Tue, Jan 27, 2026
[Archive]
Volume 9, Issue 3 (August 2022)
IJML 2022, 9(3): 187-197 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Marjani A, Hesamizadeh K, Bokharaei-Salim F, Khanaliha K, Karbalaie Niya M H, Habib Z et al . Distribution of Epstein-Barr Virus and Human Herpesvirus 8 Co-Infections among Human Immunodeficiency Virus -1 Positive Patients. IJML 2022; 9 (3) :187-197
URL: http://ijml.ssu.ac.ir/article-1-435-en.html
URL: http://ijml.ssu.ac.ir/article-1-435-en.html
Arezoo Marjani 

 , Khashayar Hesamizadeh
, Khashayar Hesamizadeh 

 , Farah Bokharaei-Salim
, Farah Bokharaei-Salim 

 , Khadijeh Khanaliha
, Khadijeh Khanaliha 

 , Mohammad Hadi Karbalaie Niya
, Mohammad Hadi Karbalaie Niya 

 , Zahra Habib
, Zahra Habib 

 , Maryam Esghaei *
, Maryam Esghaei * 




 , Khashayar Hesamizadeh
, Khashayar Hesamizadeh 

 , Farah Bokharaei-Salim
, Farah Bokharaei-Salim 

 , Khadijeh Khanaliha
, Khadijeh Khanaliha 

 , Mohammad Hadi Karbalaie Niya
, Mohammad Hadi Karbalaie Niya 

 , Zahra Habib
, Zahra Habib 

 , Maryam Esghaei *
, Maryam Esghaei * 


Department of Virology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
Full-Text [PDF 421 kb]
(529 Downloads)
| Abstract (HTML) (1711 Views)
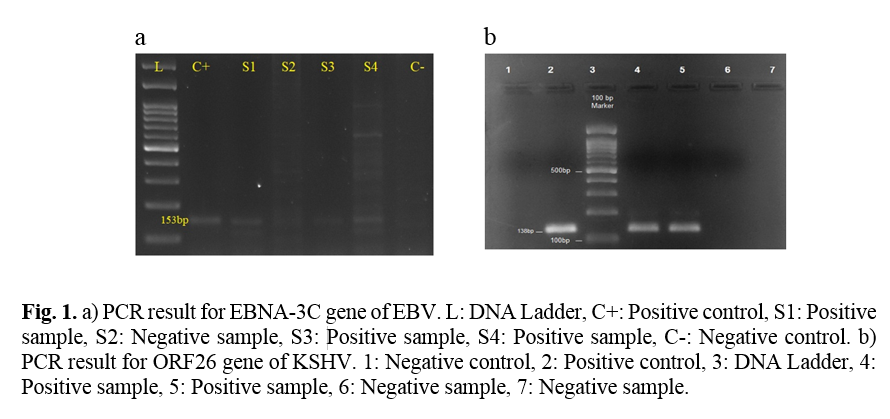
Full-Text: (659 Views)
Introduction
The Human Immunodeficiency Virus/ Acquired Immunodeficiency Syndrome (HIV/ AIDS) remains a significant problem worldwide. So far, 770'000 patients worldwide have died from HIV and HIV-related diseases. Viral suppression is accomplished by combining antiretroviral therapy, including a complex of at least three or more Antiretroviral drugs. However, a prolonged lifespan has been observed among HIV-infected patients with the availability of Highly Active Antiviral Therapy (HAART) [1, 2]. However, opportunistic infections (OIs) continue to be the leading cause of mortality among HIV/AIDS-infected patients worldwide.
OIs related to HIV-infected individuals negatively affect the quality of life and accelerate progress towards AIDS in these patients, and, as a result, they drastically decrease the effects of treatment with ART. Following infection with HIV, the host
immune system is weakened; consequently, opportunistic infections and malignancies threaten the patient [3]. In these patients, co-infection with opportunistic viruses, such as Epstein-Barr virus (EBV) (or human herpes virus (HHV)-4 and HHV-8 or Kaposi sarcoma-associated herpesvirus (KSHV), occurs [4]. Herpesviruses like EBV are ubiquitous, but KSHV does not have this feature. The prevalence of KSHV in sub-Saharan Africa has been reported to be high (> 50%); however, it is uncommon in Asia, European countries, and the United States (< 10%) [5, 6]. EBV and KSHV are classified as members of the herpesviral family, gammaherpesvirus subfamily. Gammaherpesviruses have a great tropism to lymphocytes [7]. Among HIV/AIDS individuals, KSHV infection can lead to Kaposi’s sarcoma. This malignancy is caused by cells on the blood vessels or lymph nodes that may spread to other organs [3]. Interestingly, EBV and KSHV can cause latent infection, during which EBV and KSHV can express non-coding RNAs and proteins that cause cellular proliferation, which is essential for their life cycle. Generally, an efficient immune system prevents this mechanism, while in an immunocompromised or suppressed immune system, OIs such as EBV and
KSHV could establish malignancies and lymphoproliferative disorders [8]. The EBV involves cancers such as Hodgkin's lymphoma, gastric cancer, nasopharyngeal carcinomas, and Burkitt's lymphoma [9]. In addition, the role of the KSHV has been observed in multicentric Castleman disease, Kaposi sarcoma, and primary effusion lymphoma [6].
Previous studies in the Iranian population have provided limited information on EBV and KSHV among HIV-1-infected individuals [10]. Therefore, the present study was carried out to evaluate the prevalence of EBV and KSHV infections in Iranian HIV-1-positive patients with and without HAART therapy.
Materials and Methods
Study design
A cross-sectional study was conducted to determine the prevalence of EBV and KSHV infection in saliva obtained from HIV-infected patients in the Iranian population with and without HAART therapy. Data were collected from patients who underwent treatment at the hospitals affiliated with the Iran University of Medical Sciences, Tehran, Iran, from 2018 to 2019. All participants were asked to sign the informed consent in accordance with the Helsinki declaration. The study was approved by the Ethical Committee of the Research Deputy at Iran University of Medical Sciences, Tehran, Iran (code no: IR.IUMS.FMD.REC. 1398.018).
Study population
Inclusion criteria were being positive for HIV infection based on the data repository, not being at the end stage of the disease (AIDS), and agreeing to participate in the study by signing informed consent. Excluded patients were individuals without detectable HIV viral load and those who did not complete the questionnaire and datasets.
Saliva and serum samples (n=103) were collected from all the patients participating in the study. The laboratory information was collected at admission during the research period. Of all 103 included patients, 59 (57.3%) were male. A professional practitioner filled out the questionnaire for each patient or used the data repository. Also, the patient's demographic characteristics, including gender, age, age category, liver enzyme levels, and CD4 count, were recorded.
Sample preparation
About 3 ml of saliva samples were collected from patients into sterile tubes and stored at −70 °C until use. In addition, 5 ml of whole blood was collected from each patient, and the serum was separated via centrifugation after clotting.
Serologic tests
Anti-EBV IgG Enzyme-Linked Immunosorbent Assay (ELISA) was performed using the Anti-Epstein Barr virus (EBV-VCA) IgG Human ELISA Kit (ab108730, Abcam, Cambridge, United Kingdom), according to kit instructions. The absorbance value of 0.150–1.300 was considered the cut-off point. The positive samples had values more than the cut-off point. Anti-KSHV/HHV8 IgG ELISA testing was accomplished using the human herpes virus type 8 IgG antibody (HHV8-Ab-IgG) ELISA Kit (MBS2800428, San Diego, California, United States), according to the kit instructions. The positive results were reported by OD sample ≥ 0.10 and negative values by OD < 0.10.
DNA extraction
According to the manufacturer's instructions, DNA was extracted from saliva using a DNA extraction kit (QIAamp® DNA Mini Kit, Qiagen, GmbH, Germany). The quantity, quantification, and purity of the extracted DNA (OD 260/280 nm) were determined using the Nano Drop™ 1000 Spectrophotometer by Thermo Fisher Scientific. The extracted genomic DNA was stored at -20 ˚C until use.
Polymerase chain reaction (PCR)
In the present study, the EBV EBNA-3 gene was detected using conventional PCR, and KSHV minor capsid protein (encoded by ORF26) was detected via nested-PCR. In each PCR reaction test, 200-500 μg/ μl of DNA was used. The primers used are shown in Table 1 [11, 12].
The first round of nested PCR for KSHV was accomplished in a 25μL mixture including 200-500 μg/μl of each extracted DNA, 1.5 U of Taq DNA polymerase, 2.5 μL of 10X PCR Buffer, 10 pM of forward and reverse primers (First round primers), 200 μM mix dNTPs, and 1.5 mM MgCl2 concentration as well as distilled water added to the rest of the volume. Amplification was performed that included pre-denaturation at 95 °C for 5 min (1 cycle), and 35 cycles of denaturation at 95 °C for 40 seconds, annealing at 55 °C or 45 seconds, and extension at 72 °C for 40 seconds, followed by a post extension at 72 °C for 8 min. Then, using the inner primer pair, 200-500 μg/ μl of PCR product of the first amplification was added to the second stage of PCR amplification. In the second round of nested-PCR, the same process was accomplished, as demonstrated in the first round, using the second round primers. The second round was performed using the following protocol: pre-denaturation at 95 °C for 5min (1 cycle), 35 cycles of denaturation at 95 °C for 35 seconds, annealing at 55 °C or 40 seconds, and extension at 72 °C for 35 seconds, followed by a post extension at 72 °C for 8 min.
For EBV PCR, a 25 μL mixture including 200-500 μg/ μl of sample or control, 12.5 μl of master mix, 0.5 μl of forward and reverse primers, and 9 μl distilled water were added. Amplification was performed, which included pre-denaturation at 94 °C for 10 min (1 cycle), 35 cycles of denaturation at 94 °C for 30 seconds, annealing at 58 °C or 30 seconds, and extension at 72 °C for 30 seconds, followed by a final extension at 72 °C for 5 min.
Using agarose gel electrophoresis, PCR products, and DNA ladder along with positive and negative controls could be visualized.
Quality control
Each ELISA assay for EBV and KSHV qualified by the cut-off standards. In this regard, the EBV cut-off was valued as greater than 1.3 for positive results, and for the KSHV, it was greater than 0.1.
Also, a positive and a negative sample were used for the extraction quality control, then used as a control for PCR. Nucleotide sequencing was used as a confirmatory test for PCR results. One of the positive PCR products from each virus was sequenced; raw data were trimmed by CLC workbench five bioinformatics software and confirmed by Basic Local Alignment Search Tool online software (https://blast.ncbi.nlm.nih.gov).
Statistical analysis
Statistical analyses were performed using SPSS (SPSS Inc, Chicago, IL, USA), version 20. Chi-square and appropriate parametric tests were applied to analyze gender, age category, and patients under treatment with HAART.
Results
The mean age ± SD of the patients was 43.9 ± 16.1 years (range: 18-82 years). Of 103 participants, 59 (57.3%) were male and the age groups were 10-19 years 4.9% (n = 5), 20-29 years 18.4% (n=19), 30-39 years 22.3% (n = 23), 40-49 years 14.6% (n = 15), and 50-89 years 39.8% (n = 41). Different age categories and positive and negative results are shown in Table 2. The mean CD4 count ± SD of all 103 patients was 532.47 ± 212 cells/μL (range 142 - 995 cells/μL), and the mean aspartate aminotransferase ± SD was 16.50 ± 6.97 IU/L (range 8-35 IU/L) and the mean alanine aminotransferase ± SD was 16.07 ± 6.33 IU/L (range 7-33 IU/L). The present study observed no significant relationship between demographic variables, including age, CD4 count, aspartate aminotransferase, alanine aminotransferase, and gender.
Using the PCR results, KSHV DNA was found in 19 (18.4%) patients and EBV DNA in 61 (59.2%) (Figure 1). Moreover, KSHV antibody and EBV were detected in 73 (70.9%) and 97 (94.2%) patients, respectively (Table 3).
71.8% (74/103) of HIV-infected participants were under treatment with HAART, and 27.2% (28/103) were new cases. The frequency rates of different laboratory tests in HIV-infected patients in these two groups are shown in Table 4.
No statistically significant association was found between gender and KSHV antibody in serum, EBV antibody in serum, KSHV DNA in saliva, and EBV DNA in saliva. Also, no statistically significant association was found between the age category and KSHV antibody, EBV antibody, KSHV DNA, and EBV DNA. In addition, no statistically significant association was observed between patients under treatment with HAART, KSHV, and EBV antibodies. Meanwhile, a significant relationship was found between patients under treatment with HAART, KSHV DNA, and EBV DNA (Table 4).
OIs related to HIV-infected individuals negatively affect the quality of life and accelerate progress towards AIDS in these patients, and, as a result, they drastically decrease the effects of treatment with ART. Following infection with HIV, the host
immune system is weakened; consequently, opportunistic infections and malignancies threaten the patient [3]. In these patients, co-infection with opportunistic viruses, such as Epstein-Barr virus (EBV) (or human herpes virus (HHV)-4 and HHV-8 or Kaposi sarcoma-associated herpesvirus (KSHV), occurs [4]. Herpesviruses like EBV are ubiquitous, but KSHV does not have this feature. The prevalence of KSHV in sub-Saharan Africa has been reported to be high (> 50%); however, it is uncommon in Asia, European countries, and the United States (< 10%) [5, 6]. EBV and KSHV are classified as members of the herpesviral family, gammaherpesvirus subfamily. Gammaherpesviruses have a great tropism to lymphocytes [7]. Among HIV/AIDS individuals, KSHV infection can lead to Kaposi’s sarcoma. This malignancy is caused by cells on the blood vessels or lymph nodes that may spread to other organs [3]. Interestingly, EBV and KSHV can cause latent infection, during which EBV and KSHV can express non-coding RNAs and proteins that cause cellular proliferation, which is essential for their life cycle. Generally, an efficient immune system prevents this mechanism, while in an immunocompromised or suppressed immune system, OIs such as EBV and
KSHV could establish malignancies and lymphoproliferative disorders [8]. The EBV involves cancers such as Hodgkin's lymphoma, gastric cancer, nasopharyngeal carcinomas, and Burkitt's lymphoma [9]. In addition, the role of the KSHV has been observed in multicentric Castleman disease, Kaposi sarcoma, and primary effusion lymphoma [6].
Previous studies in the Iranian population have provided limited information on EBV and KSHV among HIV-1-infected individuals [10]. Therefore, the present study was carried out to evaluate the prevalence of EBV and KSHV infections in Iranian HIV-1-positive patients with and without HAART therapy.
Materials and Methods
Study design
A cross-sectional study was conducted to determine the prevalence of EBV and KSHV infection in saliva obtained from HIV-infected patients in the Iranian population with and without HAART therapy. Data were collected from patients who underwent treatment at the hospitals affiliated with the Iran University of Medical Sciences, Tehran, Iran, from 2018 to 2019. All participants were asked to sign the informed consent in accordance with the Helsinki declaration. The study was approved by the Ethical Committee of the Research Deputy at Iran University of Medical Sciences, Tehran, Iran (code no: IR.IUMS.FMD.REC. 1398.018).
Study population
Inclusion criteria were being positive for HIV infection based on the data repository, not being at the end stage of the disease (AIDS), and agreeing to participate in the study by signing informed consent. Excluded patients were individuals without detectable HIV viral load and those who did not complete the questionnaire and datasets.
Saliva and serum samples (n=103) were collected from all the patients participating in the study. The laboratory information was collected at admission during the research period. Of all 103 included patients, 59 (57.3%) were male. A professional practitioner filled out the questionnaire for each patient or used the data repository. Also, the patient's demographic characteristics, including gender, age, age category, liver enzyme levels, and CD4 count, were recorded.
Sample preparation
About 3 ml of saliva samples were collected from patients into sterile tubes and stored at −70 °C until use. In addition, 5 ml of whole blood was collected from each patient, and the serum was separated via centrifugation after clotting.
Serologic tests
Anti-EBV IgG Enzyme-Linked Immunosorbent Assay (ELISA) was performed using the Anti-Epstein Barr virus (EBV-VCA) IgG Human ELISA Kit (ab108730, Abcam, Cambridge, United Kingdom), according to kit instructions. The absorbance value of 0.150–1.300 was considered the cut-off point. The positive samples had values more than the cut-off point. Anti-KSHV/HHV8 IgG ELISA testing was accomplished using the human herpes virus type 8 IgG antibody (HHV8-Ab-IgG) ELISA Kit (MBS2800428, San Diego, California, United States), according to the kit instructions. The positive results were reported by OD sample ≥ 0.10 and negative values by OD < 0.10.
DNA extraction
According to the manufacturer's instructions, DNA was extracted from saliva using a DNA extraction kit (QIAamp® DNA Mini Kit, Qiagen, GmbH, Germany). The quantity, quantification, and purity of the extracted DNA (OD 260/280 nm) were determined using the Nano Drop™ 1000 Spectrophotometer by Thermo Fisher Scientific. The extracted genomic DNA was stored at -20 ˚C until use.
Polymerase chain reaction (PCR)
In the present study, the EBV EBNA-3 gene was detected using conventional PCR, and KSHV minor capsid protein (encoded by ORF26) was detected via nested-PCR. In each PCR reaction test, 200-500 μg/ μl of DNA was used. The primers used are shown in Table 1 [11, 12].
The first round of nested PCR for KSHV was accomplished in a 25μL mixture including 200-500 μg/μl of each extracted DNA, 1.5 U of Taq DNA polymerase, 2.5 μL of 10X PCR Buffer, 10 pM of forward and reverse primers (First round primers), 200 μM mix dNTPs, and 1.5 mM MgCl2 concentration as well as distilled water added to the rest of the volume. Amplification was performed that included pre-denaturation at 95 °C for 5 min (1 cycle), and 35 cycles of denaturation at 95 °C for 40 seconds, annealing at 55 °C or 45 seconds, and extension at 72 °C for 40 seconds, followed by a post extension at 72 °C for 8 min. Then, using the inner primer pair, 200-500 μg/ μl of PCR product of the first amplification was added to the second stage of PCR amplification. In the second round of nested-PCR, the same process was accomplished, as demonstrated in the first round, using the second round primers. The second round was performed using the following protocol: pre-denaturation at 95 °C for 5min (1 cycle), 35 cycles of denaturation at 95 °C for 35 seconds, annealing at 55 °C or 40 seconds, and extension at 72 °C for 35 seconds, followed by a post extension at 72 °C for 8 min.
For EBV PCR, a 25 μL mixture including 200-500 μg/ μl of sample or control, 12.5 μl of master mix, 0.5 μl of forward and reverse primers, and 9 μl distilled water were added. Amplification was performed, which included pre-denaturation at 94 °C for 10 min (1 cycle), 35 cycles of denaturation at 94 °C for 30 seconds, annealing at 58 °C or 30 seconds, and extension at 72 °C for 30 seconds, followed by a final extension at 72 °C for 5 min.
Using agarose gel electrophoresis, PCR products, and DNA ladder along with positive and negative controls could be visualized.
Quality control
Each ELISA assay for EBV and KSHV qualified by the cut-off standards. In this regard, the EBV cut-off was valued as greater than 1.3 for positive results, and for the KSHV, it was greater than 0.1.
Also, a positive and a negative sample were used for the extraction quality control, then used as a control for PCR. Nucleotide sequencing was used as a confirmatory test for PCR results. One of the positive PCR products from each virus was sequenced; raw data were trimmed by CLC workbench five bioinformatics software and confirmed by Basic Local Alignment Search Tool online software (https://blast.ncbi.nlm.nih.gov).
Statistical analysis
Statistical analyses were performed using SPSS (SPSS Inc, Chicago, IL, USA), version 20. Chi-square and appropriate parametric tests were applied to analyze gender, age category, and patients under treatment with HAART.
Results
The mean age ± SD of the patients was 43.9 ± 16.1 years (range: 18-82 years). Of 103 participants, 59 (57.3%) were male and the age groups were 10-19 years 4.9% (n = 5), 20-29 years 18.4% (n=19), 30-39 years 22.3% (n = 23), 40-49 years 14.6% (n = 15), and 50-89 years 39.8% (n = 41). Different age categories and positive and negative results are shown in Table 2. The mean CD4 count ± SD of all 103 patients was 532.47 ± 212 cells/μL (range 142 - 995 cells/μL), and the mean aspartate aminotransferase ± SD was 16.50 ± 6.97 IU/L (range 8-35 IU/L) and the mean alanine aminotransferase ± SD was 16.07 ± 6.33 IU/L (range 7-33 IU/L). The present study observed no significant relationship between demographic variables, including age, CD4 count, aspartate aminotransferase, alanine aminotransferase, and gender.
Using the PCR results, KSHV DNA was found in 19 (18.4%) patients and EBV DNA in 61 (59.2%) (Figure 1). Moreover, KSHV antibody and EBV were detected in 73 (70.9%) and 97 (94.2%) patients, respectively (Table 3).
71.8% (74/103) of HIV-infected participants were under treatment with HAART, and 27.2% (28/103) were new cases. The frequency rates of different laboratory tests in HIV-infected patients in these two groups are shown in Table 4.
No statistically significant association was found between gender and KSHV antibody in serum, EBV antibody in serum, KSHV DNA in saliva, and EBV DNA in saliva. Also, no statistically significant association was found between the age category and KSHV antibody, EBV antibody, KSHV DNA, and EBV DNA. In addition, no statistically significant association was observed between patients under treatment with HAART, KSHV, and EBV antibodies. Meanwhile, a significant relationship was found between patients under treatment with HAART, KSHV DNA, and EBV DNA (Table 4).
Table 1. The list of primers used to PCR and Nested-PCR
| Sequence 5′ to 3′ | Base pair (bp) | Gene | Virus | |
| F-AGA AGG GGA GCG TGT GTT GT R-GGC TCG TTT TTG ACG TCG GC |
153 |
EBNA3C | EBV | |
| F-AGC CGA AAG GAT TCC ACC AT R-TCC GTG TTG TCT ACG TCC AG |
First round | 233 | Minor-capsid/ ORF26 | KSHV |
| F-TAT TCT GCA GCA GCT GTT GG R- TCT ACG TCC AGA CGA TAT GTG C |
Second round | 138 | ||
EBV= Epstein-Barr virus; KSHV= Kaposi sarcoma-associated herpesvirus
Table 2. KSHV DNA, EBV DNA, KSHV antibodies, and EBV antibodies in different age categories
| Age categories | KSHV DNA | EBV DNA | KSHV antibody | EBV antibody | ||||
| Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | |
| 10-19 | 2 | 3 | 3 | 2 | 3 | 2 | 5 | 0 |
| 20-29 | 5 | 14 | 10 | 9 | 13 | 5 | 18 | 1 |
| 30-39 | 5 | 18 | 16 | 7 | 17 | 6 | 22 | 1 |
| 40-49 | 3 | 12 | 12 | 3 | 11 | 4 | 14 | 1 |
| 50-89 | 4 | 36 | 20 | 20 | 29 | 10 | 38 | 1 |
KSHV= Kaposi sarcoma-associated herpesvirus; EBV= Epstein-Barr virus;
Table 3. Demographic parameters for all participants
| Parameters | Male | Female | Total | P- Value | |
| No. | 59 (57.3) | 44 (42.7) | 103 (100%) | - | |
| Age | 46.5 ± 16.3 | 40.3 ± 15.3 | 43.9 ± 16.1 | 0.250* | |
| CD4 count (cells/μL) | 520.7 ± 213.6 | 548.2 ± 213.4 | 532.47 ± 212 | 0.380* | |
| AST (IU/L) | 17.3 ± 7.7 | 15.4 ± 5.8 | 16.50 ± 6.97 | 0.745* | |
| ALT (IU/L) | 16.8 ± 6.6 | 15.1 ± 5.9 | 16.07 ± 6.33 | 0.799* | |
| PCR KSHV | Positive | 11 (18.6%) | 8 (18.2%) | 19 (18.4%) | 0.996 |
| Negative | 48 (81.4%) | 35 (79.5%) | 83 (80.6%) | ||
| PCR EBV | Positive | 33 (55.9%) | 28 (63.6%) | 61 (59.2%) | 0.492 |
| Negative | 25 (42.4%) | 16 (36.4%) | 41 (39.8%) | ||
| KSHV antibody | Positive | 40 (67.8%) | 33 (75.0%) | 73 (70.9%) | 0.464 |
| Negative | 17 (28.8%) | 10 (22.7%) | 27 (26.2%) | ||
| EBV antibody | Positive | 55 (93.2%) | 42 (95.5%) | 97 (94.2%) | 0.468 |
| Negative | 3 (5.1%) | 1 (2.3%) | 4 (3.9%) | ||
| * Chi-Square | |||||
AST= Aspartate aminotransferase; ALT= Alanine aminotransferase; EBV= Epstein-Barr virus; KSHV= Kaposi sarcoma-associated herpesvirus
Table 4. Frequency of different laboratory tests in HIV- infected patients under treatment with HAART or not under HAART treatment
| HAART treatment | KSHV DNA | EBV DNA | KSHV antibody | EBV antibody | ||||
| Yes | Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative |
| 8 (10.8%) | 65 (87.8%) | 40 (54.1%) | 34 (45.9%) | 55 (74.3%) | 17 (23.0%) | 69 (93.2%) | 3 (4.1%) | |
| No | 11 (39.3%) | 17 (60.7%) | 21 (75.0%) | 6 (21.4%) | 18 (64.3%) | 9 (32.1%) | 27 (96.4%) | 1 (3.6%) |
| P value | 0.001 | 0.031 | 0.328 | 0.892 | ||||
HAART= Highly active antiretroviral therapy

Discussion
According to previous studies, HIV/AIDS is currently a health problem in Iran [13, 14]. So, opportunistic infections such as KSHV and EBV have become important as common HIV/AIDS-related malignancies. In the present study, we examined HIV-1 positive individuals' blood and saliva specimens to understand the prevalence of KSHV and EBV using PCR and ELISA. Generally, we found the following frequency rates: KSHV antibody in serum 70.9%, EBV antibody in serum 94.2%, KSHV DNA in saliva 18.4%, and EBV DNA in saliva 59.2%. Statistically, we have found a significant relationship between being under treatment with HAART and the presence of KSHV and EBV DNA in saliva.
In one study conducted by Hesamizadeh et al. in Iran on 109 HIV-infected individuals, KSHV DNA was detected in plasma and peripheral blood mononuclear cells (PBMC) specimens of 3.6% (n = 4) and 5.5% (n = 6) HIV-infected patients, respectively. The present study detected KSHV DNA in saliva specimens of 18.4% (n = 19) of HIV-infected patients. Our results demonstrated that the prevalence of KSHV DNA in the saliva is higher than that reported by Hesamizadeh K et al. in a similar population. In addition, in our study, the age range of the participants (18-82 years) was not similar to that of Hesamizadeh et al.'s study (2-64 years).
Furthermore, our findings in the current study did not support the results reported by Hesamizadeh K et al. regarding the percentage of patients with the HAART regimen. We had 71.8% (74/103) of the patient under HAART therapy, while Hesamizadeh et al. had only 33% [11]. Thus, the existing differences in the results could be reasonable.
In another study conducted in Iran, KSHV DNA was not reported in HIV-infected patients [10]. In Iran, KSHV DNA in PBMC and plasma samples have been reported to be 5.5% and 3.6%, respectively [11]. In the present investigation, the prevalence of EBV and KSHV indicated that these infections are common in HIV-1 infected individuals in the Iranian population, which is possible because of similar transmission routes of HIV-1 and KSHV infections. However, the results of our study differ from those of some published studies performed in Iran [10, 11].
In samples obtained from 175 patients with nasopharyngeal carcinoma, EBV DNA was observed in 80% of the cases [15]. In Japan, using PCR assay, EBV in saliva was reported to be 48.5% and 15% in individuals with periodontitis and uninfected individuals, respectively [16]. EBV and KSHV DNA in saliva were evaluated in Uganda, too. Shedding of EBV among mothers' saliva was reported to be 72%, and KSHV was 22%; in children's saliva, EBV was 85%, and KSHV was 40%. In saliva, EBV shedding was higher than KSHV shedding [17]. In another study, among HIV-infected individuals, the prevalence rates of EBV DNA, KSHV DNA, Cytomegalovirus DNA, and HSV-1 DNA were reported to be 90%, 57%, 31%, and 16%, respectively [18]. In the Brazilian Amazon region, KSHV DNA in saliva samples was reported to be 23.7% [19], and in Nigeria, the prevalence of HHV8 was 62% in HIV-infected individuals [20]. One study argued that the high prevalence of HHV8 among HIV-1 infected individuals was related to the transmission route of HHV8 by sexual contact [20]. Another study demonstrated that saliva is one of the risk factors associated with HHV infections [18]. Kaposi's sarcoma-associated herpesvirus infection is transmitted through important and different routes, including sexual transmission, saliva, organ transplantation, and blood transfusion [11]. In this study, the main route of KSHV transmission among HIV-1 infected patients was sexual contact. The present study's findings confirmed the findings reported by a previous study conducted by Ogoina et al. [20]. Among men who had sex with men (MSM) with HIV infection, oral shedding of KSHV associated with Human Papillomavirus was reported [21]. In a total of 193 MSM with HIV infection, 76.2% EBV DNA in saliva was reported [22]. In saliva specimens obtained from HIV-infected individuals under treatment with antiretrovirals, the prevalence rates of EBV, KSHV, and CMV DNA were reported to be 73%, 24%, and 27%, respectively [23].
In Brazil, among HIV-infected patients, KSHV DNA was reported in 75% of the individuals studied [24] and 40% in saliva samples [25]. In this country, among HIV-infected patients with or without a HAART regimen, HHV infection in saliva was reported to be common [24]. In another study on HIV-infected individuals, HHV-8 DNA was reported in 5 out of 30 (17%) patients [26]. However, the current study's findings are consistent with those reported from various populations related to this infection [24, 26].
The present study's findings seem to be specifically different from those reported in a study conducted in Indonesia [27]. In our study, KSHV antibodies were detected in 73 out of 103 (70.9%) patients, while in Indonesia, this frequency was reported as 7 out of 91 (7.7%). Differences may be explained by rational, geographical, and habitual differences between the two populations, which need further investigation.
Moreover, the current research results do not support those reported in a previous study conducted in Turkey. In our study, KSHV antibodies were reported in 73 out of 103 (70.9%) patients, while in the study from Turkey, KSHV IgG antibodies were detected in 44 out of 173 (25.4%) individuals infected with HIV [28].
The prevalence of EBV and KSHV in participants of the current study reveals that asymptomatic viral shedding in the saliva is a usual phenomenon. One of the Iranian public health goals is to make antiretroviral drugs accessible and free of cost for all HIV-1 individuals [29]. Worldwide access to HAART has decreased mortality from HIV/AIDS-related illnesses, including opportunistic diseases [1]. The present study, similar to other studies, confirms the presumption that HAART has low efficacy on EBV and KSHV shedding in saliva [30]. Nevertheless, our results demonstrated that EBV and KSHV shedding is high in the saliva of HIV-1 infected patients. These findings are consistent with other data that indicated high EBV and KSHV salivary shedding in immunosuppressed HIV-1 infected patients [18, 31, 32].
Generally, EBV has a lifelong persistent and latent infection. Almost half of the children under the age of 5 are infected with EBV, and as many as 90% of adults deal with this virus at some point in their lives. Thus, it is important to consider the patients' age to analyze and evaluate primary infection or reactivation of EBV. Numerous clinical studies have shown that the prevalence of EBV varies (17.5% to 90%) from country to country. In HIV-infected individuals, the shedding rate of EBV is very high [22]. In children, primary EBV infections are typically asymptomatic, which usually manifests very mild symptoms or symptoms very similar to other viral infections [33].
Nevertheless, the primary infection of EBV in youths results in approximately half of the patients suffering from infectious mononucleosis. Another study reported that serological methods for identifying EBV infection were less sensitive than other methods, such as EBV real-time PCR [34]. HAART reduces opportunistic EBV infection frequency in HIV-positive patients [35]. In China, the presence of EBV DNA was reported in the saliva of 100% of HIV infected population without HAART [36]. The prevalence of EBV infection can vary in different age ranges. In children, EBV infection prevalence was reported to be more than 50% under the age of 3 and more than 90% at the age of 8 and above [33]. It seems that the results of similar studies are affected by variables, including different groups in different populations and individuals' levels of sexual health.
Commonly, infection with herpesviruses, especially EBV and KSHV, happens in early life, and antibodies can be detected lifelong. Although our results showed that most of our samples had positive antibody results, molecular tests such as PCR, which shows a recent infection, may not be positive in all cases and the same result as IgG antibody results.
A major methodological strength of the present study was the simultaneous assessment of EBV and KSHV DNA/antibodies in saliva specimens obtained from HIV-infected individuals. Nevertheless, our research has some limitations that should be considered before any generalizations. Small sample sizes are one of the limitations of the current study. Also, we did not compare HIV-infected patients and those without HIV infection. Another limitation of the present study was the lack of viral load tests for EBV and KSHV. Simultaneous evaluation of EBV DNA and KSHV DNA in saliva specimens from individuals living with HIV was performed in a few studies. Therefore, we could not make more extensive comparisons due to the limited number of these studies. Moreover, limitations of the salivary-based test include possible cross-reactivity of antibodies with those produced against other viruses. Furthermore, this test is indicated for surveillance and not for early diagnosis.
Conclusion
Our findings showed that the presence of KSHV and EBV DNA in the saliva is high in HIV-infected patients in Iran. It may contribute to further complications in these patients, especially in immunocompromised or immunosuppressed ones. Further studies with a greater sample size and control group should be conducted to obtain more complete results.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgments
The authors and researchers would like to thank Keyvan laboratory, Tehran, Iran, for providing PCR samples and controls. This research was supported by the Iran University of Medical Sciences (Grant number: 14401).
In one study conducted by Hesamizadeh et al. in Iran on 109 HIV-infected individuals, KSHV DNA was detected in plasma and peripheral blood mononuclear cells (PBMC) specimens of 3.6% (n = 4) and 5.5% (n = 6) HIV-infected patients, respectively. The present study detected KSHV DNA in saliva specimens of 18.4% (n = 19) of HIV-infected patients. Our results demonstrated that the prevalence of KSHV DNA in the saliva is higher than that reported by Hesamizadeh K et al. in a similar population. In addition, in our study, the age range of the participants (18-82 years) was not similar to that of Hesamizadeh et al.'s study (2-64 years).
Furthermore, our findings in the current study did not support the results reported by Hesamizadeh K et al. regarding the percentage of patients with the HAART regimen. We had 71.8% (74/103) of the patient under HAART therapy, while Hesamizadeh et al. had only 33% [11]. Thus, the existing differences in the results could be reasonable.
In another study conducted in Iran, KSHV DNA was not reported in HIV-infected patients [10]. In Iran, KSHV DNA in PBMC and plasma samples have been reported to be 5.5% and 3.6%, respectively [11]. In the present investigation, the prevalence of EBV and KSHV indicated that these infections are common in HIV-1 infected individuals in the Iranian population, which is possible because of similar transmission routes of HIV-1 and KSHV infections. However, the results of our study differ from those of some published studies performed in Iran [10, 11].
In samples obtained from 175 patients with nasopharyngeal carcinoma, EBV DNA was observed in 80% of the cases [15]. In Japan, using PCR assay, EBV in saliva was reported to be 48.5% and 15% in individuals with periodontitis and uninfected individuals, respectively [16]. EBV and KSHV DNA in saliva were evaluated in Uganda, too. Shedding of EBV among mothers' saliva was reported to be 72%, and KSHV was 22%; in children's saliva, EBV was 85%, and KSHV was 40%. In saliva, EBV shedding was higher than KSHV shedding [17]. In another study, among HIV-infected individuals, the prevalence rates of EBV DNA, KSHV DNA, Cytomegalovirus DNA, and HSV-1 DNA were reported to be 90%, 57%, 31%, and 16%, respectively [18]. In the Brazilian Amazon region, KSHV DNA in saliva samples was reported to be 23.7% [19], and in Nigeria, the prevalence of HHV8 was 62% in HIV-infected individuals [20]. One study argued that the high prevalence of HHV8 among HIV-1 infected individuals was related to the transmission route of HHV8 by sexual contact [20]. Another study demonstrated that saliva is one of the risk factors associated with HHV infections [18]. Kaposi's sarcoma-associated herpesvirus infection is transmitted through important and different routes, including sexual transmission, saliva, organ transplantation, and blood transfusion [11]. In this study, the main route of KSHV transmission among HIV-1 infected patients was sexual contact. The present study's findings confirmed the findings reported by a previous study conducted by Ogoina et al. [20]. Among men who had sex with men (MSM) with HIV infection, oral shedding of KSHV associated with Human Papillomavirus was reported [21]. In a total of 193 MSM with HIV infection, 76.2% EBV DNA in saliva was reported [22]. In saliva specimens obtained from HIV-infected individuals under treatment with antiretrovirals, the prevalence rates of EBV, KSHV, and CMV DNA were reported to be 73%, 24%, and 27%, respectively [23].
In Brazil, among HIV-infected patients, KSHV DNA was reported in 75% of the individuals studied [24] and 40% in saliva samples [25]. In this country, among HIV-infected patients with or without a HAART regimen, HHV infection in saliva was reported to be common [24]. In another study on HIV-infected individuals, HHV-8 DNA was reported in 5 out of 30 (17%) patients [26]. However, the current study's findings are consistent with those reported from various populations related to this infection [24, 26].
The present study's findings seem to be specifically different from those reported in a study conducted in Indonesia [27]. In our study, KSHV antibodies were detected in 73 out of 103 (70.9%) patients, while in Indonesia, this frequency was reported as 7 out of 91 (7.7%). Differences may be explained by rational, geographical, and habitual differences between the two populations, which need further investigation.
Moreover, the current research results do not support those reported in a previous study conducted in Turkey. In our study, KSHV antibodies were reported in 73 out of 103 (70.9%) patients, while in the study from Turkey, KSHV IgG antibodies were detected in 44 out of 173 (25.4%) individuals infected with HIV [28].
The prevalence of EBV and KSHV in participants of the current study reveals that asymptomatic viral shedding in the saliva is a usual phenomenon. One of the Iranian public health goals is to make antiretroviral drugs accessible and free of cost for all HIV-1 individuals [29]. Worldwide access to HAART has decreased mortality from HIV/AIDS-related illnesses, including opportunistic diseases [1]. The present study, similar to other studies, confirms the presumption that HAART has low efficacy on EBV and KSHV shedding in saliva [30]. Nevertheless, our results demonstrated that EBV and KSHV shedding is high in the saliva of HIV-1 infected patients. These findings are consistent with other data that indicated high EBV and KSHV salivary shedding in immunosuppressed HIV-1 infected patients [18, 31, 32].
Generally, EBV has a lifelong persistent and latent infection. Almost half of the children under the age of 5 are infected with EBV, and as many as 90% of adults deal with this virus at some point in their lives. Thus, it is important to consider the patients' age to analyze and evaluate primary infection or reactivation of EBV. Numerous clinical studies have shown that the prevalence of EBV varies (17.5% to 90%) from country to country. In HIV-infected individuals, the shedding rate of EBV is very high [22]. In children, primary EBV infections are typically asymptomatic, which usually manifests very mild symptoms or symptoms very similar to other viral infections [33].
Nevertheless, the primary infection of EBV in youths results in approximately half of the patients suffering from infectious mononucleosis. Another study reported that serological methods for identifying EBV infection were less sensitive than other methods, such as EBV real-time PCR [34]. HAART reduces opportunistic EBV infection frequency in HIV-positive patients [35]. In China, the presence of EBV DNA was reported in the saliva of 100% of HIV infected population without HAART [36]. The prevalence of EBV infection can vary in different age ranges. In children, EBV infection prevalence was reported to be more than 50% under the age of 3 and more than 90% at the age of 8 and above [33]. It seems that the results of similar studies are affected by variables, including different groups in different populations and individuals' levels of sexual health.
Commonly, infection with herpesviruses, especially EBV and KSHV, happens in early life, and antibodies can be detected lifelong. Although our results showed that most of our samples had positive antibody results, molecular tests such as PCR, which shows a recent infection, may not be positive in all cases and the same result as IgG antibody results.
A major methodological strength of the present study was the simultaneous assessment of EBV and KSHV DNA/antibodies in saliva specimens obtained from HIV-infected individuals. Nevertheless, our research has some limitations that should be considered before any generalizations. Small sample sizes are one of the limitations of the current study. Also, we did not compare HIV-infected patients and those without HIV infection. Another limitation of the present study was the lack of viral load tests for EBV and KSHV. Simultaneous evaluation of EBV DNA and KSHV DNA in saliva specimens from individuals living with HIV was performed in a few studies. Therefore, we could not make more extensive comparisons due to the limited number of these studies. Moreover, limitations of the salivary-based test include possible cross-reactivity of antibodies with those produced against other viruses. Furthermore, this test is indicated for surveillance and not for early diagnosis.
Conclusion
Our findings showed that the presence of KSHV and EBV DNA in the saliva is high in HIV-infected patients in Iran. It may contribute to further complications in these patients, especially in immunocompromised or immunosuppressed ones. Further studies with a greater sample size and control group should be conducted to obtain more complete results.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgments
The authors and researchers would like to thank Keyvan laboratory, Tehran, Iran, for providing PCR samples and controls. This research was supported by the Iran University of Medical Sciences (Grant number: 14401).
References
- HIV, Key facts, Fact sheets, world health organization. 27 July 2022. URL: https://www.who.int/news-room/fact-sheets/detail/hiv-aids.
- Vahabpour R, Bokharaei-Salim F, Kalantari S, Garshasbi S, Monavari SH, Esghaei M, et al. HIV-1 genetic diversity and transmitted drug resistance frequency among Iranian treatment-naive, sexually infected individuals. Archiv Virol. 2017; 162(6): 1477-485.
- Oktafiani D, Megasari NLA, Fitriana E, Nasronudin N, Lusida MI, Soetjipto S. Detection of human herpesvirus-8 antigen in HIV-infected patients in East Java, Indonesia. African journal of infectious diseases. 2018;12(2):43-6.
- Brooks GF. Jawetz, Melnick, & Adelberg's medical microbiology/Geo. F. Brooks...[et al.]: New York; Chicago: McGraw Hill Medical; 2010.
- Pinzone MR, Berretta M, Cacopardo B, Nunnari G, editors. Epstein-Barr virus–and Kaposi sarcoma-associated herpesvirus–related malignancies in the setting of human immunodeficiency virus infection. Seminars in oncology; 2015.
- Gonçalves PH, Uldrick TS, Yarchoan R. HIV-associated Kaposi sarcoma and related diseases. AIDS (London, England). 2017; 31(14): 1903.
- Grinde B. Herpesviruses: latency and reactivation–viral strategies and host response. Journal of Oral Microbiology 2013; 5(1): 22766.
- Cesarman E. Gammaherpesvirus and lympho-proliferative disorders in immunocompromised patients. Cancer letters 2011; 305(2): 163-74.
- Young LS, Yap LF, Murray PG. Epstein–Barr virus: more than 50 years old and still providing surprises. Nature Reviews Cancer 2016; 16(12): 789.
- Ramezani A, Saboori E, Azadmanesh K, Mohraz M, Kazemimanesh M, Karami A, et al. No evidence of human herpesvirus 8 among Iranian patients infected with HIV. Iranian Journal of Public Health 2016; 45(7): 935.
- Hesamizadeh K, Keyvani H, Bokharaei-Salim F, Monavari SH, Esghaei M, Sefidi FJ. Molecular epidemiology of Kaposi’s sarcoma-associated herpes virus, and risk factors in HIV-infected patients in Tehran, 2014. Iranian Red Crescent Medical Journal 2016; 18(11): 32603.
- Tabibzadeh A, Niya MHK, Esghaei M, Bokharaei-Salim F, Ataei-Pirkooh A, Kiani SJ, et al. Molecular epidemiology of epstein-barr virus (ebv) in patients with hematologic malignancies. Asian Pacific Journal of Cancer Prevention 2020; 21(3): 693.
- Shushtari ZJ, Sajjadi H, Forouzan AS, Salimi Y, Dejman M. Disclosure of HIV status and social support among people living with HIV. Iranian Red Crescent Medical Journal 2014; 16(8): 11856.
- Joulaei H, Lankarani KB, Kazerooni PA, Marzban M. Number of HIV-infected cases in Iran: true or just an iceberg. Indian journal of sexually transmitted diseases and AIDS. 2017; 38(2): 157.
- Pow EH, Law MY, Tsang PC, Perera RA, Kwong DL. Salivary Epstein-Barr virus DNA level in patients with nasopharyngeal carcinoma following radiotherapy. Oral oncology 2011; 47(9): 879-82.
- Idesawa M, Sugano N, Ikeda K, Oshikawa M, Takane M, Seki K, et al. Detection of Epstein–Barr virus in saliva by real‐time PCR. Oral Microbiology and Immunology 2004; 19(4): 230-32.
- Newton R, Labo N, Wakeham K, Marshall V, Roshan R, Nalwoga A, et al. Determinants of gammaherpesvirus shedding in saliva among Ugandan children and their mothers. The Journal of Infectious Diseases 2018; 218(6): 892-900.
- Miller CS, Berger JR, Mootoor Y, Avdiushko SA, Zhu H, Kryscio RJ. High prevalence of multiple human herpesviruses in saliva from human immunodeficiency virus-infected persons in the era of highly active antiretroviral therapy. Journal of Clinical Microbiology 2006; 44(7): 2409-415.
- de Souza VA, Sumita LM, Nascimento M-C, Oliveira J, Mascheretti M, Quiroga M, et al. Human herpesvirus-8 infection and oral shedding in Amerindian and non-Amerindian populations in the Brazilian Amazon region. Journal of Infectious Diseases 2007; 196(6): 844-52.
- Ogoina D, Onyemelukwe G, Musa B, Babadoko A. Seroprevalence and determinants of human herpes virus 8 infection in adult Nigerians with and without HIV-1 infection. African Health Sciences 2011; 11(2): 158-62.
- Parisi SG, Cruciani M, Scaggiante R, Boldrin C, Andreis S, Dal Bello F, et al. Anal and oral human papillomavirus (HPV) infection in HIV-infected subjects in northern Italy: a longitudinal cohort study among men who have sex with men. BMC infectious diseases 2011; 11(1): 1-9.
- Scaggiante R, Andreis S, Basso M, Franchin E, Franzetti M, Del Vecchio C, et al. Epstein–Barr and cytomegalovirus DNA salivary shedding correlate with long‐term plasma HIV RNA detection in HIV‐infected men who have sex with men. Journal of Medical Virology 2016; 88(7): 1211-221.
- Jacobson MA, Ditmer DP, Sinclair E, Martin JN, Deeks SG, Hunt P, et al. Human herpesvirus replication and abnormal CD8+ T cell activation and low CD4+ T cell counts in antiretroviral-suppressed HIV-infected patients. PloS one 2009; 4(4): 5277.
- Carvalho KSS, Silvestre EdA, Maciel SdS, Lira HIG, Galvão RAdS, Soares MJdS, et al. PCR detection of multiple human herpesvirus DNA in saliva from HIV-infected individuals in Teresina, State of Piauí, Brazil. Revista da Sociedade Brasileira de Medicina Tropical 2010; 43(6): 620-23.
- de França TRT, de Araújo RA, Ribeiro CMB, Leao JC. Salivary shedding of HHV‐8 in people infected or not by human immunodeficiency virus 1. Journal of Oral Pathology & Medicine 2011; 40(1): 97-102.
- Triantos D, Horefti E, Paximadi E, Kyriakopoulou Z, Karakassiliotis G, Papanastasiou K, et al. Presence of human herpes virus‐8 in saliva and non‐lesional oral mucosa in HIV‐infected and oncologic immunocompromised patients. Oral Micro-biology and Immunology 2004; 19(3): 201-204.
- Oktafiani D, Megasari NLA, Fitriana E, Lusida MI. Human herpes virus 8 antibodies in HIV-positive patients in Surabaya, Indonesia. Infectious Disease Reports 2020; 12(11): 101-104.
- Altuğlu İ, Yolcu A, Öcek ZA, Sertöz Y, Gökengin D. Investigation of human herpesvirus-8 seroprevalence in blood donors and HIV-positive patients admitted to Ege University Medical School Hospital, Turkey. Mikrobiyoloji Bulteni 2016; 50(1): 104-11.
- Islamic Republic of Iran AIDS Progress Report On Monitoring of the United Nations General Assembly Special Session on HIV and AIDS. National AIDS Committee Secretariat, Ministry of Health and Medical Education. Available at: https://www.unaids.org/sites/default/files/country/documents/IRN_narrative_report_2015.pdf. [Access date: Mar 2015]
- Gandhi M, Koelle D, Ameli N, Bacchetti P, Greenspan J, Navazesh M, et al. prevalence of human herpesvirus-8 salivary shedding in HIV increases with CD4 count. Journal of Dental Research 2004; 83(8): 639-43.
- Grande SR, Imbronito AV, Okuda OS, Lotufo RFM, Magalhães MHG, Nunes FD. Herpes viruses in periodontal compromised sites: comparison between HIV‐positive and‐negative patients. Journal of Clinical Periodontology 2008; 35(10): 838-45.
- Griffin E, Krantz E, Selke S, Huang ML, Wald A. Oral mucosal reactivation rates of herpesviruses among HIV‐1 seropositive persons. Journal of Medical Virology 2008; 80(7): 1153-159.
- Xiong G, Zhang B, Huang My, Zhou H, Chen LZ, Feng QS, et al. Epstein-Barr virus (EBV) infection in Chinese children: a retrospective study of age-specific prevalence. PLoS One 2014; 9(6): 99857.
- Cao P, Zhang M, Wang W, Dai Y, Sai B, Sun J, et al. Fluorescence in situ hybridization is superior for monitoring Epstein Barr viral load in infectious mononucleosis patients. BMC Infectious Diseases 2017; 17(1): 323.
- Chakraborty N, Bhattacharyya S, De C, Mukherjee A, Bhattacharya D, Santra S, et al. Incidence of multiple Herpesvirus infection in HIV seropositive patients, a big concern for Eastern Indian scenario. Virology Journal 2010; 7(1): 147.
- Yan Y, Ren Y, Chen R, Hu J, Ji Y, Yang J, et al. Evaluation of Epstein-Barr virus salivary shedding in HIV/AIDS patients and HAART use: A retrospective cohort study. Virologica Sinica 2018; 33(3): 227-33.
Type of Study: Research |
Subject:
Virology
Received: 2021/10/10 | Accepted: 2022/08/16 | Published: 2022/10/15
Received: 2021/10/10 | Accepted: 2022/08/16 | Published: 2022/10/15
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





