabilities. HA supports the migration, survival, and differentiation of stem cells. HA supports the proper formation of the matrix by differentiating stem cells to become articular. It suggests that if used in vivo, such a device can be integrated into a joint defect site and healed.
Nevertheless, it is necessary to have appropriate molecular signals to support the repair and healing of joint lesions. Synergistic growth factors must be added to maintain and improve the induction of regenerative articular cartilage, thus preventing fibroids from returning to the cartilage or endochondral ossification. Under these circumstances, the HA matrix with other scaffolds could be a viable alternative to promote better regeneration of articular cartilage.
Conflicts of interest
The authors have no conflict of interest to declare.
Acknowledgments
Not applicable.
Although the widespread use of HA hydrogels is important in biomedical applications, the effect of HA on cellular behavior, especially through cell surface receptors such as CD44, has been poorly studied. We reviewed articles that examined hyaluronic acid scaffolds for cell differentiation into cartilage and their effect on surface receptors.
HA adhesion has a large effect on the hMSC response, leading to increased cell proliferation, proliferation, and the formation of focal adhesions. HA parameters have been shown to affect hMSC cartilage formation, as seen through gene expression profiles, potentially by activating cytoskeletal organization and cell ability. HA fibrous hydrogels are a promising alternative to non-fibrous hydrogels for regenerative strategies that can be used in the future. HA can direct articular cartilage. Combining this material with other natural and synthetic scaffolds has been shown to have cartilage induction capabilities. HA supports the migration, survival, and differentiation of stem cells. HA supports the proper formation of the matrix by differentiating stem cells to become articular. It suggests that if used in vivo, such a device can be integrated into a joint defect site and healed.
Nevertheless, it is necessary to have appropriate molecular signals to support the repair and healing of joint lesions. Synergistic growth factors must be added to maintain and improve the induction of regenerative articular cartilage, thus preventing fibroids from returning to the cartilage or endochondral ossification. Under these circumstances, the HA matrix with other scaffolds could be a viable alternative to promote better regeneration of articular cartilage.
Conflicts of interest
The authors have no conflict of interest to declare.
Acknowledgments
Not applicable.
References
- Vilela C, Correia C, Oliveira JM, Sousa RA, Espregueira-Mendes J, Reis RL. Cartilage repair using hydrogels: a critical review of in vivo experimental designs. ACS Biomaterials Science & Engineering. 2015; 1(9): 726-39.
- Szychlinska MA, Stoddart MJ, D'Amora U, Ambrosio L, Alini M, Musumeci G. Mesenchymal stem cell-based cartilage regeneration approach and cell senescence: can we manipulate cell aging and function? Tissue Engineering Part B: Reviews. 2017; 23(6): 529-39.
- Feng Q, Wei K, Zhang K, Yang B, Tian F, Wang G, et al. One-pot solvent exchange preparation of non-swellable, thermoplastic, stretchable, and adhesive supramolecular hydrogels based on dual synergistic physical cross-linking. NPG Asia Materials 2018; 10(1): 455.
- Makris EA, Gomoll AH, Malizos KN, Hu JC, Athanasiou KA. Repair and tissue engineering techniques for articular cartilage. Nature Reviews Rheumatology. 2015; 11(1): 21.
- Wang Z, Wang Z, Lu WW, Zhen W, Yang D, Peng S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Materials 2017; 9(10): 435.
- Fakhari A, Berkland C. Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment. Acta biomaterialia. 2013; 9(7): 7081-92.
- Kim IL, Mauck RL, Burdick JA. Hydrogel design for cartilage tissue engineering: a case study with hyaluronic acid. Biomaterials 2011; 32(34): 8771-782.
- Laurent TC, Fraser JR. Hyaluronan. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 1992; 6(7): 2397-404.
- Moreland LW. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis research & therapy 2003; 5(2): 54-67.
- Chung C, Burdick JA. Engineering cartilage tissue. Advanced drug delivery reviews 2008; 60(2): 243-62.
- Chung C, Beecham M, Mauck RL, Burdick JA. The influence of degradation characteristics of hyaluronic acid hydrogels on in vitro neocartilage formation by mesenchymal stem cells. Biomaterials 2009; 30(26): 4287-96.
- Kim IL, Khetan S, Baker BM, Chen CS, Burdick JA. Fibrous hyaluronic acid hydrogels that direct MSC chondrogenesis through mechanical and adhesive cues. Biomaterials. 2013; 34(22): 5571-580.
- Chung C, Burdick JA. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue engineering Part A. 2009; 15(2): 243-54.
- Hashemibeni B, Mardani M, Bahrami M, Valiani A, Mehr MS, Pourentezari M. Comparison of fibrin and PLGA/fibrin Scaffolds for chondrogenesis of human adipose derived stem cells by icariin. Journal of Kerman University of Medical Sciences 2020; 27(1): 14-23.
- Hashemibeni B, Pourentezari M, Valiani A, Zamani M, Mardani M. Effect of icariin on the chondrogenesis of human adipose derived stem cells on poly (lactic-co-glycolic) acid/fibrin composite scaffold. Int J Adv Biotech Res. 2017; 8(2): 595-605.
- Randolph MA, Anseth K, Yaremchuk MJ. Tissue engineering of cartilage. Clinics in Plastic Surgery 2003; 30(4): 519-37.
- Marlovits S, Hombauer M, Truppe M, Vecsei V, Schlegel W. Changes in the ratio of type-I and type-II collagen expression during monolayer culture of human chondrocytes. The Journal of Bone and Joint Surgery 2004; 86(2): 286-95.
- Narcisi R, Cleary MA, Brama PA, Hoogduijn MJ, Tüysüz N, ten Berge D, et al. Long-term expansion, enhanced chondrogenic potential, and suppression of endochondral ossification of adult human MSCs via WNT signaling modulation. Stem Cell Reports 2015; 4(3): 459-72.
- Eger W, Schumacher BL, Mollenhauer J, Kuettner KE, Cole AA. Human knee and ankle cartilage explants: catabolic differences. Journal of Orthopaedic Research 2002; 20(3): 526-34.
- Bian L, Zhai DY, Tous E, Rai R, Mauck RL, Burdick JA. Enhanced MSC chondrogenesis following delivery of TGF-β3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials 2011; 32(27): 6425-434.
- Hashemibeni B, Mardani M, Valiani A, Pourentezari M, Anvari M, Yadegari M, et al. Effects of avocado/soybean on the chondrogenesis of human adipose-derived stem cells cultured on polylactic-co-glycolic acid/fibrin hybrid scaffold. Journal of Applied Biotechnology Reports 2019; 6(4): 145-50.
- Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell and Tissue Research 2007; 327(3): 449-62.
- Fellows CR, Matta C, Zakany R, Khan IM, Mobasheri A. Adipose, bone marrow and synovial joint-derived mesenchymal stem cells for cartilage repair. Frontiers in Genetics 2016; 20(2): 213.
- Madry H, Rey-Rico A, Venkatesan JK, Johnstone B, Cucchiarini M. Transforming growth factor beta-releasing scaffolds for cartilage tissue engineering. Tissue Engineering Part B: Reviews 2014; 20(2): 106-25.
- Anvari M, Dortaj H, Hashemibeni B, Pourentezari M. Application of some herbal medicine used for the treatment of osteoarthritis and chondrogenesis. Traditional and Integrative Medicine 2020; 5(3): 126-149.
- Nikolova MP, Chavali MS. Recent advances in biomaterials for 3D scaffolds: A review. Bioactive materials 2019; 4: 271-92.
- Park IK, Cho CS. Stem cell-assisted approaches for cartilage tissue engineering. International Journal of Stem Cells 2010; 3(2): 96.
- Ahmed TA, Hincke MT. Strategies for articular cartilage lesion repair and functional restoration. Tissue Engineering Part B: Reviews. 2010; 16(3): 305-29.
- Stoop R. Smart biomaterials for tissue engineering of cartilage. Injury 2008; 39(1): 77-87.
- Schulz RM, Bader A. Cartilage tissue engineering and bioreactor systems for the cultivation and stimulation of chondrocytes. European Biophysics Journal 2007; 36(4-5): 539-68.
- Bian L, Fong JV, Lima EG, Stoker AM, Ateshian GA, Cook JL, et al. Dynamic mechanical loading enhances functional properties of tissue-engineered cartilage using mature canine chondrocytes. Tissue Engineering Part A 2010; 16(5): 1781-90.
- Ikada Y. Challenges in tissue engineering. Journal of the Royal Society Interface 2006; 3(10): 589-601.
- Kaczmarek B, Sionkowska A, Gołyńska M, Polkowska I, Szponder T, Nehrbass D, et al. In vivo study on scaffolds based on chitosan, collagen, and hyaluronic acid with hydroxyapatite. International Journal of Biological Macromolecules 2018; 118:938-44.
- Rodríguez-Vázquez M, Vega-Ruiz B, Ramos-Zúñiga R, Saldaña-Koppel DA, Quiñones-Olvera LF. Chitosan and its potential use as a scaffold for tissue engineering in regenerative medicine. BioMed Research International 2015; 2015: 821279.
- Dicker KT, Gurski LA, Pradhan-Bhatt S, Witt RL, Farach-Carson MC, Jia X. Hyaluronan: a simple polysaccharide with diverse biological functions. Acta Biomaterialia 2014; 10(4): 1558-70.
- Snetkov P, Zakharova K, Morozkina S, Olekhnovich R, Uspenskaya M. Hyaluronic acid: the influence of molecular weight on structural, physical, physico-chemical, and degradable properties of biopolymer. Polymers 2020; 12(8): 1800.
- Amorim S, Reis CA, Reis RL, Pires RA. Extracellular matrix mimics using hyaluronan-based biomaterials. Trends in Biotechnology 2020; 39(1): 90-104.
- Miri AK, Li NY, Avazmohammadi R, Thibeault SL, Mongrain R, Mongeau L. Study of extracellular matrix in vocal fold biomechanics using a two-phase model. Biomechanics and modeling in mechanobiology 2015; 14(1): 49-57.
- Sparavigna A. Role of the extracellular matrix in skin aging and dedicated treatment-state of the art. Plastic and Aesthetic Research 2020; 7: 14.
- Maiz-Fernández S, Pérez-Álvarez L, Ruiz-Rubio L, Pérez González R, Sáez-Martínez V, Ruiz Pérez J, et al. Synthesis and characterization of covalently crosslinked pH-responsive hyaluronic acid nanogels: effect of synthesis parameters. Polymers 2019; 11(4): 742.
- Romanò C, De Vecchi E, Bortolin M, Morelli I, Drago L. Hyaluronic acid and its composites as a local antimicrobial/antiadhesive barrier. Journal of Bone and Joint Infection 2017; 2(1): 63.
- Lin W, Liu Z, Kampf N, Klein J. The role of hyaluronic acid in cartilage boundary lubrication. Cells 2020; 9(7): 1606.
- Hu Y, Wang Y, Tong Y. Optic perineuritis secondary to hyaluronic acid injections: a case report. BMC Ophthalmology. 2019; 19(1): 241.
- Huang L, Wang Y, Liu H, Huang J. Local injection of high-molecular hyaluronan promotes wound healing in old rats by increasing angiogenesis. Oncotarget 2018; 9(9): 8241.
- Sharifian Z, Hashemibeni B, Pourentezari M, Valiani A, Mardani M, Rarani MZ, et al. Comparison of PLGA/fibrin and PLGA/hyaluronic acid scaffolds for chondrogenesis of human adipose-derived stem cells. International Journal of Medical Laboratory 2020; 7(2): 128-137.
- Park YD, Tirelli N, Hubbell JA. Photopolymerized hyaluronic acid-based hydrogels and interpenetrating networks. Biomaterials. 2003; 24(6): 893-900.
- Menaa F, Menaa A, Menaa B. Hyaluronic acid and derivatives for tissue engineering. J Biotechnol Biomater S. 2011; 3:1.
- Lee JY, Spicer AP. Hyaluronan: a multifunctional, megaDalton, stealth molecule. Current Opinion in Cell Biology 2000; 12(5): 581-6.
- Collins MN, Birkinshaw C. Comparison of the effectiveness of four different cross-linking agents with hyaluronic acid hydrogel films for tissue-culture applications. Journal of Applied Polymer Science. 2007; 104(5): 3183-191.
- Pescosolido L, Schuurman W, Malda J, Matricardi P, Alhaique F, Coviello T, et al. Hyaluronic acid and dextran-based semi-IPN hydrogels as biomaterials for bioprinting. Biomacromolecules 2011; 12(5): 1831-838.
- Stern R, Kogan G, Jedrzejas MJ, Šoltés L. The many ways to cleave hyaluronan. Biotechnology advances 2007; 25(6): 537-57.
- Hachet E, Van Den Berghe Hln, Bayma E, Block MR, Auzély-Velty R. Design of biomimetic cell-interactive substrates using hyaluronic acid hydrogels with tunable mechanical properties. Biomacromolecules 2012; 13(6): 1818-827.
- Mayol L, Biondi M, Russo L, Malle BM, Schwach-Abdellaoui K, Borzacchiello A. Amphiphilic hyaluronic acid derivatives toward the design of micelles for the sustained delivery of hydrophobic drugs. Carbohydrate Polymers 2014; 102: 110-16.
- Borzacchiello A, Russo L, Malle BM, Schwach-Abdellaoui K, Ambrosio L. Hyaluronic acid based hydrogels for regenerative medicine applications. BioMed Research International 2015; 2015: 871218.
- Yuan T, He L, Yang J, Zhang L, Xiao Y, Fan Y, et al. Conjugated icariin promotes tissue-engineered cartilage formation in hyaluronic acid/collagen hydrogel. Process Biochemistry 2015; 50(12): 2242-250.
- Mondal S, Haridas N, Letha SS, Vijith V, Rajmohan G, Rosemary M. Development of injectable high molecular weight hyaluronic acid hydrogels for cartilage regeneration. Journal of Macromolecular Science, Part A. 2016; 53(8): 507-14.
- Chen CH, Kuo CY, Wang YJ, Chen JP. Dual function of glucosamine in gelatin/ hyaluronic acid cryogel to modulate scaffold mechanical properties and to maintain chondrogenic phenotype for cartilage tissue engineering. International Journal of Molecular Sciences 2016; 17(11): 1957.
- Mahapatra C, Jin GZ, Kim HW. Alginate-hyaluronic acid-collagen composite hydrogel favorable for the culture of chondrocytes
and their phenotype maintenance. Tissue Engineering and Regenerative Medicine 2016; 13(5): 538-46. - Park H, Kim SW, Lee JW, Lee KY. Injectable hydrogels prepared from partially oxidized hyaluronate and glycol chitosan for chondrocyte encapsulation. Carbohydrate Polymers 2017; 157: 1281-287.
- Amann E, Wolff P, Breel E, van Griensven M, Balmayor ER. Hyaluronic acid facilitates chondrogenesis and matrix deposition of human adipose derived mesenchymal stem cells and human chondrocytes co-cultures. Acta Biomaterialia 2017; 52: 130-44.
- Liu C, Liu D, Wang Y, Li Y, Li T, Zhou Z, et al. Glycol chitosan/oxidized hyaluronic acid hydrogels functionalized with cartilage extracellular matrix particles and incorporating BMSCs for cartilage repair. Artificial Cells, Nanomedicine, and Biotechnology. 2018; 46(S1): 721-32.
- Lin H, Beck AM, Shimomura K, Sohn J,
Fritch MR, Deng Y, et al. Optimization of photocrosslinked gelatin/hyaluronic acid hybrid scaffold for the repair of cartilage defect. Journal of Tissue Engineering and Regenerative Medicine 2019; 13(8): 1418-29. - Jooybar E, Abdekhodaie MJ, Alvi M, Mousavi A, Karperien M, Dijkstra PJ. An injectable platelet lysate-hyaluronic acid hydrogel supports cellular activities and induces chondrogenesis of encapsulated mesenchymal stem cells. Acta Biomaterialia 2019; 83: 233-44.
- Wang J, Sun X, Zhang Z, Wang Y, Huang C, Yang C, et al. Silk fibroin/collagen/hyaluronic acid scaffold incorporating pilose antler polypeptides microspheres for cartilage tissue engineering. Materials Science and Engineering: Part C 2019; 94: 35-44.
- Ren Y, Zhang H, Qin W, Du B, Liu L, Yang J. A collagen mimetic peptide-modified hyaluronic acid hydrogel system with enzymatically mediated degradation for mesenchymal stem cell differentiation. Materials Science and Engineering: Part C 2020; 108: 110276.
- Tsanaktsidou E, Kammona O, Labude N, Neuss S, Krüger M, Kock L, et al. Biomimetic cell-laden MeHA hydrogels for the regeneration of cartilage tissue. Polymers 2020; 12(7): 1598.
Type of Study: Research |
Subject:
Genetics/ Biotechnology
Received: 2021/05/9 | Accepted: 2022/02/28 | Published: 2021/03/29
Received: 2021/05/9 | Accepted: 2022/02/28 | Published: 2021/03/29
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




