Sat, Nov 29, 2025
[Archive]
Volume 11, Issue 2 (May 2024)
IJML 2024, 11(2): 152-161 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hashemibeni B, Katani M, Zolfaghari B, Soleimani M, Valiani A, Pourentezari M. Cartilage-Specific Protein Expression in Adipose-Derived Stem Cells Treated with Pomegranate Seed Extract and Soybean/Avocado in Fibrin Scaffolds. IJML 2024; 11 (2) :152-161
URL: http://ijml.ssu.ac.ir/article-1-537-en.html
URL: http://ijml.ssu.ac.ir/article-1-537-en.html
Batool Hashemibeni 
 , Mehri Katani
, Mehri Katani 
 , Behzad Zolfaghari
, Behzad Zolfaghari 
 , Mitra Soleimani
, Mitra Soleimani 
 , Ali Valiani
, Ali Valiani 
 , Majid Pourentezari *
, Majid Pourentezari * 


 , Mehri Katani
, Mehri Katani 
 , Behzad Zolfaghari
, Behzad Zolfaghari 
 , Mitra Soleimani
, Mitra Soleimani 
 , Ali Valiani
, Ali Valiani 
 , Majid Pourentezari *
, Majid Pourentezari * 

Department of Biology & Anatomical Sciences, Faculty of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. 4 Yazd Neuroendocrine Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
Full-Text [PDF 301 kb]
(204 Downloads)
| Abstract (HTML) (448 Views)
Introduction


Discussion
References
Full-Text: (133 Views)
Introduction
Cartilage is specialized tissue without blood vessels and has main cells called chondrocytes scattered in the extracellular matrix (ECM), rich in collagen (Col) and proteoglycans. Based on the biochemical and structural properties of the ECM, there are three types of cartilage: hyaline, elastic, and fibrous [1-3]. Chondrocytes in Lacuna cannot proliferate and migrate, so this tissue cannot be adequately repaired, and cartilage tissue damage is one of the global problems [3]. Osteoarthritis is one of the common diseases of middle age and old age, associated with thinning and degeneration of articular cartilage and eventual destruction of the underlying bone [4]. In this disease, damage to the cartilage collagen network and a decrease in aggrecans cause cartilage destruction [5]. It is estimated that 10% of people over the age of 60 suffer from rheumatoid disease [6].
Tissue engineering is a potential scientific field in the medical sciences that seeks permanent and efficient keys to repair tissue damage that affects millions of people worldwide each year. By integrating the principles and methods of engineering and medical sciences, tissue engineering is searching for tissue production, or an efficient organ that can be a suitable alternative to repair, replace or improve the function of damaged tissue or organs [7]. Regulation of cell phenotype, cell proliferation, induction of in vitro differentiation, and proper scaffold design are essential factors in cartilage tissue engineering (CTE) [8]. Success in CTE depends on three basic elements:1- Select a cell source that can produce new tissue with hyaline cartilage tissue characteristics; 2- The use of inducible growth factors, cytokines, hormones, or physical stimuli that cause cells to differentiate and produce a cell-like ECM, 3- Selection of suitable biomaterials as scaffolds to allow cell proliferation and chondrogenic differentiation [9, 10].
Among cells, adipose-derived stem cells (ADSCs), due to their high potential for differentiation into different cell lines, as well as high proliferation rate, longer lifespan, less invasive action during harvesting, maintaining differentiation ability in a variety of culture media and scaffolds, as well, ease of collection is a good candidate for tissue engineering and is widely used as a source of stem cells in this field [9-11].
Fibrin is a natural biomaterial formed from the polymerization of fibrinogen by thrombin [12]. Other properties of fibrin include biocompatibility, biodegradability, high culture efficiency, and proper cell distribution [13]. The existence of such benefits and its role in the natural process of recovery and regeneration has made fibrin a better choice as a scaffold for transferring various materials to cells than artificial scaffolds. It should be noted that the relatively rapid decomposition and poor strength of fibrin are limitations of its use as a scaffold [10].
Important molecules are used in CTE, and their role is to induce cartilage and maintain the phenotype of cartilage cells. Growth factors are important in regulating stem cell differentiation and are widely used in CTE and repair. These factors are important in proliferation, apoptosis, and cell differentiation and regulate the cell cycle and immune system [14]. The growth factors mainly used today to differentiate chondrogenesis are transforming growth factor beta (TGFβ)-1 and TGF-β3 [9- 15, 16]. These growth factors not only cause cells to express hyaline cartilage-specific markers (such as Col II) in high amounts but inevitably lead to increased hypertrophic differentiation or the formation of fibrous cartilage [13]. In addition, their high cost, rapid degradation, and short half-life limit their widespread use, especially in clinical settings [17]. Therefore, researchers in the field of CTE are looking for materials to replace these growth factors. Soybean/ avocado unsaponifiable (ASU) contains avocado and soybean extract in a ratio of 1 avocado to 2 soybeans prepared unsaponifiable with the brand name Piasclidine [16]. The main constituents of ASU are phytosterols β-sitosterol, campesterol, and stigmasterol [18]. ASU has anabolic, anti-catabolic, and supportive effects on cartilage. This compound prevents cartilage destruction and builds cartilage by inhibiting certain molecules. ASU stimulates the expression of Col II and aggrecan by inhibiting inflammatory cytokines [Interleukin (IL)-1, IL-6, IL-8, tumor necrosis factor-alpha, Prostaglandin E2] in chondrocytes and also inhibits the production and activity of matrix metallopeptidases (MMP) [19, 20]. The β-sitosterol in ASU has anti-inflammatory, antioxidant, and analgesic effects [21].
Pomegranate has special chemical compounds with medicinal, antioxidant, and anti-inflammatory properties. Pomegranate seed extract (PSE) contains Prodelphinidin, anthocyanins, ellagic acid, gallic acid, and pomegranate seed oil also contains punicic ellagic acid, steroidal estrogen, oestrone and non-steroidal phytoestrogens [22]. Anthocyanins are polyphenolic compounds with antioxidant and anti-inflammatory activity [23]. Studies have shown that Prodelphinidin inhibits prostaglandins E2 and produces Col II [24].
Materials and Methods
Preparation of ASU and PSE
The avocado/soy mixture is prepared as a pure powder from Prarin Pars Company, which has the approval of the Deputy Minister of Food and Drugs of the Ministry of Health.
Pomegranates were obtained from Najaf Abad city, Isfahan province, and were approved by the Department of Pharmacognosy, Faculty of Pharmacy, University of Isfahan, before use. They were peeled, and their arils were collected and dried. The husks were dried powdered, and their extract was obtained using the percolation method. The extract was concentrated at 45 °C by applying a rotary evaporator, and first, freeze-dried, then stored at -20 °C [25].
Isolation and proliferation of hADSCs and cell culture on fibrin scaffold
Adipose tissue was prepared after obtaining written consent from the operating room and after washing under the influence of collagenase IA at the rate of 1 mg/gr of fat for 40 minutes. After tissue analysis, in order to neutralize the enzyme, equivalent to the volume of enzyme used, dulbecco's modified eagle medium (DMEM) (Gibco) culture medium containing penicillin/ streptomycin 1%, fetal bovine serum (FBS) (sigma) 10% was added. Then, by centrifugation of the resulting suspension (1400 rpm for 10 minutes), the supernatant was drained along with the fat cells, and DMEM + 10% FBS was added to the resulting cell sediment. In the final stage, the cells were cultured in a 25 cm2 flask in DMEM + 1% penicillin/streptomycin + 10% FBS in an incubator at 37 °C, 5% CO2, and relative humidity. After 24 hours, the extra cells were emptied by changing the medium. The medium was changed every 4 days. When the cells reached 80% confluence, they were detached using 0.05% trypsin/0.53 mM ethylenediaminetetraacetic acid (EDTA) (Sigma). Passage P3 cells were then seeded into scaffolds [10].
Preparation of thrombin and fibrinogen
In order to prepare thrombin, a bag of fresh frozen plasma (FFP) was prepared from the blood bank of Isfahan province, and after being placed for 10 minutes in a Ben Marie device at 37 °C, it was melted. After disinfecting the bag with 70% alcohol, the contents were transferred to a 50 ml Falcon tube; 16 ml of FFP was transferred to each Falcon tube. Then, 10 ml ampoules of calcium gluconate were added to each Falcon tube, and the resulting suspension was incubated for 60-90 minutes. After centrifugation of the resulting mixture (2200 rpm for 10 minutes), the clear contents of the upper part of each tube containing thrombin were emptied and stored at -80 °C by eliciting in 1 ml amounts [13].
The precipitate bag was prepared from the blood bank of Isfahan province and placed in Ben Marie at 37 °C for 10 minutes. After disinfecting the outer surface of the bag with 70% alcohol, the contents were transferred to a 15 ml Falcon tube under sterile conditions and stored at -80 °C.
Cell transfer to fibrin scaffold and chondrogenic differentiation
The resulting cell suspension was counted after separating the cells from the third passage from the flasks. Then 300 μl of fibrinogen was transferred to plate wells in 24 houses, and thrombin was added immediately after adding 50 μl of culture medium containing one million cells, equivalent to the volume of fibrinogen. The plate was immobilized under the hood for 10 minutes to form a fibrin scaffold. Cells transferred to fibrin scaffolds were classified into three groups: 1- ASU group, under the influence of chondrogenic induction medium with ASU with a concentration of 10 μg/ml. 2- PSE group, under the influence of chondrogenic induction medium containing PSE with a 100 μg/ml concentration. 3- A chondrogenic induction medium influenced the control group without growth factor. Finally, 1 ml of chondrogenic medium (according to the group in it) was added to each well, and the plate containing fibrin scaffold was kept in an incubator at 37 °C and 5% CO2, and the medium was replaced every three days. The induction period was considered to be 14 days, and then the fibrin scaffold and cells were used for further studies [13].
3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) technique
After induction of chondrogenesis, each well was drained on the 14th day, and after washing with PBS, 400 μl of DMEM and 40 μl of MTT (5 mg/ml) were added to the wells. The plate was incubated at 37 °C and 5% CO2 for 4 hours. Based on this, the yellow MTT, after entering the living cell, was regenerated by the mitochondrial dehydrogenase enzyme of the cells and formed a purple precipitate of formazan dye. The pure medium was then drained with MTT, and after adding 400 μl of dimethyl sulfoxide (DMSO), it was kept in the dark for one hour and pipetted. DMSO creates a purple color by dissolving formazan crystals. In the end, one hundred microliters of the resulting solution were transferred to plate 96 cells, and enzyme-linked immunosorbent assay read their optical absorption (wavelength 540 nm). This operation was repeated three times for each sample [26].
Western blot technique
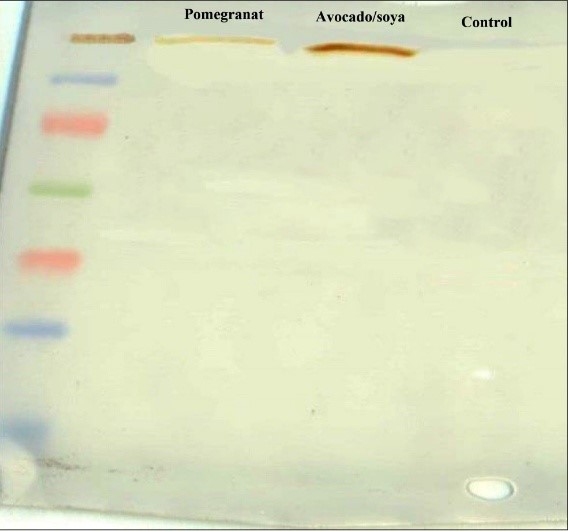
The radio-immune precipitation assay buffer was used to lyse cultured cells for protein extraction. Then, protein samples were electrophoresed at 70 V for 120 minutes on 7% sodium dodecyl-sulfate polyacrylamide with 5% stacking gel. The proteins were later transformed with a nitrocellulose paper at 40 mA for 120 minutes. The nitrocellulose blot was blocked with 4% (W/V) dry milk solution for 3 hours. The blot was washed in tween-tris-buffered saline and then incubated with collagen type I monoclonal antibody (Abcam) at a 1:1000 dilution overnight. Finally, the goat anti-mouse secondary antibody was added at a dilution of 1:5000 for 3 hours. After final washing, the protein bands were detected with diaminobenzidine [9].
Statistical analysis
The Kolmogorov-Smirnov test was used to assess the normality of the data distribution. Statistical analysis was performed using SPSS software, with one-way ANOVA and post hoc Tukey tests applied to analyze the data.
Results
After primary culture of the cell mass and observation under an inverted microscope, the cells were spherical and floating. However, after a few hours, they began to stick to the bottom of the flask, and their shape gradually changed from spherical to polygonal, star-shaped, and spindle-shaped with short, slightly cytoplasmic appendages. After 24 hours of culture and switching medium, floating cells such as red blood cells and white and fat particles were evacuated. Cells attached to the bottom of the flask, which were mononuclear, proliferated by successive mitotic divisions and occupied the bottom of the flask after 5 to 7 days. As the cells proliferated and increased in number, the stellar and palate shapes decreased, and most of the cells became spindle-shaped. With more cells proliferating and the confluence of 80% of the bottom of the flask, the cells were trypsinized and transferred to new culture dishes (Fig. 1).
MTT result
The results of MTT in different groups showed that the survival rate in the two groups of PSE and ASU was significantly lower than in the control group (p ≤ 0.05). The survival rate of the PSE group was lower than other groups, which was not significant compared to the ASU group (p ≤ 0.05), and the control group was significant (p ≤ 0.01) (Fig. 2).
Western blot results
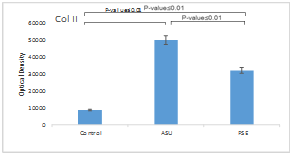
Quantitative analysis of Col II protein production showed that ASU (p ≤ 0.01) and PSE (p ≤ 0.05) groups had a significant increase compared to the control group, which was 5.7 times for the ASU group and 3.5 times for the PSE group. The production of this protein in the ASU group was significantly increased compared to the PSE group (p ≤ 0.01) (Fig. 3).
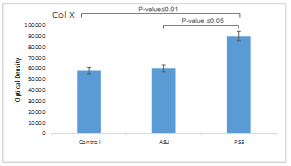
Quantitative results of Col X protein production showed that the production of this protein in the PSE group was significantly increased compared to the ASU and control groups (p ≤ 0.05). This decrease was about 1.5 times compared to ASU and control groups (Fig. 4).
Tissue engineering is a potential scientific field in the medical sciences that seeks permanent and efficient keys to repair tissue damage that affects millions of people worldwide each year. By integrating the principles and methods of engineering and medical sciences, tissue engineering is searching for tissue production, or an efficient organ that can be a suitable alternative to repair, replace or improve the function of damaged tissue or organs [7]. Regulation of cell phenotype, cell proliferation, induction of in vitro differentiation, and proper scaffold design are essential factors in cartilage tissue engineering (CTE) [8]. Success in CTE depends on three basic elements:1- Select a cell source that can produce new tissue with hyaline cartilage tissue characteristics; 2- The use of inducible growth factors, cytokines, hormones, or physical stimuli that cause cells to differentiate and produce a cell-like ECM, 3- Selection of suitable biomaterials as scaffolds to allow cell proliferation and chondrogenic differentiation [9, 10].
Among cells, adipose-derived stem cells (ADSCs), due to their high potential for differentiation into different cell lines, as well as high proliferation rate, longer lifespan, less invasive action during harvesting, maintaining differentiation ability in a variety of culture media and scaffolds, as well, ease of collection is a good candidate for tissue engineering and is widely used as a source of stem cells in this field [9-11].
Fibrin is a natural biomaterial formed from the polymerization of fibrinogen by thrombin [12]. Other properties of fibrin include biocompatibility, biodegradability, high culture efficiency, and proper cell distribution [13]. The existence of such benefits and its role in the natural process of recovery and regeneration has made fibrin a better choice as a scaffold for transferring various materials to cells than artificial scaffolds. It should be noted that the relatively rapid decomposition and poor strength of fibrin are limitations of its use as a scaffold [10].
Important molecules are used in CTE, and their role is to induce cartilage and maintain the phenotype of cartilage cells. Growth factors are important in regulating stem cell differentiation and are widely used in CTE and repair. These factors are important in proliferation, apoptosis, and cell differentiation and regulate the cell cycle and immune system [14]. The growth factors mainly used today to differentiate chondrogenesis are transforming growth factor beta (TGFβ)-1 and TGF-β3 [9- 15, 16]. These growth factors not only cause cells to express hyaline cartilage-specific markers (such as Col II) in high amounts but inevitably lead to increased hypertrophic differentiation or the formation of fibrous cartilage [13]. In addition, their high cost, rapid degradation, and short half-life limit their widespread use, especially in clinical settings [17]. Therefore, researchers in the field of CTE are looking for materials to replace these growth factors. Soybean/ avocado unsaponifiable (ASU) contains avocado and soybean extract in a ratio of 1 avocado to 2 soybeans prepared unsaponifiable with the brand name Piasclidine [16]. The main constituents of ASU are phytosterols β-sitosterol, campesterol, and stigmasterol [18]. ASU has anabolic, anti-catabolic, and supportive effects on cartilage. This compound prevents cartilage destruction and builds cartilage by inhibiting certain molecules. ASU stimulates the expression of Col II and aggrecan by inhibiting inflammatory cytokines [Interleukin (IL)-1, IL-6, IL-8, tumor necrosis factor-alpha, Prostaglandin E2] in chondrocytes and also inhibits the production and activity of matrix metallopeptidases (MMP) [19, 20]. The β-sitosterol in ASU has anti-inflammatory, antioxidant, and analgesic effects [21].
Pomegranate has special chemical compounds with medicinal, antioxidant, and anti-inflammatory properties. Pomegranate seed extract (PSE) contains Prodelphinidin, anthocyanins, ellagic acid, gallic acid, and pomegranate seed oil also contains punicic ellagic acid, steroidal estrogen, oestrone and non-steroidal phytoestrogens [22]. Anthocyanins are polyphenolic compounds with antioxidant and anti-inflammatory activity [23]. Studies have shown that Prodelphinidin inhibits prostaglandins E2 and produces Col II [24].
Materials and Methods
Preparation of ASU and PSE
The avocado/soy mixture is prepared as a pure powder from Prarin Pars Company, which has the approval of the Deputy Minister of Food and Drugs of the Ministry of Health.
Pomegranates were obtained from Najaf Abad city, Isfahan province, and were approved by the Department of Pharmacognosy, Faculty of Pharmacy, University of Isfahan, before use. They were peeled, and their arils were collected and dried. The husks were dried powdered, and their extract was obtained using the percolation method. The extract was concentrated at 45 °C by applying a rotary evaporator, and first, freeze-dried, then stored at -20 °C [25].
Isolation and proliferation of hADSCs and cell culture on fibrin scaffold
Adipose tissue was prepared after obtaining written consent from the operating room and after washing under the influence of collagenase IA at the rate of 1 mg/gr of fat for 40 minutes. After tissue analysis, in order to neutralize the enzyme, equivalent to the volume of enzyme used, dulbecco's modified eagle medium (DMEM) (Gibco) culture medium containing penicillin/ streptomycin 1%, fetal bovine serum (FBS) (sigma) 10% was added. Then, by centrifugation of the resulting suspension (1400 rpm for 10 minutes), the supernatant was drained along with the fat cells, and DMEM + 10% FBS was added to the resulting cell sediment. In the final stage, the cells were cultured in a 25 cm2 flask in DMEM + 1% penicillin/streptomycin + 10% FBS in an incubator at 37 °C, 5% CO2, and relative humidity. After 24 hours, the extra cells were emptied by changing the medium. The medium was changed every 4 days. When the cells reached 80% confluence, they were detached using 0.05% trypsin/0.53 mM ethylenediaminetetraacetic acid (EDTA) (Sigma). Passage P3 cells were then seeded into scaffolds [10].
Preparation of thrombin and fibrinogen
In order to prepare thrombin, a bag of fresh frozen plasma (FFP) was prepared from the blood bank of Isfahan province, and after being placed for 10 minutes in a Ben Marie device at 37 °C, it was melted. After disinfecting the bag with 70% alcohol, the contents were transferred to a 50 ml Falcon tube; 16 ml of FFP was transferred to each Falcon tube. Then, 10 ml ampoules of calcium gluconate were added to each Falcon tube, and the resulting suspension was incubated for 60-90 minutes. After centrifugation of the resulting mixture (2200 rpm for 10 minutes), the clear contents of the upper part of each tube containing thrombin were emptied and stored at -80 °C by eliciting in 1 ml amounts [13].
The precipitate bag was prepared from the blood bank of Isfahan province and placed in Ben Marie at 37 °C for 10 minutes. After disinfecting the outer surface of the bag with 70% alcohol, the contents were transferred to a 15 ml Falcon tube under sterile conditions and stored at -80 °C.
Cell transfer to fibrin scaffold and chondrogenic differentiation
The resulting cell suspension was counted after separating the cells from the third passage from the flasks. Then 300 μl of fibrinogen was transferred to plate wells in 24 houses, and thrombin was added immediately after adding 50 μl of culture medium containing one million cells, equivalent to the volume of fibrinogen. The plate was immobilized under the hood for 10 minutes to form a fibrin scaffold. Cells transferred to fibrin scaffolds were classified into three groups: 1- ASU group, under the influence of chondrogenic induction medium with ASU with a concentration of 10 μg/ml. 2- PSE group, under the influence of chondrogenic induction medium containing PSE with a 100 μg/ml concentration. 3- A chondrogenic induction medium influenced the control group without growth factor. Finally, 1 ml of chondrogenic medium (according to the group in it) was added to each well, and the plate containing fibrin scaffold was kept in an incubator at 37 °C and 5% CO2, and the medium was replaced every three days. The induction period was considered to be 14 days, and then the fibrin scaffold and cells were used for further studies [13].
3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) technique
After induction of chondrogenesis, each well was drained on the 14th day, and after washing with PBS, 400 μl of DMEM and 40 μl of MTT (5 mg/ml) were added to the wells. The plate was incubated at 37 °C and 5% CO2 for 4 hours. Based on this, the yellow MTT, after entering the living cell, was regenerated by the mitochondrial dehydrogenase enzyme of the cells and formed a purple precipitate of formazan dye. The pure medium was then drained with MTT, and after adding 400 μl of dimethyl sulfoxide (DMSO), it was kept in the dark for one hour and pipetted. DMSO creates a purple color by dissolving formazan crystals. In the end, one hundred microliters of the resulting solution were transferred to plate 96 cells, and enzyme-linked immunosorbent assay read their optical absorption (wavelength 540 nm). This operation was repeated three times for each sample [26].
Western blot technique
The radio-immune precipitation assay buffer was used to lyse cultured cells for protein extraction. Then, protein samples were electrophoresed at 70 V for 120 minutes on 7% sodium dodecyl-sulfate polyacrylamide with 5% stacking gel. The proteins were later transformed with a nitrocellulose paper at 40 mA for 120 minutes. The nitrocellulose blot was blocked with 4% (W/V) dry milk solution for 3 hours. The blot was washed in tween-tris-buffered saline and then incubated with collagen type I monoclonal antibody (Abcam) at a 1:1000 dilution overnight. Finally, the goat anti-mouse secondary antibody was added at a dilution of 1:5000 for 3 hours. After final washing, the protein bands were detected with diaminobenzidine [9].
Statistical analysis
The Kolmogorov-Smirnov test was used to assess the normality of the data distribution. Statistical analysis was performed using SPSS software, with one-way ANOVA and post hoc Tukey tests applied to analyze the data.
Results
After primary culture of the cell mass and observation under an inverted microscope, the cells were spherical and floating. However, after a few hours, they began to stick to the bottom of the flask, and their shape gradually changed from spherical to polygonal, star-shaped, and spindle-shaped with short, slightly cytoplasmic appendages. After 24 hours of culture and switching medium, floating cells such as red blood cells and white and fat particles were evacuated. Cells attached to the bottom of the flask, which were mononuclear, proliferated by successive mitotic divisions and occupied the bottom of the flask after 5 to 7 days. As the cells proliferated and increased in number, the stellar and palate shapes decreased, and most of the cells became spindle-shaped. With more cells proliferating and the confluence of 80% of the bottom of the flask, the cells were trypsinized and transferred to new culture dishes (Fig. 1).
MTT result
The results of MTT in different groups showed that the survival rate in the two groups of PSE and ASU was significantly lower than in the control group (p ≤ 0.05). The survival rate of the PSE group was lower than other groups, which was not significant compared to the ASU group (p ≤ 0.05), and the control group was significant (p ≤ 0.01) (Fig. 2).
Western blot results
Quantitative analysis of Col II protein production showed that ASU (p ≤ 0.01) and PSE (p ≤ 0.05) groups had a significant increase compared to the control group, which was 5.7 times for the ASU group and 3.5 times for the PSE group. The production of this protein in the ASU group was significantly increased compared to the PSE group (p ≤ 0.01) (Fig. 3).
Quantitative results of Col X protein production showed that the production of this protein in the PSE group was significantly increased compared to the ASU and control groups (p ≤ 0.05). This decrease was about 1.5 times compared to ASU and control groups (Fig. 4).
(A) 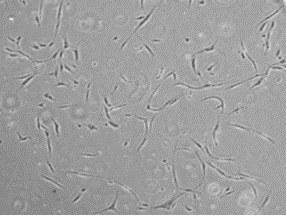 (B)
(B) 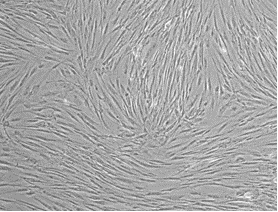
 (B)
(B) 
Fig. 1. Morphology of hADSCs at different passages: (A) First passage and (B) Third passage.

Fig. 2. Survival rates of differentiated cells in control, unsaponifiable soybean/ avocado (ASU), and Pomegranate seed extract (PSE) groups, with comparative analysis.


Fig. 3. Expression of Col II protein in control, unsaponifiable soybean/ avocado (ASU), and Pomegranate seed extract (PSE) groups, with comparative analysis. β-Actin was used as an internal control.


Fig. 4. Expression of Col X protein in control, unsaponifiable soybean/ avocado (ASU), and Pomegranate seed extract (PSE) groups, with comparative analysis. β-actin was used as an internal control.
Discussion
Subcutaneous adipose tissue can be used as a source of stem cells quickly, without invasive methods, and with a small amount of it, many stem cells can be obtained [27]. Therefore, this study used subcutaneous fat tissue to achieve hADSCs. Fibrin scaffold is a type of natural scaffold made from blood. The fibrin scaffold allows migration, reproduction, the transfer of food, the transmission of molecular messages, and the excretion of metabolic substances [10, 28]. Fibrin is compatible with the immune system, is non-toxic, and does not cause reactions such as necrosis and fibrosis in the body [29]. In 2008, Yang et al. compared the proliferation, survival, and apoptosis of vertebral disc cells in fibrin and alginate scaffolds were lower in fibrin scaffolds than in alginate scaffolds [30]. Similar to the previous study, Girandon et al. examined the proliferation and survival rate of fat-derived stem cells in both fibrin and alginate scaffolds and found that the proliferation and survival rates of these cells were higher in fibrin scaffolds than in alginate scaffolds; Also, the rate of apoptosis in this scaffold was less than that of alginate scaffold [31]. Therefore, due to its suitable properties, fibrin was used as a scaffold in the present study.
One of the factors affecting chondrogenesis is TGF-βs, which, due to its high cost and low half-life and the development of chondrocytes towards hypertrophy [13, 32], is necessary to achieve a new and suitable composition to replace it. Therefore, In the present study, we examined the effect of two ASU and PSE compounds on ADSC's chondrogenesis in fibrin scaffold. ASU contains avocado and soybean extract, which stimulates the expression of Col ІІ and cartilaginous aggrecans and inhibits IL-1β [9]. These polyphenol compounds have antioxidant and anti-inflammatory properties [19, 20].
The present study evaluated stem cells implanted in fibrin scaffold after 14 days for proliferation and cell survival. The results showed that PSE and ASU significantly reduced survival in both groups compared with the control group. However, there was no significant difference between the PSE and ASU groups. In the induction of differentiation, proliferation in stem cells usually decreases, and the expression of genes specific to differentiated cells begins. Therefore, since no inducer factors were in the control group, cell proliferation significantly increased compared to the PSE and ASU groups affected by the Inducer factors. As in our previous study, we evaluated the effect of ASU and icariin on inducing chondrogenesis in hADSCs in a poly(lactic-co-glycolic) acid /fibrin hybrid scaffold. The results showed a significant reduction in cell proliferation and survival in the groups treated with ASU, icariin, and TGF-β3 compared to the control group. Although the study scaffold was poly(lactic-co-glycolic) acid /fibrin, the results of the MTT method were similar to the present study [9].
In this study, using semi-quantitative analysis of Image J software, the expression of Col II and X genes in hADSCs in the fibrin scaffold was investigated, and it was shown that the expression of Col II protein in the ASU group was higher than the PSE group, and the effect of ASU as a chondrogenic stimulant was more significant than the effect of PSE. The expression level of Col II protein in the ASU group was approximately 1.5 times that of the PSE group, according to the present study by Lippiello et al. In 2008, in vitro examined the effect of ASU on bovine cartilage chondrocytes and stated that ASU causes the production of Col ІІ protein [18].
PSE prevents cartilage cell damage and changes in matrix proteoglycans in osteoarthritis joints. PSE inhibits proinflammatory cytokines and MAPK, which MAPK phosphorylates certain transcription factors and activates MMP [33]. Hadipour-Jahromy et al. Injected monoid acetate into the tibiofemoral joint of mice to evaluate the effect of PSE on osteoarthritis joints, they reported that PSE caused less damage to the cartilage structure and also inhibited MMP and preserved knee proteoglycans and protected knee cartilage [34].
Col X is a well-known osteogenic marker associated with hypertrophic chondrocytes. In their study, Ismaili et al. explored the impact of piasclidine on Col X gene expression during the chondrogenic differentiation of hADSCs cultured in fibrin scaffolds. We examined the effect of ASU on Western blotting on Col X protein expression, but Ismaili examined the effect of ASU on Col X gene expression using real-time polymerase chain reaction. In the Ismaili study, the Col X gene was expressed more in the TGF-β group than in the ASU group, and in our study, the Col X protein was expressed more in the PSE group than in the ASU group [16].
Conclusion
According to the results, ASU and PSE are two important factors in the induction of chondrogenesis in hADSCs in fibrin scaffold because they increase the primary and specific markers for transparent cartilage. ASU's impact on chondrogenesis of hADSCs in fibrin scaffold is more significant than that of PSE.
Ethical Considerations
Ethics code received from the Research Council of
Esfehan University of Medical Sciences (IR.MUI.REC.1395.3.265).
Funding
This study was supported financially by the Islamic Azad University, Shiraz, Iran.
Conflict of Interest
There are no conflicts of interest based on the author's approval.
Acknowledgments
We would like to acknowledge Isfahan University of Medical Sciences for providing us with the necessary resources and facilities to carry out this project.
Authors’ Contributions
H.B, Z.B and P.M participated in the study idea and design. H. B was the supervisor of the project, and K.M, V.A and H.B were the advisers for the research performance. S.M and P.M also accomplished analysis and interpretation of the results. All authors revised and approved the final manuscript version.
One of the factors affecting chondrogenesis is TGF-βs, which, due to its high cost and low half-life and the development of chondrocytes towards hypertrophy [13, 32], is necessary to achieve a new and suitable composition to replace it. Therefore, In the present study, we examined the effect of two ASU and PSE compounds on ADSC's chondrogenesis in fibrin scaffold. ASU contains avocado and soybean extract, which stimulates the expression of Col ІІ and cartilaginous aggrecans and inhibits IL-1β [9]. These polyphenol compounds have antioxidant and anti-inflammatory properties [19, 20].
The present study evaluated stem cells implanted in fibrin scaffold after 14 days for proliferation and cell survival. The results showed that PSE and ASU significantly reduced survival in both groups compared with the control group. However, there was no significant difference between the PSE and ASU groups. In the induction of differentiation, proliferation in stem cells usually decreases, and the expression of genes specific to differentiated cells begins. Therefore, since no inducer factors were in the control group, cell proliferation significantly increased compared to the PSE and ASU groups affected by the Inducer factors. As in our previous study, we evaluated the effect of ASU and icariin on inducing chondrogenesis in hADSCs in a poly(lactic-co-glycolic) acid /fibrin hybrid scaffold. The results showed a significant reduction in cell proliferation and survival in the groups treated with ASU, icariin, and TGF-β3 compared to the control group. Although the study scaffold was poly(lactic-co-glycolic) acid /fibrin, the results of the MTT method were similar to the present study [9].
In this study, using semi-quantitative analysis of Image J software, the expression of Col II and X genes in hADSCs in the fibrin scaffold was investigated, and it was shown that the expression of Col II protein in the ASU group was higher than the PSE group, and the effect of ASU as a chondrogenic stimulant was more significant than the effect of PSE. The expression level of Col II protein in the ASU group was approximately 1.5 times that of the PSE group, according to the present study by Lippiello et al. In 2008, in vitro examined the effect of ASU on bovine cartilage chondrocytes and stated that ASU causes the production of Col ІІ protein [18].
PSE prevents cartilage cell damage and changes in matrix proteoglycans in osteoarthritis joints. PSE inhibits proinflammatory cytokines and MAPK, which MAPK phosphorylates certain transcription factors and activates MMP [33]. Hadipour-Jahromy et al. Injected monoid acetate into the tibiofemoral joint of mice to evaluate the effect of PSE on osteoarthritis joints, they reported that PSE caused less damage to the cartilage structure and also inhibited MMP and preserved knee proteoglycans and protected knee cartilage [34].
Col X is a well-known osteogenic marker associated with hypertrophic chondrocytes. In their study, Ismaili et al. explored the impact of piasclidine on Col X gene expression during the chondrogenic differentiation of hADSCs cultured in fibrin scaffolds. We examined the effect of ASU on Western blotting on Col X protein expression, but Ismaili examined the effect of ASU on Col X gene expression using real-time polymerase chain reaction. In the Ismaili study, the Col X gene was expressed more in the TGF-β group than in the ASU group, and in our study, the Col X protein was expressed more in the PSE group than in the ASU group [16].
Conclusion
According to the results, ASU and PSE are two important factors in the induction of chondrogenesis in hADSCs in fibrin scaffold because they increase the primary and specific markers for transparent cartilage. ASU's impact on chondrogenesis of hADSCs in fibrin scaffold is more significant than that of PSE.
Ethical Considerations
Ethics code received from the Research Council of
Esfehan University of Medical Sciences (IR.MUI.REC.1395.3.265).
Funding
This study was supported financially by the Islamic Azad University, Shiraz, Iran.
Conflict of Interest
There are no conflicts of interest based on the author's approval.
Acknowledgments
We would like to acknowledge Isfahan University of Medical Sciences for providing us with the necessary resources and facilities to carry out this project.
Authors’ Contributions
H.B, Z.B and P.M participated in the study idea and design. H. B was the supervisor of the project, and K.M, V.A and H.B were the advisers for the research performance. S.M and P.M also accomplished analysis and interpretation of the results. All authors revised and approved the final manuscript version.
References
- Hunziker EB, Lippuner K, Keel M, Shintani N. An educational review of cartilage repair: precepts & practice–myths & misconceptions–progress & prospects. Osteoarthritis and Cartilage 2015; 23(3): 334-50.
- Theocharis AD, Manou D, Karamanos NK. The extracellular matrix as a multitasking player in disease. The FEBS Journal 2019; 286(15): 2830-869.
- Jiang S, Guo W, Tian G, Luo X, Peng L, Liu S, et al. Clinical application status of articular cartilage regeneration techniques: Tissue-engineered cartilage brings new hope. Stem Cells International 2020; 2020: 5690252.
- Courties A, Kouki I, Soliman N, Mathieu S, Sellam J. Osteoarthritis year in review 2024: Epidemiology and therapy. Osteoarthritis and Cartilage 2024; 32(11): 1397-404.
- Kloppenburg M, Berenbaum F. Osteoarthritis year in review 2019: epidemiology and therapy. Osteoarthritis and Cartilage 2020; 28(3): 242-48.
- Contartese D, Tschon M, De Mattei M, Fini M. Sex specific determinants in osteoarthritis: A systematic review of preclinical studies. International Journal of Molecular Sciences 2020; 21(10): 3696.
- Ali Q, Malik S, Malik A, Hafeez MN, Salman S. Role of modern technologies in tissue engineering. Archives of Neuroscience 2020; 7(1): 90394.
- Chen M, Jiang Z, Zou X, You X, Cai Z, Huang J. Advancements in tissue engineering for articular cartilage regeneration. Heliyon 2024; 10(3): 25400.
- Hashemibeni B, Mardani M, Valiani A, Pourentezari M, Anvari M, Yadegari M, et al. Effects of avocado/soybean on the chondrogenesis of human adipose-derived stem cells cultured on polylactic-co-glycolic acid/ fibrin hybrid scaffold. Journal of Applied Biotechnology Reports 2019; 6(4): 145-50.
- Hashemibeni B, Mardani M, Bahrami M, Valiani A, Mehr MS, Pourentezari M. Comparison of fibrin and PLGA/ fibrin scaffolds for chondrogenesis of human adipose derived stem cells by icariin. J Kerman Univ Med Sci. 2020; 27: 14-23.
- Yuan C, Song W, Jiang X, Wang Y, Li C, Yu W, et al. Adipose-derived stem cell-based optimization strategies for musculoskeletal regeneration: recent advances and perspectives. Stem Cell Research & Therapy 2024; 15(1): 91.
- Al Kayal T, Losi P, Pierozzi S, Soldani G. A new method for fibrin-based electrospun/ sprayed scaffold fabrication. Scientific Reports 2020; 10(1): 1-4.
- Valiani A, Izadi M, Bahramian H, Esfandiari E, Hashemibeni B. Comparison between the effect of kartogenin and TGFβ3 on chondrogenesis of human adipose-derived stem cells in fibrin scaffold. Bratislavske Lekarske Listy 2017; 118(10): 591-97.
- Endo K, Fujita N, Nakagawa T, Nishimura R. Comparison of the effect of growth factors on chondrogenesis of canine mesenchymal stem cells. Journal of Veterinary Medical Science 2019; 81(8): 1211-218.
- Stevens MM, Marini RP, Martin I, Langer R, Shastri VP. FGF‐2 enhances TGF‐β1‐induced periosteal chondrogenesis. Journal of Orthopaedic Research 2004; 22(5): 1114-119.
- Hashemibeni B, Valiani A, Esmaeli M, Kazemi M, Aliakbari M, Iranpour FG. Comparison of the efficacy of piascledine and transforming growth factor β1 on chondrogenic differentiation of human adipose-derived stem cells in fibrin and fibrin-alginate scaffolds. Iranian Journal of Basic Medical Sciences 2018; 21(2): 212.
- Chen MJ, Whiteley JP, Please CP, Ehlicke F, Waters SL, Byrne HM. Identifying chondrogenesis strategies for tissue engineering of articular cartilage. Journal of Tissue Engineering. 2019; 10: 2041731419842431.
- Lippiello L, Nardo JV, Harlan R, Chiou T. Metabolic effects of avocado/ soy unsaponifiables on articular chondrocytes. Evidence-Based Complementary and Alternative Medicine 2008; 5(2): 191-97.
- Ownby SL, Fortuno LV, Au AY, Grzanna MW, Rashmir-Raven AM, Frondoza CG. Expression of proinflammatory mediators is inhibited by an avocado/ soybean unsaponifiables and epigallocatechin gallate combination. Journal of Inflammation 2014; 11(1): 8.
- Au R, Al-Talib T, Au A, Phan P, Frondoza C. Avocado soybean unsaponifiables (ASU) suppress TNF-α, IL-1β, COX-2, iNOS gene expression, and prostaglandin E2 and nitric oxide production in articular chondrocytes and monocyte/ macrophages. Osteoarthritis and Cartilage 2007; 15(11): 1249-55.
- Babu S, Jayaraman S. An update on β-sitosterol: A potential herbal nutraceutical for diabetic management. Biomedicine & Pharmacotherapy 2020; 131:110702.
- Viuda‐Martos M, Fernández‐López J, Pérez‐Álvarez J. Pomegranate and its many functional components as related to human health: a review. Comprehensive Reviews in Food Science and Food Safety 2010; 9(6): 635-54.
- Miguel MG. Anthocyanins: Antioxidant and/or anti-inflammatory activities. Journal of Applied Pharmaceutical Science 2011; 1(6): 7-15.
- Garbacki N, Angenot L, Bassleer C, Damas J, Tits M. Effects of prodelphinidins isolated from Ribes nigrum on chondrocyte metabolism and COX activity. Naunyn-Schmiedeberg's Archives of Pharmacology 2002; 365(6): 434-41.
- Teimourinejad A, Hashemibeni B, Salehi H, Mostafavi FS, Kazemi M, Bahramian H. Chondrogenic activity of two herbal products; pomegranate fruit extract and avocado/soybean unsaponifiable. Research in Pharmaceutical Sciences 2020; 15(4): 358-66.
- Kabiri A, Esfandiari E, Esmaeili A, Hashemibeni B, Pourazar A, Mardani M. Platelet-rich plasma application in chondrogenesis. Advanced Biomedical Research 2014; 3: 138.
- Esfandiari E, Roshankhah S, Mardani M, Hashemibeni B, Naghsh E, Kazemi M, et al. The effect of high frequency electric field on enhancement of chondrogenesis in human adipose-derived stem cells. Iranian Journal of Basic Medical Sciences 2014; 17(8): 571.
- Ahmed TA, Dare EV, Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Engineering Part B: Reviews 2008; 14(2): 199-215.
- Malafaya PB, Silva GA, Reis RL. Natural–origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Advanced Drug Delivery Reviews 2007; 59(4-5): 207-33.
- Yang SH, Wu CC, Shih TTF, Chen PQ, Lin FH. Three‐dimensional culture of human nucleus pulposus cells in fibrin clot: comparisons on cellular proliferation and matrix synthesis with cells in alginate. Artificial Organs 2008; 32(1): 70-3.
- Girandon L, Kregar-Velikonja N, Bozikov K, Barlic A. In vitro models for adipose tissue engineering with adipose-derived stem cells using different scaffolds of natural origin. Folia Biol (Praha). 2011; 57(2): 47-56.
- Hashemibeni B, Pourentezari M, Valiani A, Zamani M, Mardani M. Effect of icariin on the chondrogenesis of human adipose derived stem cells on poly (lactic-co-glycolic) acid/fibrin composite scaffold. Int J Adv Biotech Res. 2017; 8(2): 595-605.
- Rahimi HR, Arastoo M, Ostad SN. A comprehensive review of Punica granatum (pomegranate) properties in toxicological, pharmacological, cellular and molecular biology researches. Iranian Journal of Pharmaceutical Research 2012; 11(2): 385.
- Hadipour‐Jahromy M, Mozaffari‐Kermani R. Chondroprotective effects of pomegranate juice on monoiodoacetate‐induced osteoarthritis of the knee joint of mice. Phytother Res. 2010; 24(2): 182-5.
Type of Study: Research |
Subject:
General
Received: 2024/12/2 | Accepted: 2025/02/16 | Published: 2025/03/8
Received: 2024/12/2 | Accepted: 2025/02/16 | Published: 2025/03/8
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




