Fri, Feb 6, 2026
[Archive]
Volume 11, Issue 4 (November 2024)
IJML 2024, 11(4): 318-333 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Kamayi Z, Galedari M. Impact of Interval Training and Prosopis Farcta on Intestinal GLP-1 Gene Expression in Diabetic Male Rats. IJML 2024; 11 (4) :318-333
URL: http://ijml.ssu.ac.ir/article-1-546-en.html
URL: http://ijml.ssu.ac.ir/article-1-546-en.html
Islamic Azad University, Science & Research Branch of Ahvaz, Iran
Full-Text [PDF 433 kb]
(84 Downloads)
| Abstract (HTML) (191 Views)
ΔΔCT = (CT Target− CT Reference) Time X − (CT Target− CT Reference) Time 0
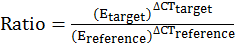
(ΔCT Reference= CT Control – CT Treatment, ΔCT Target= Ct Control– Ct Treatment)
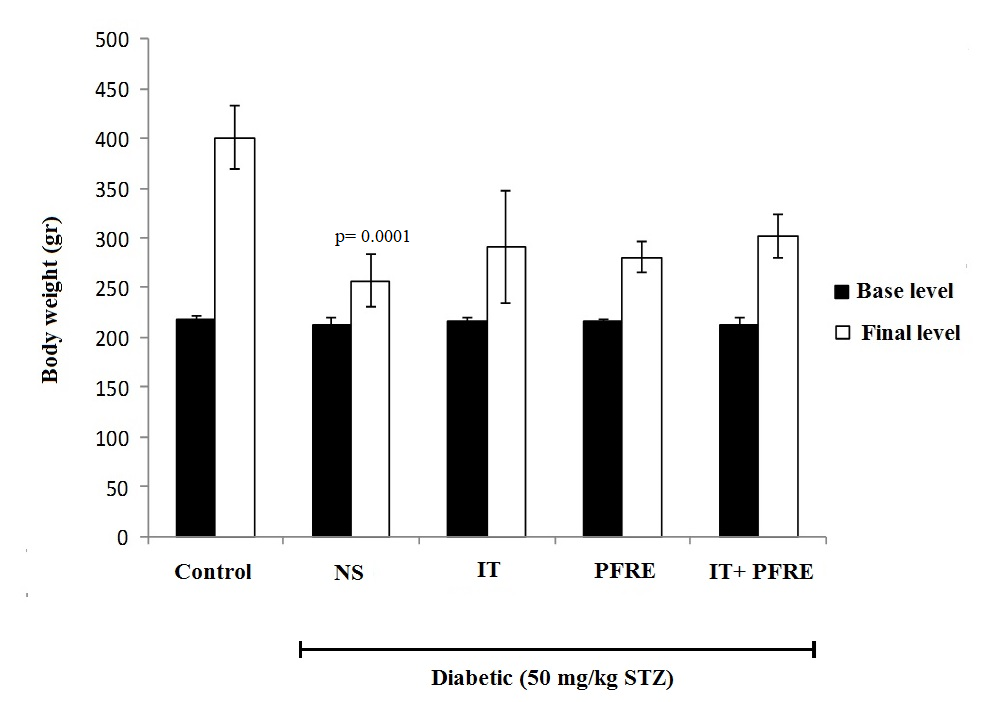
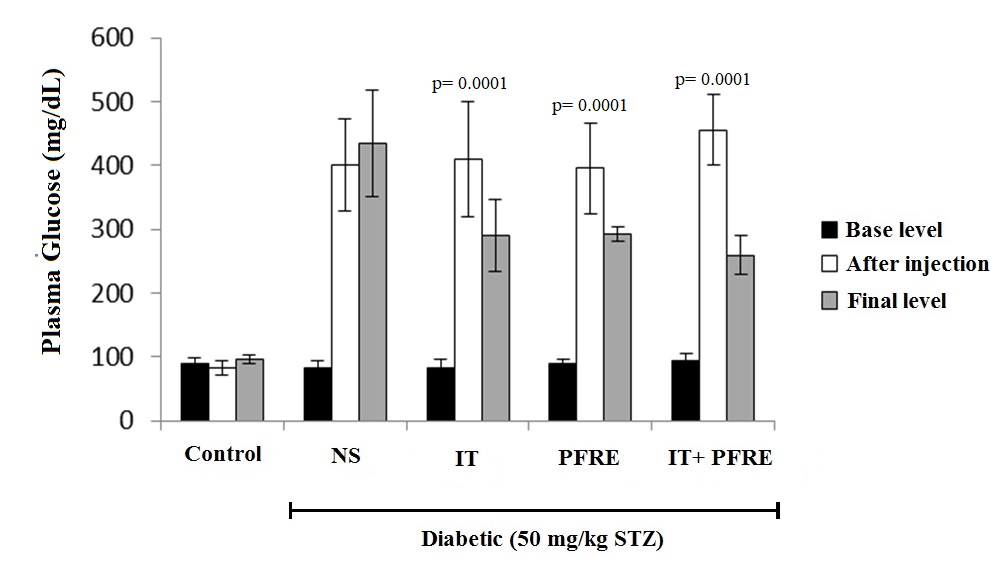
Fig. 2. The effect of intermittent training and PFRE administration on the blood glucose changes in the studied groups
Data are expressed as mean ± standard deviation
NS= Streptozotocin-induced diabetic rats; IT= Intermittent training; PFRE= Prosopis farcta root extract

Discussion
References
Full-Text: (49 Views)
Introduction
diabetes mellitus is a metabolic disorder, and its prevalence has reached an alarming level with the increase in obesity worldwide, particularly in developing countries [1]. The prevalence of diabetes in the world was 4% in 1995, and it will reach 5% by 2025, so that the number of people with diabetes will reach 300 million in 2025, from 135 million in 1995 [2]. Diabetes mellitus is characterized by impaired insulin secretion, insulin resistance, and excessive production of glucose by the liver [3]. The elevation of blood glucose levels in diabetic patients leads to serious complications such as nephropathy, retinopathy, neuropathy, cardiovascular disease, and even death [4].
Diabetes mellitus is a multifactorial syndrome, and various factors play a role in the onset and progression of this disease. Gene mutations, aging, some environmental factors, and underlying diseases are the most determining factors [5]. Among these factors, the role of the incretin hormone Glucagon-like peptide-1 (GLP-1) and its receptors (GLP-1R) is important in insulin secretion from pancreatic cells. Incretins are digestive hormones that generally increase the secretion of insulin, decrease the secretion, and the rate of gastric emptying, and create a feeling of satiety [6]. GLP-1 is secreted from the intestinal L cells after food enters the intestine and blood glucose increases. This hormone increases insulin levels and decreases blood sugar in direct and indirect ways. GLP-1 increases insulin gene expression and its synthesis directly through activation of GLP-1 receptors on pancreatic cells, and on the other hand, indirectly through the vagus nerve system and hepatic portal vein, increases insulin secretion. Research shows that in type 2 diabetes, GLP-1 secretion is disrupted, so improving blood GLP-1 levels can increase insulin levels and regulate blood glucose [7, 8].
In the presence of increased blood glucose concentration, rapid and higher cAMP production is mediated by stimulation of GLP-1R-dependent plasma membrane-bound adenylyl cyclase, increasing insulin secretion from β-cells [9]. Although plasma membrane-bound adenylyl cyclase activity is also increased in response to other high glucose-dependent pathways, it is affected to a much lesser extent than GLP-1R [10].
Streptozotocin induced diabetes is one of the chemical models of experimental diabetes in which Streptozotocin is used to destroy beta cells [11]. Streptozotocin was extracted from a species of Streptomyces (Streptomyces achromogenes) in 1956 by the researchers of Upjohn Pharmaceutical Company as an antibiotic and has a wide antimicrobial spectrum. Streptozotocin is used clinically in the treatment of beta-cell malignancy and also as a laboratory agent to induce diabetes. Structurally, this substance is a nitrous urea glucosamine, and due to its structural similarity to glucose, it enters beta cells and selectively destroys them [11, 12]. The availability and relatively low cost of medicinal plants, as well as fewer side effects than chemical drugs, have caused them to be widely considered all over the world. Prosopis farcta root extract (PFRE) belongs to the Leguminosae family and the Mimosoideae subfamily, which is native to dry and semi-arid regions of America, Asia, and Africa [13]. The effective compounds found in this plant are: 5-hydroxyl tryptamine (alkaloid), L-arabinose, lectin, quercetin, tryptamine, and apigenin [14]. Literature review showed that this plant has anti-diabetic, antioxidant, anti-inflammatory, anti-apoptotic, anti-spasm, and soothing properties [14, 15]. According to previous research, prescribing the hydroalcoholic extract of the fruit and the root of the PFRE plant has been able to reduce blood glucose by changing the expression level of the pyruvate kinase gene [16].
In 2019, an experimental study was conducted to investigate the effect of Prosopis farcta extract on the gene expression of some of the main genes of the glycolysogen pathway involved in insulin signaling pyruvate kinase, phosphofructokinase, glucokinase and insulin signaling phosphotidylinositol 3 kinase, insulin receptor substrate, glucose transporter2 (GluT2) in HePG2 cells. In this study, HePG2 cells were cultured and treated with Prosopis farcta extract (leaves, roots, fruits).
The results showed that the expression of glucokinase, phosphofructo, and pyruvate kinase was upregulated, and the expression of GluT2, PI3-kinase, and insulin receptor substrate genes was upregulated compared to control cells. It seems that the effect on diabetes of Prosopis farcta on diabetes is not more than the glycolysis pathway. This mechanism acts by increasing gene expression in the insulin signaling pathway [17].
Several studies have investigated the effect of exercise interventions on blood glucose levels, serum insulin, insulin resistance, hormonal or genetic factors in patients with type 2 diabetes and healthy people. But the findings are slightly contradictory; nevertheless, all studies have reported a significant reduction in blood glucose levels as a result of sports activities [18, 19]. Moreover, exercise training may be somewhat effective as a physiological regulator of GLP-1 function or secretion [20]. In the study of Lee et al., 12 weeks of low-intensity aerobic training led to a decrease in glucose and serum leptin levels, as well as a significant increase in GLP-1 in adolescent boys with type 2 diabetes [21]. But in the study of Ueda et al, serum GLP-1 levels did not change significantly after 12 weeks of exercise compared to before the exercise program [22]. So, this study was designed to investigate the effect of six weeks of interval training and consumption of PFRE and the combination of the two interventions on gene expression of GLP-1 in the intestine of diabetic male rats..
Materials and Methods
Drugs and chemicals
Streptozotocin, with a purity greater than 99%, was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). All of the Assay Kits were purchased from ZellBio (Germany). All other chemicals used were of analytical grade.
Animals
Fifty male Wistar rats (10 weeks old and weighing between 220 -250 g) were obtained from the laboratory animal reproduction and breeding center of Ahvaz Jundishapur University of Medical Sciences. Before starting the experiment, the rats were placed in a new housing space for seven days, with free access to water and standard animal food. During the experiment, the rats were kept in polycarbonate cages with dimensions of 25 x 50 cm (5 mice in one cage) with a temperature of 22 ± 2 °C and a relative humidity of 50 ± 5%. The dark and light cycle was 12:12 with light starting at 7 am. Animals had free access to standard food and water.
Plant extraction
The Prosopis farcta plant was harvested in the winter of 2018, from its natural habitats in Shushtar city, Khuzestan province, Iran, and the plant species was confirmed by the Faculty of Botany of Shahid Chamran University of Ahvaz. The plant was dried at room temperature (25-26 °C), away from direct sunlight, then the root of the dried plant was ground and used for the next steps. Then the extract was prepared by the maceration method. First, the ground roots were soaked in 70% ethanol in a dark environment for 72 hours with occasional shaking. The solution was filtered using filter paper (Whatman No. 2) and then concentrated under vacuum in a rotary evaporator. The concentrated extract was completely dried in Pyrex plates for 24 hours in an oven at 40 °C. The harvested extract was stored at 4 °C. Then the extract was dissolved in distilled water at a concentration of 10% and fed orally to the animals at a dose of 300 mg /kg of body weight. For other groups, a similar volume of distilled water was gavage so that the stress caused by gavage was similar in all animals.
Study design
In this experimental study, fifty male Wistar rats were randomly divided into five groups, including: 1) control group, 2) diabetic group, 3) diabetic + intermittent exercise group, 4) diabetic PFRE group, 5) diabetic + combination of intermittent exercise and PFRE consumption group. After the end of the intervention, sampling was done. The timing of measurements, interventions, and sampling is presented in Table 1.
Diabetes Induction
In order to induce diabetes mellitus in rats, a single dose of 50 mg/kg of Streptozotocin (dissolved in sodium citrate solvent and citric acid) was injected intraperitoneally after an overnight fast (without food). 48 hours after Streptozotocin injection, and after 12 hours of fasting, a blood sample was taken by creating a scratch at the end of the rat’s tail, and the fasting blood sugar was measured using a glucometer (Accu-Chek Active, Roche, Germany). The animals receiving Streptozotocin with a glucose concentration higher than 250 mg/dL were considered diabetic rats. To equalize the stress in non-diabetic rats, only the sodium citrate solvent was injected for them. Also, to ensure the diabetes status, blood sugar was measured every week using tail vein blood.
Measurement of the maximum running speed
First, the rats were adapted to training on the treadmill for three days. To perform the maximum speed test, the rats were fasted for 4 hours before the test, and the test was performed between 9 and 11 am.
The animals performed the warm-up program at a speed of 5 meters/min for 5 minutes and then started the test at a speed of 9 meters/min (5 minutes). In the continuation of the test, the speed of the rotating tape was increased by 2 meters/min every 2 minutes until the animals became exhausted. The criterion of animal retardation was the inability to return to running on the treadmill within 10 seconds [23].
Interval training protocol
Intermittent training, including running on the rodent treadmill at a speed of 24 to 34 m/min, equivalent to 75 to 100% of the maximum oxygen consumption, was performed six days a week for six weeks [24]. Due to the obesity of the animals, the training started with a lower intensity than the original protocol. Before starting the training period, the animals first ran on the treadmill for a week to familiarize themselves with the training. Overall, during the six weeks, the animals performed 5-12 repetitions of 60 seconds at an intensity of 24-34 m/min with active rest intervals of 75 seconds (Table 2). All training sessions started with a 5-minute warm-up (at a speed of 5 m/min) and ended with a 5-minute cool-down.
Diabetes mellitus is a multifactorial syndrome, and various factors play a role in the onset and progression of this disease. Gene mutations, aging, some environmental factors, and underlying diseases are the most determining factors [5]. Among these factors, the role of the incretin hormone Glucagon-like peptide-1 (GLP-1) and its receptors (GLP-1R) is important in insulin secretion from pancreatic cells. Incretins are digestive hormones that generally increase the secretion of insulin, decrease the secretion, and the rate of gastric emptying, and create a feeling of satiety [6]. GLP-1 is secreted from the intestinal L cells after food enters the intestine and blood glucose increases. This hormone increases insulin levels and decreases blood sugar in direct and indirect ways. GLP-1 increases insulin gene expression and its synthesis directly through activation of GLP-1 receptors on pancreatic cells, and on the other hand, indirectly through the vagus nerve system and hepatic portal vein, increases insulin secretion. Research shows that in type 2 diabetes, GLP-1 secretion is disrupted, so improving blood GLP-1 levels can increase insulin levels and regulate blood glucose [7, 8].
In the presence of increased blood glucose concentration, rapid and higher cAMP production is mediated by stimulation of GLP-1R-dependent plasma membrane-bound adenylyl cyclase, increasing insulin secretion from β-cells [9]. Although plasma membrane-bound adenylyl cyclase activity is also increased in response to other high glucose-dependent pathways, it is affected to a much lesser extent than GLP-1R [10].
Streptozotocin induced diabetes is one of the chemical models of experimental diabetes in which Streptozotocin is used to destroy beta cells [11]. Streptozotocin was extracted from a species of Streptomyces (Streptomyces achromogenes) in 1956 by the researchers of Upjohn Pharmaceutical Company as an antibiotic and has a wide antimicrobial spectrum. Streptozotocin is used clinically in the treatment of beta-cell malignancy and also as a laboratory agent to induce diabetes. Structurally, this substance is a nitrous urea glucosamine, and due to its structural similarity to glucose, it enters beta cells and selectively destroys them [11, 12]. The availability and relatively low cost of medicinal plants, as well as fewer side effects than chemical drugs, have caused them to be widely considered all over the world. Prosopis farcta root extract (PFRE) belongs to the Leguminosae family and the Mimosoideae subfamily, which is native to dry and semi-arid regions of America, Asia, and Africa [13]. The effective compounds found in this plant are: 5-hydroxyl tryptamine (alkaloid), L-arabinose, lectin, quercetin, tryptamine, and apigenin [14]. Literature review showed that this plant has anti-diabetic, antioxidant, anti-inflammatory, anti-apoptotic, anti-spasm, and soothing properties [14, 15]. According to previous research, prescribing the hydroalcoholic extract of the fruit and the root of the PFRE plant has been able to reduce blood glucose by changing the expression level of the pyruvate kinase gene [16].
In 2019, an experimental study was conducted to investigate the effect of Prosopis farcta extract on the gene expression of some of the main genes of the glycolysogen pathway involved in insulin signaling pyruvate kinase, phosphofructokinase, glucokinase and insulin signaling phosphotidylinositol 3 kinase, insulin receptor substrate, glucose transporter2 (GluT2) in HePG2 cells. In this study, HePG2 cells were cultured and treated with Prosopis farcta extract (leaves, roots, fruits).
The results showed that the expression of glucokinase, phosphofructo, and pyruvate kinase was upregulated, and the expression of GluT2, PI3-kinase, and insulin receptor substrate genes was upregulated compared to control cells. It seems that the effect on diabetes of Prosopis farcta on diabetes is not more than the glycolysis pathway. This mechanism acts by increasing gene expression in the insulin signaling pathway [17].
Several studies have investigated the effect of exercise interventions on blood glucose levels, serum insulin, insulin resistance, hormonal or genetic factors in patients with type 2 diabetes and healthy people. But the findings are slightly contradictory; nevertheless, all studies have reported a significant reduction in blood glucose levels as a result of sports activities [18, 19]. Moreover, exercise training may be somewhat effective as a physiological regulator of GLP-1 function or secretion [20]. In the study of Lee et al., 12 weeks of low-intensity aerobic training led to a decrease in glucose and serum leptin levels, as well as a significant increase in GLP-1 in adolescent boys with type 2 diabetes [21]. But in the study of Ueda et al, serum GLP-1 levels did not change significantly after 12 weeks of exercise compared to before the exercise program [22]. So, this study was designed to investigate the effect of six weeks of interval training and consumption of PFRE and the combination of the two interventions on gene expression of GLP-1 in the intestine of diabetic male rats..
Materials and Methods
Drugs and chemicals
Streptozotocin, with a purity greater than 99%, was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). All of the Assay Kits were purchased from ZellBio (Germany). All other chemicals used were of analytical grade.
Animals
Fifty male Wistar rats (10 weeks old and weighing between 220 -250 g) were obtained from the laboratory animal reproduction and breeding center of Ahvaz Jundishapur University of Medical Sciences. Before starting the experiment, the rats were placed in a new housing space for seven days, with free access to water and standard animal food. During the experiment, the rats were kept in polycarbonate cages with dimensions of 25 x 50 cm (5 mice in one cage) with a temperature of 22 ± 2 °C and a relative humidity of 50 ± 5%. The dark and light cycle was 12:12 with light starting at 7 am. Animals had free access to standard food and water.
Plant extraction
The Prosopis farcta plant was harvested in the winter of 2018, from its natural habitats in Shushtar city, Khuzestan province, Iran, and the plant species was confirmed by the Faculty of Botany of Shahid Chamran University of Ahvaz. The plant was dried at room temperature (25-26 °C), away from direct sunlight, then the root of the dried plant was ground and used for the next steps. Then the extract was prepared by the maceration method. First, the ground roots were soaked in 70% ethanol in a dark environment for 72 hours with occasional shaking. The solution was filtered using filter paper (Whatman No. 2) and then concentrated under vacuum in a rotary evaporator. The concentrated extract was completely dried in Pyrex plates for 24 hours in an oven at 40 °C. The harvested extract was stored at 4 °C. Then the extract was dissolved in distilled water at a concentration of 10% and fed orally to the animals at a dose of 300 mg /kg of body weight. For other groups, a similar volume of distilled water was gavage so that the stress caused by gavage was similar in all animals.
Study design
In this experimental study, fifty male Wistar rats were randomly divided into five groups, including: 1) control group, 2) diabetic group, 3) diabetic + intermittent exercise group, 4) diabetic PFRE group, 5) diabetic + combination of intermittent exercise and PFRE consumption group. After the end of the intervention, sampling was done. The timing of measurements, interventions, and sampling is presented in Table 1.
Diabetes Induction
In order to induce diabetes mellitus in rats, a single dose of 50 mg/kg of Streptozotocin (dissolved in sodium citrate solvent and citric acid) was injected intraperitoneally after an overnight fast (without food). 48 hours after Streptozotocin injection, and after 12 hours of fasting, a blood sample was taken by creating a scratch at the end of the rat’s tail, and the fasting blood sugar was measured using a glucometer (Accu-Chek Active, Roche, Germany). The animals receiving Streptozotocin with a glucose concentration higher than 250 mg/dL were considered diabetic rats. To equalize the stress in non-diabetic rats, only the sodium citrate solvent was injected for them. Also, to ensure the diabetes status, blood sugar was measured every week using tail vein blood.
Measurement of the maximum running speed
First, the rats were adapted to training on the treadmill for three days. To perform the maximum speed test, the rats were fasted for 4 hours before the test, and the test was performed between 9 and 11 am.
The animals performed the warm-up program at a speed of 5 meters/min for 5 minutes and then started the test at a speed of 9 meters/min (5 minutes). In the continuation of the test, the speed of the rotating tape was increased by 2 meters/min every 2 minutes until the animals became exhausted. The criterion of animal retardation was the inability to return to running on the treadmill within 10 seconds [23].
Interval training protocol
Intermittent training, including running on the rodent treadmill at a speed of 24 to 34 m/min, equivalent to 75 to 100% of the maximum oxygen consumption, was performed six days a week for six weeks [24]. Due to the obesity of the animals, the training started with a lower intensity than the original protocol. Before starting the training period, the animals first ran on the treadmill for a week to familiarize themselves with the training. Overall, during the six weeks, the animals performed 5-12 repetitions of 60 seconds at an intensity of 24-34 m/min with active rest intervals of 75 seconds (Table 2). All training sessions started with a 5-minute warm-up (at a speed of 5 m/min) and ended with a 5-minute cool-down.
Table 1. Procedure of treating animals in different groups
| Obtaining animals | Acquaintance | Examination | Induction of diabetes | Groups | Duration and type of intervention | Sampling |
| 50 male Wistar rats |
Determining and isolating Untrainable rats | Performing the maximum running speed test | Intraperitoneal injection of 50 mg/kg Streptozotocin |
Control group | (standard regime) without activity | End of the 6th week |
| Diabetes group | Diabetes induction | |||||
| Diabetes + interval training group | Diabetes induction: 6 weeks of interval training | |||||
| Diabetes + PFRE group | Diabetes induction: 6 weeks of PFRE consumption | |||||
| Diabetes group + combination of exercise and PFRE | Diabetes induction: Six weeks of training along with the use of PFRE |
PFRE = Prosopis farcta root extract
Table 2. Interval training protocol
| Repetition × Light duration (light intensity) × Heavy duration (heavy intensity) | Weak |
| 5×1min(24m/min) ×75sec(5m/min) | First week |
| 8×1min(24m/min) ×75sec(5m/min) | Second week |
| 10×1min(28m/min) ×75sec (5m/min) | Third week |
| 10×1min(30m/min) ×75sec (5m/min) | Fourth week |
| 12×1min(30m/min) ×75sec (5m/min) | Fifth week |
| 12×1min(34m/min) ×75sec (5m/min) | Sixth week |
Sample collection
48 hours after the last training session and after 12 hours of fasting, the animals were anesthetized using a combination of ketamine (100 mg/kg/ip) & xylazine (50 mg/kg/ip). To minimize the effect of the circadian rhythm, all samplings were done between 9 and 11 am. Blood samples were taken directly from the heart. Then the abdomen of the animals was opened, and the duodenal part of the rats' intestine was removed, and the extra tissues were washed with distilled water and immediately frozen in liquid nitrogen and stored at -80°C until analysis.
Serum biochemical assays
The blood specimens were collected in a gel-filled clot tube. After blood clotting, the samples were centrifuged for 15 minutes at 3,000 rpm, and the serum was separated and stored at -20 °C until use for the biochemical assays. Fasting blood sugar (FBS) was measured using the Auto Analyzer (Alpha Classic AT Plus, IRAN).
Measurement of GLP-1 gene expression by the real-time polymerase chain reaction (RT-PCR) method
RT-PCR technique was used to quantitatively measure GLP-1 gene expression in intestinal tissue. Briefly, first, the primer design was done, and then the total RNA was extracted from the tissues and converted into cDNA using reverse transcriptase enzyme. The resulting cDNA was treated with the DNase I enzyme to remove genomic DNA. Then the cDNA was amplified by PCR and analyzed for the expression of the mentioned gene.
Extraction of total RNA
RNA extraction from intestinal tissue was performed using Kyazol solution according to the manufacturer's protocol (Qiagen, Germany). To ensure no contamination with genomic DNA, it was exposed to DNase I (Fermentas). The purity of the extracted RNA was checked using a spectrophotometer (DPI-1, Kiagen) at a wavelength of 260 nm. The extracted RNA was kept at -80 °C until use.
cDNA synthesis
After extracting RNA with high purity and concentration, cDNA synthesis steps were performed according to the manufacturer's protocol (Fermentas, USA). Oligo dT primer (MWG-Biotech, Germany) and reverse transcription enzyme (Fermentas) were used for single-stranded cDNA synthesis.
Polymerase chain reaction
First, all designed primers were examined, and then gene expression was measured using the q-RT PCR method. For each test, the materials and primers used were poured into 48-well containers in the proportions mentioned in the manufacturer's instructions and mixed.
PCR reaction was performed using PCR master mix (Applied Biosystems) and SYBR Green in an ABI Step One thermocycler (Applied Biosystems, Sequences Detection Systems, Foster City, CA) according to the manufacturer's protocol. 40 cycles were considered for each Real-Time PCR cycle, and the temperatures of each cycle were set at 94°C for 20 seconds, 58-60°C for 30 seconds, and 72°C for 30 seconds.
The melting curve and negative control curve were evaluated to check the accuracy of PCR reactions and the presence of contamination in each reaction. The expression ratio of the studied gene was evaluated by the comparative method of threshold cycle (CT) using the following formula. The expression level of the target gene was normalized with the reference gene, and the expression of the genes of the healthy group was considered as a calibrator. E stands for efficiency and was obtained by drawing a standard curve for the gene. For specific amplification of the GLP-1 gene in real time PCR, specific primers were designed and used. The sequence of the forward primer GLP-1R was 5′-TGATGTGAGTTCTTAGTTGGAGGG-3′ and the reverse primer sequence r-glp1 was 5′-TGTGAGGATGGTTGTGAATGGTGA-3′.
48 hours after the last training session and after 12 hours of fasting, the animals were anesthetized using a combination of ketamine (100 mg/kg/ip) & xylazine (50 mg/kg/ip). To minimize the effect of the circadian rhythm, all samplings were done between 9 and 11 am. Blood samples were taken directly from the heart. Then the abdomen of the animals was opened, and the duodenal part of the rats' intestine was removed, and the extra tissues were washed with distilled water and immediately frozen in liquid nitrogen and stored at -80°C until analysis.
Serum biochemical assays
The blood specimens were collected in a gel-filled clot tube. After blood clotting, the samples were centrifuged for 15 minutes at 3,000 rpm, and the serum was separated and stored at -20 °C until use for the biochemical assays. Fasting blood sugar (FBS) was measured using the Auto Analyzer (Alpha Classic AT Plus, IRAN).
Measurement of GLP-1 gene expression by the real-time polymerase chain reaction (RT-PCR) method
RT-PCR technique was used to quantitatively measure GLP-1 gene expression in intestinal tissue. Briefly, first, the primer design was done, and then the total RNA was extracted from the tissues and converted into cDNA using reverse transcriptase enzyme. The resulting cDNA was treated with the DNase I enzyme to remove genomic DNA. Then the cDNA was amplified by PCR and analyzed for the expression of the mentioned gene.
Extraction of total RNA
RNA extraction from intestinal tissue was performed using Kyazol solution according to the manufacturer's protocol (Qiagen, Germany). To ensure no contamination with genomic DNA, it was exposed to DNase I (Fermentas). The purity of the extracted RNA was checked using a spectrophotometer (DPI-1, Kiagen) at a wavelength of 260 nm. The extracted RNA was kept at -80 °C until use.
cDNA synthesis
After extracting RNA with high purity and concentration, cDNA synthesis steps were performed according to the manufacturer's protocol (Fermentas, USA). Oligo dT primer (MWG-Biotech, Germany) and reverse transcription enzyme (Fermentas) were used for single-stranded cDNA synthesis.
Polymerase chain reaction
First, all designed primers were examined, and then gene expression was measured using the q-RT PCR method. For each test, the materials and primers used were poured into 48-well containers in the proportions mentioned in the manufacturer's instructions and mixed.
PCR reaction was performed using PCR master mix (Applied Biosystems) and SYBR Green in an ABI Step One thermocycler (Applied Biosystems, Sequences Detection Systems, Foster City, CA) according to the manufacturer's protocol. 40 cycles were considered for each Real-Time PCR cycle, and the temperatures of each cycle were set at 94°C for 20 seconds, 58-60°C for 30 seconds, and 72°C for 30 seconds.
The melting curve and negative control curve were evaluated to check the accuracy of PCR reactions and the presence of contamination in each reaction. The expression ratio of the studied gene was evaluated by the comparative method of threshold cycle (CT) using the following formula. The expression level of the target gene was normalized with the reference gene, and the expression of the genes of the healthy group was considered as a calibrator. E stands for efficiency and was obtained by drawing a standard curve for the gene. For specific amplification of the GLP-1 gene in real time PCR, specific primers were designed and used. The sequence of the forward primer GLP-1R was 5′-TGATGTGAGTTCTTAGTTGGAGGG-3′ and the reverse primer sequence r-glp1 was 5′-TGTGAGGATGGTTGTGAATGGTGA-3′.
ΔΔCT = (CT Target− CT Reference) Time X − (CT Target− CT Reference) Time 0
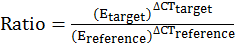
(ΔCT Reference= CT Control – CT Treatment, ΔCT Target= Ct Control– Ct Treatment)
Statistical analysis
Statistical analyses were performed using SPSS software version 22. The results were presented as mean ± standard deviation. First, the normality of data distribution was analyzed using the Kolmogorov-Smirnov test. One-way ANOVA was used to compare the means of groups, and Bonferroni's test was used as a post hoc test. The significance level for all tests was considered at p < 0.05.
Results
The effect of intermittent training and PFRE administration on body weight
ANOVA test results showed that the induction of diabetes caused a significant decrease in the weight of rats compared to healthy animals (p = 0.0001; F(4) = 30.175 ). Also, the weight of the group subjected to the combination of PFRE consumption and exercise showed a significant increase compared to the diabetic group, but no significant difference was observed between the groups of exercise, PFRE, and the combination of PFRE and exercise (Fig. 1).
The effect of intermittent training and PFRE administration on the blood glucose level
Induction of diabetes caused a significant increase in plasma glucose concentration. Also, the concentration of plasma glucose decreased significantly after six weeks of intermittent exercise, consumption of PFRE, and the combination of exercise and PFRE (p= 0.0001; F(4) = 72.542), but no significant difference was observed between the intervention groups. (Fig. 2).
The effect of intermittent training and PFRE administration on the GLP-1 gene expression
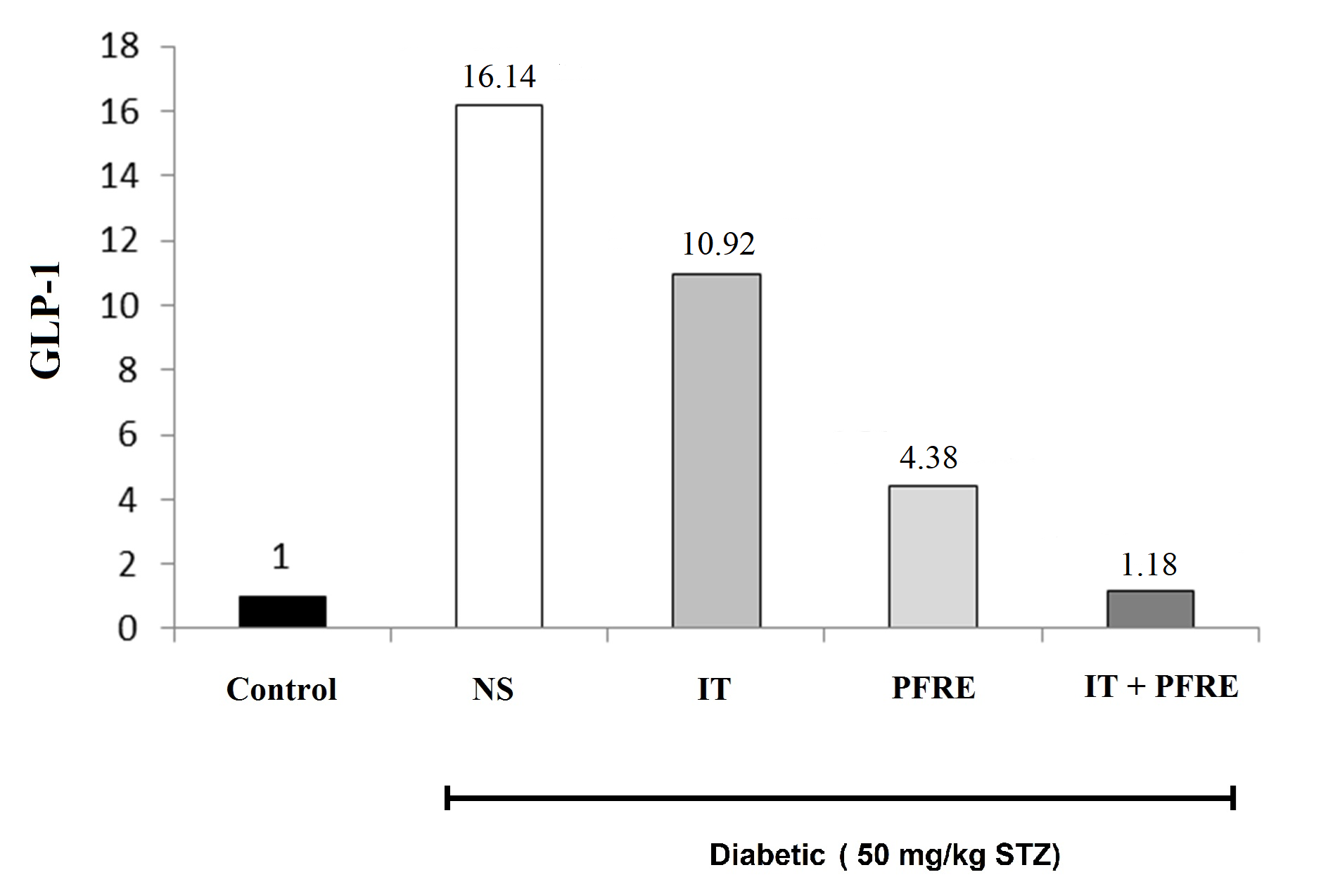
Current results showed that the induction of diabetes has increased the expression of GLP-1 by about 16 times compared to the control group. Intermittent training, PFRE consumption, and the combination of exercise and PFRE decreased the expression of GLP-1 by 32.3, 72.8, and 92.6%, respectively, compared to the diabetes group (Fig. 3). Table 3 displays the effect of intermittent training and PFRE administration on the measured parameters.
Statistical analyses were performed using SPSS software version 22. The results were presented as mean ± standard deviation. First, the normality of data distribution was analyzed using the Kolmogorov-Smirnov test. One-way ANOVA was used to compare the means of groups, and Bonferroni's test was used as a post hoc test. The significance level for all tests was considered at p < 0.05.
Results
The effect of intermittent training and PFRE administration on body weight
ANOVA test results showed that the induction of diabetes caused a significant decrease in the weight of rats compared to healthy animals (p = 0.0001; F(4) = 30.175 ). Also, the weight of the group subjected to the combination of PFRE consumption and exercise showed a significant increase compared to the diabetic group, but no significant difference was observed between the groups of exercise, PFRE, and the combination of PFRE and exercise (Fig. 1).
The effect of intermittent training and PFRE administration on the blood glucose level
Induction of diabetes caused a significant increase in plasma glucose concentration. Also, the concentration of plasma glucose decreased significantly after six weeks of intermittent exercise, consumption of PFRE, and the combination of exercise and PFRE (p= 0.0001; F(4) = 72.542), but no significant difference was observed between the intervention groups. (Fig. 2).
The effect of intermittent training and PFRE administration on the GLP-1 gene expression
Current results showed that the induction of diabetes has increased the expression of GLP-1 by about 16 times compared to the control group. Intermittent training, PFRE consumption, and the combination of exercise and PFRE decreased the expression of GLP-1 by 32.3, 72.8, and 92.6%, respectively, compared to the diabetes group (Fig. 3). Table 3 displays the effect of intermittent training and PFRE administration on the measured parameters.

Fig. 1. The effect of intermittent training and PFRE administration on the body weight in the studied groups (* significant difference was seen in the weight of the animals at the final level)
Data are expressed as mean ± standard deviation
NS= Streptozotocin-induced diabetic rats; IT= Intermittent training; PFRE= Prosopis farcta root extract
Data are expressed as mean ± standard deviation
NS= Streptozotocin-induced diabetic rats; IT= Intermittent training; PFRE= Prosopis farcta root extract

Fig. 2. The effect of intermittent training and PFRE administration on the blood glucose changes in the studied groups
Data are expressed as mean ± standard deviation
NS= Streptozotocin-induced diabetic rats; IT= Intermittent training; PFRE= Prosopis farcta root extract


Fig. 3. The effect of intermittent training and PFRE administration on the GLP-1
gene expression level in the studied groups
Data are expressed as mean ± standard deviation
NS= Streptozotocin-induced diabetic rats; IT= Intermittent training; PFRE= Prosopis farcta root extract
gene expression level in the studied groups
Data are expressed as mean ± standard deviation
NS= Streptozotocin-induced diabetic rats; IT= Intermittent training; PFRE= Prosopis farcta root extract
Table 3. The effect of intermittent training and PFRE administration on the measured parameters
| Variabls | Groups | ||||
| Control | Diabetic | Diabetic+IT | Diabetic+PFRE | Diabetic+IT+PFRE | |
| Initial weight (gr)* | 217.4 ± 4.2 | 212.8 ±7.7 | 216.6 ± 3.6 | 215.8 ± 1.81 | 213.4 ± 6.7 |
| Final weight # | 401.2 ± 32.05 | 256.4± 26.5 | 290.4 ± 56.5 | 280.8 ± 15.5 | 3020 ± 22.6 |
| Initial plasma glucose (mg/dl) & | 88.6 ± 10.5 | 82.8 ± 11.4 | 83.0 ± 12.6 | 89.8 ± 7.7 | 94.0 ± 11.6 |
| Glucose after injection (mg/dl) $ | 82.4 ± 11.00 | 400.8± 71.4 | 409.8 ± 91.1 | 395.6 ± 71.6 | 455.4 ± 54.9 |
| Final plasma glucose # | 96.6 ± 7.2 | 434.6± 82.5 | 290.4 ± 56.5 | 293.4 ± 11.2 | 259.8 ± 30.6 |
| GLP-1 | 0.00000034± 0.00000023 |
0.0000055± 0.0000015 |
0.0000037± 0.0000023 |
0.0000015± 0.0000014 |
0.000000405± 0.0000002 |
* Before starting the study
& After eight weeks of a high-fat diet and before starting to exercise and consume PFRE
# At the end of six weeks of intervention
$ 48 hours after streptozotocin injection
Data are expressed as mean ± standard deviation
IT= Intermittent training; PFRE= Prosopis farcta root extract; GLP-1= Glucagon-like peptide-1
& After eight weeks of a high-fat diet and before starting to exercise and consume PFRE
# At the end of six weeks of intervention
$ 48 hours after streptozotocin injection
Data are expressed as mean ± standard deviation
IT= Intermittent training; PFRE= Prosopis farcta root extract; GLP-1= Glucagon-like peptide-1
Discussion
GLP-1 is a peptide hormone consisting of 30 amino acids. GLP-1 plays a crucial role in glucose homeostasis and insulin secretion. It is produced by the L cells in the small intestine and released into the bloodstream in response to food intake [7].
GLP-1 binds to the GLP-1 receptor, which is expressed on various tissues, including the pancreas, brain, and gastrointestinal tract. It increases insulin secretion directly by affecting its receptors on pancreatic beta cells and indirectly through the vagus nerve system and hepatic portal vein. When GLP-1 binds to its receptor on pancreatic beta cells, it activates a signaling cascade that leads to increased insulin secretion and decreased glucagon secretion. This helps to lower blood glucose levels by promoting glucose uptake and storage in tissues.
In addition to direct effects on insulin secretion, GLP-1 stimulates glucose consumption and is also able to increase the proliferation of pancreatic beta cells [25]. GLP-1 also slows down gastric emptying and reduces appetite by acting on receptors in the brain. This helps to control food intake and promote weight loss. In addition to its effects on glucose regulation and appetite, GLP-1 has been shown to have cardiovascular benefits. It can improve endothelial function, reduce inflammation, and lower blood pressure [7].
Type 2 diabetes results from a decrease in beta-cell function and an increase in insulin resistance. Moreover, in type 2 diabetes, GLP-1 secretion is disturbed, so the increase and improvement of blood GLP-1 level can cause an increase in insulin and blood glucose regulation [26]. Therefore, the present study was conducted to investigate the potential effect of intermittent exercise and consumption of PFRE on GLP-1 gene expression in an experimental model of diabetes.
The findings of the present study showed that induction of diabetes caused a significant increase in plasma glucose concentration and an increase in GLP-1 gene expression about 16 times in the control group.
The authors concluded that due to the significant increase in blood sugar levels in the diabetic group (positive control), the level of GLP-1 hormone has increased in response to increased blood glucose to stimulate more insulin secretion. Exercise improves glucose metabolism and insulin sensitivity, but its effect on GLP-1 gene expression depends on several factors, including the duration, intensity, and frequency of exercise [27]. So, more research is needed to understand the relationship between intermittent exercise and GLP-1 expression. Our findings showed that six weeks of intermittent training caused a significant decrease in plasma glucose concentration and no significant change in GLP-1 gene expression.
Several studies have investigated the effect of exercise interventions on blood glucose levels, serum insulin, insulin resistance, hormonal or genetic factors in patients with type 2 diabetes and healthy people. But the findings are slightly contradictory; nevertheless, all studies have reported a significant reduction in blood glucose levels as a result of sports activities [18, 19]. In addition, various results have been reported regarding the effect of exercise on GLP levels. For example, in the study of Ueda et al, serum GLP-1 levels did not change significantly after 12 weeks of exercise compared to before the exercise program [22].
In the study of Abdolfathi et al, performing acute aerobic activity with moderate intensity to the point of exhaustion immediately and 24 hours after the activity did not affect serum GLP-1, glucose, and insulin levels in women with T2D [28], which are in line with the results of the present study. However, in the study of Lee et al., 12 weeks of low-intensity aerobic training led to a decrease in glucose and serum leptin levels, as well as a significant increase in GLP-1 in adolescent boys with type 2 diabetes [21]. By examining and comparing these studies more closely, some possible reasons for this difference in results can be attributed to the intensity, duration, volume, and type of exercise.
In addition, previous research has shown that the intensity and duration of exercise are effective in the changes of inflammatory indices in type 2 diabetic patients [29]. The authors concluded that in the present study, insufficient duration, intensity, and volume of exercise may play a role in the lack of effect of exercise on GLP-1 gene expression.
Currently, the direct role of the herbal product on GLP-1 gene expression is not clear. However, some studies have suggested that certain flavonoids and other herbal ingredients may indirectly affect GLP-1 levels and activity. For example, some studies have shown that flavonoids found in fruits and vegetables, such as quercetin and catechins, may improve glucose regulation by increasing GLP-1 secretion and enhancing its effects on insulin secretion and glucose uptake [30]. Similarly, natural antioxidants such as resveratrol have been shown to improve insulin sensitivity and glucose metabolism, possibly by increasing GLP-1 activity [31]. Overall, while there is no direct relationship between herbal ingredients and GLP-1 gene expression, these compounds may have beneficial effects on glucose regulation and metabolic health through their interactions with GLP-1 signaling pathways.
The findings of the present study indicated that six weeks of consumption of PFRE caused a significant decrease in plasma glucose concentration and a decrease in GLP-1 gene expression.
In a similar study, Heidar Lashkari et al. investigated the effects of the metabolic extract of Prosopis farcta seed on blood glucose levels in diabetic rats [32]. They reported that this extract had a significant effect in reducing blood glucose levels in the diabetic rats, which was consistent with the results of the present study.
Different mechanisms have been mentioned regarding the anti-diabetic effects of the Prosopis farcta extract. Some researchers suggested that the anti-diabetic activity might be attributed to the presence of flavonoids, tannins, and other polyphenolic compounds in the extract [33]. It has also been suggested that this extract increases insulin secretion, protects pancreatic beta cells from damage, improves insulin sensitivity, and enhances insulin signaling and glucose uptake. Additionally, the extract may have antioxidant properties that contribute to its anti-diabetic effects [34-36].
There is limited research on the specific effects of combining exercise and natural products on diabetic control and GLP-1 gene expression. However, recent studies have suggested that the combination of interval exercise and herbal products may have a synergistic effect on diabetes complications.
For example, in a study conducted by Ghalavand et al, the effects of interval training and nettle supplementation on glycemic control and blood pressure in men with type 2 diabetes were investigated. Their findings showed that interval training and nettle supplementation were effective strategies for managing type 2 diabetes symptoms, and combining them provided even greater benefits [37].
In another study, Nouri et al investigated the effects of resveratrol supplementation and exercise on diabetic biomarkers in the liver of diabetic rats. The results showed that both interventions reduced apoptosis, improved lipid profile, and increased the expression of genes related to the regulation of glucose and lipid metabolism. In addition, combining resveratrol supplementation with exercise showed better results [38].
The results of the present study showed that the combination of six weeks of interval training with the use of PFRE at the same time decreased blood sugar, and with a decrease in blood sugar, the expression of the GLP-1 gene decreased significantly.
Fujiwara et al believe that combining drug therapy with lifestyle interventions such as diet and exercise can lead to greater improvements in blood glucose control, GLP-1 levels, and other health outcomes in people with type 2 diabetes [39]. Overall, while more research is needed to fully understand the effects of combining exercise and herbal supplements on GLP-1 gene expression, these interventions may have synergistic benefits for improving glucose regulation and metabolic health. The authors of this study conclude that exercise, along with the consumption of the extract, has reduced blood sugar in diabetic animals, and this itself has reduced the expression of the GLP-1 gene through a feedback mechanism. The literature review showed that the combination of intermittent exercise and herbal products may work through multiple mechanisms to improve glucose regulation and reduce blood sugar levels, including: Increased insulin sensitivity, enhanced glucose uptake, increased GLP-1 activity, reduced inflammation, and Improved mitochondrial function. Exercise can increase insulin sensitivity, allowing the body to use insulin more effectively to lower blood sugar levels. Herbal products such as cinnamon and fenugreek have also been shown to improve insulin sensitivity [40, 41]. Exercise can increase glucose uptake by muscle cells, which can help to lower blood sugar levels. Some herbal products, such as berberine, have also been shown to enhance glucose uptake [42]. Exercise has been shown to increase GLP-1 activity, and some herbal products, such as bitter melon and ginseng, have also been shown to stimulate GLP-1 secretion [39, 43]. Chronic inflammation can contribute to insulin resistance and impaired glucose metabolism. Exercise has anti-inflammatory effects, and some herbal products, such as turmeric and ginger, have also been shown to have anti-inflammatory properties [44, 45]. Mitochondria are the energy-producing organelles in cells, and impaired mitochondrial function can contribute to insulin resistance and impaired glucose metabolism. Exercise has been shown to improve mitochondrial function, and some herbal products, such as ashwagandha, have also been shown to enhance mitochondrial function [46, 47].
The anti-diabetic effect of PFRE can be attributed to the effective compounds of this plant, including quercetin and apigenin. Quercetin, a natural flavonoid, stimulates GLP-1 release through TAS2R38-mediated PLC signaling from enteroendocrine NCI-H716 cells. Quercetin significantly enhanced GLP-1 secretion, which was attenuated by TAS2R38 siRNA and PLC inhibitors [48].
Quercetin decreases blood glucose levels, improves glucose tolerance, and enhances pancreatic β-cell function via various mechanistic pathways, such as AMPK, which regulates GLUT4 expression in adipose tissue and muscles. It also regulates glycaemia by reducing GLUT2 expression and sodium-dependent glucose uptake in the gut, as well as lowering glucose absorption. It also inhibits the release of pro-inflammatory mediators, such as interleukin-1β, interleukin-4, interleukin-6, and tumor necrosis factor-α., preventing pancreatic β-cell damage. Quercetin has been shown to improve insulin sensitivity, glucose metabolism, and insulin secretion in diabetic animal models by promoting pancreatic β-cell proliferation. It inhibits the α-glucosidase and dipeptidyl peptidase-IV enzymes, which prolong the half-life of GLP-1 and glucose-dependent insulinotropic polypeptide [49].
Apigenin, a natural bioflavonoid, possesses the ability to inhibit α-glucosidase activity, cause stimulation of insulin action and secretion, Amplifies glucose-stimulated insulin secretion through the PKA-MEK signaling cascade without directly affecting K-ATP channels [50]. It can be concluded that each of the effective compounds of PFRE in lowering blood sugar can affect lowering blood sugar through one or more pathways, of which GLP-1 gene expression is one of these pathways. Therefore, PFRE can be effective in lowering blood sugar by increasing GLP-1 gene expression.
Despite the efforts to control disturbing and interfering factors, some things were out of the researcher's control. One of these factors was the stress caused by the treadmill and the nature of the animals' activity on the treadmill, which may have affected the results of the present study. Another factor was the injury caused by the friction between the animal's foot and the conveyor belt, as well as the tail injury of the animals in the training group, which probably caused inflammation in the body of these animals and may have affected the results of the present study. Gavage of animals is another thing that causes stress to animals.
It is suggested that to better investigate the mechanism of blood sugar reduction and the effect of GLP-1 gene expression on it, the expression of other genes, including TCF7L2, which affect regulating GLP-1 activity, should be used. Also, given that the differences in the results of studies investigating the effect of exercise on GLP-1 gene expression can be attributed to the intensity, duration, volume, and type of exercise, it is suggested that exercise training be performed with different intensities and durations, because in this study, due to the obesity of the animals, the training started with a lower intensity than the original protocol.
Conclusion
In general, the results of the present study showed that intermittent exercise, PFRE consumption, and the combination of 2 interventions reduce blood sugar. Also, this decrease in blood sugar decreased the expression of GLP-1 through a feedback mechanism. However, further research is needed to fully understand the mechanisms underlying this effect and to determine the optimal combination of exercise and herbal products for maximizing GLP-1 expression.
Ethical Considerations
After the study, the animals were euthanized easily using standard conventional methods. Moreover, all experiments were done according to the Animal Ethics Committee Guidelines of Islamic Azad University - Science & Research Branch of Ahvaz, Iran.
Funding
The corresponding author covered the experiment and other associated costs.
Conflict of Interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the Laboratory Animal Breeding and Reproduction Center, Jundishapur University of Ahvaz, and the Faculty of Botany, Shahid Chamran University of Ahvaz.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Authors' Contributions
Z.K. and M.G. were responsible for designing the study protocol, conducting the literature review, providing feedback on the manuscripts, writing the manuscript and improving the interpretation of the results. M.G were responsible for analyzing data. Z.K was responsible for writing the manuscript, assembling data, and interpreting analyses.
GLP-1 binds to the GLP-1 receptor, which is expressed on various tissues, including the pancreas, brain, and gastrointestinal tract. It increases insulin secretion directly by affecting its receptors on pancreatic beta cells and indirectly through the vagus nerve system and hepatic portal vein. When GLP-1 binds to its receptor on pancreatic beta cells, it activates a signaling cascade that leads to increased insulin secretion and decreased glucagon secretion. This helps to lower blood glucose levels by promoting glucose uptake and storage in tissues.
In addition to direct effects on insulin secretion, GLP-1 stimulates glucose consumption and is also able to increase the proliferation of pancreatic beta cells [25]. GLP-1 also slows down gastric emptying and reduces appetite by acting on receptors in the brain. This helps to control food intake and promote weight loss. In addition to its effects on glucose regulation and appetite, GLP-1 has been shown to have cardiovascular benefits. It can improve endothelial function, reduce inflammation, and lower blood pressure [7].
Type 2 diabetes results from a decrease in beta-cell function and an increase in insulin resistance. Moreover, in type 2 diabetes, GLP-1 secretion is disturbed, so the increase and improvement of blood GLP-1 level can cause an increase in insulin and blood glucose regulation [26]. Therefore, the present study was conducted to investigate the potential effect of intermittent exercise and consumption of PFRE on GLP-1 gene expression in an experimental model of diabetes.
The findings of the present study showed that induction of diabetes caused a significant increase in plasma glucose concentration and an increase in GLP-1 gene expression about 16 times in the control group.
The authors concluded that due to the significant increase in blood sugar levels in the diabetic group (positive control), the level of GLP-1 hormone has increased in response to increased blood glucose to stimulate more insulin secretion. Exercise improves glucose metabolism and insulin sensitivity, but its effect on GLP-1 gene expression depends on several factors, including the duration, intensity, and frequency of exercise [27]. So, more research is needed to understand the relationship between intermittent exercise and GLP-1 expression. Our findings showed that six weeks of intermittent training caused a significant decrease in plasma glucose concentration and no significant change in GLP-1 gene expression.
Several studies have investigated the effect of exercise interventions on blood glucose levels, serum insulin, insulin resistance, hormonal or genetic factors in patients with type 2 diabetes and healthy people. But the findings are slightly contradictory; nevertheless, all studies have reported a significant reduction in blood glucose levels as a result of sports activities [18, 19]. In addition, various results have been reported regarding the effect of exercise on GLP levels. For example, in the study of Ueda et al, serum GLP-1 levels did not change significantly after 12 weeks of exercise compared to before the exercise program [22].
In the study of Abdolfathi et al, performing acute aerobic activity with moderate intensity to the point of exhaustion immediately and 24 hours after the activity did not affect serum GLP-1, glucose, and insulin levels in women with T2D [28], which are in line with the results of the present study. However, in the study of Lee et al., 12 weeks of low-intensity aerobic training led to a decrease in glucose and serum leptin levels, as well as a significant increase in GLP-1 in adolescent boys with type 2 diabetes [21]. By examining and comparing these studies more closely, some possible reasons for this difference in results can be attributed to the intensity, duration, volume, and type of exercise.
In addition, previous research has shown that the intensity and duration of exercise are effective in the changes of inflammatory indices in type 2 diabetic patients [29]. The authors concluded that in the present study, insufficient duration, intensity, and volume of exercise may play a role in the lack of effect of exercise on GLP-1 gene expression.
Currently, the direct role of the herbal product on GLP-1 gene expression is not clear. However, some studies have suggested that certain flavonoids and other herbal ingredients may indirectly affect GLP-1 levels and activity. For example, some studies have shown that flavonoids found in fruits and vegetables, such as quercetin and catechins, may improve glucose regulation by increasing GLP-1 secretion and enhancing its effects on insulin secretion and glucose uptake [30]. Similarly, natural antioxidants such as resveratrol have been shown to improve insulin sensitivity and glucose metabolism, possibly by increasing GLP-1 activity [31]. Overall, while there is no direct relationship between herbal ingredients and GLP-1 gene expression, these compounds may have beneficial effects on glucose regulation and metabolic health through their interactions with GLP-1 signaling pathways.
The findings of the present study indicated that six weeks of consumption of PFRE caused a significant decrease in plasma glucose concentration and a decrease in GLP-1 gene expression.
In a similar study, Heidar Lashkari et al. investigated the effects of the metabolic extract of Prosopis farcta seed on blood glucose levels in diabetic rats [32]. They reported that this extract had a significant effect in reducing blood glucose levels in the diabetic rats, which was consistent with the results of the present study.
Different mechanisms have been mentioned regarding the anti-diabetic effects of the Prosopis farcta extract. Some researchers suggested that the anti-diabetic activity might be attributed to the presence of flavonoids, tannins, and other polyphenolic compounds in the extract [33]. It has also been suggested that this extract increases insulin secretion, protects pancreatic beta cells from damage, improves insulin sensitivity, and enhances insulin signaling and glucose uptake. Additionally, the extract may have antioxidant properties that contribute to its anti-diabetic effects [34-36].
There is limited research on the specific effects of combining exercise and natural products on diabetic control and GLP-1 gene expression. However, recent studies have suggested that the combination of interval exercise and herbal products may have a synergistic effect on diabetes complications.
For example, in a study conducted by Ghalavand et al, the effects of interval training and nettle supplementation on glycemic control and blood pressure in men with type 2 diabetes were investigated. Their findings showed that interval training and nettle supplementation were effective strategies for managing type 2 diabetes symptoms, and combining them provided even greater benefits [37].
In another study, Nouri et al investigated the effects of resveratrol supplementation and exercise on diabetic biomarkers in the liver of diabetic rats. The results showed that both interventions reduced apoptosis, improved lipid profile, and increased the expression of genes related to the regulation of glucose and lipid metabolism. In addition, combining resveratrol supplementation with exercise showed better results [38].
The results of the present study showed that the combination of six weeks of interval training with the use of PFRE at the same time decreased blood sugar, and with a decrease in blood sugar, the expression of the GLP-1 gene decreased significantly.
Fujiwara et al believe that combining drug therapy with lifestyle interventions such as diet and exercise can lead to greater improvements in blood glucose control, GLP-1 levels, and other health outcomes in people with type 2 diabetes [39]. Overall, while more research is needed to fully understand the effects of combining exercise and herbal supplements on GLP-1 gene expression, these interventions may have synergistic benefits for improving glucose regulation and metabolic health. The authors of this study conclude that exercise, along with the consumption of the extract, has reduced blood sugar in diabetic animals, and this itself has reduced the expression of the GLP-1 gene through a feedback mechanism. The literature review showed that the combination of intermittent exercise and herbal products may work through multiple mechanisms to improve glucose regulation and reduce blood sugar levels, including: Increased insulin sensitivity, enhanced glucose uptake, increased GLP-1 activity, reduced inflammation, and Improved mitochondrial function. Exercise can increase insulin sensitivity, allowing the body to use insulin more effectively to lower blood sugar levels. Herbal products such as cinnamon and fenugreek have also been shown to improve insulin sensitivity [40, 41]. Exercise can increase glucose uptake by muscle cells, which can help to lower blood sugar levels. Some herbal products, such as berberine, have also been shown to enhance glucose uptake [42]. Exercise has been shown to increase GLP-1 activity, and some herbal products, such as bitter melon and ginseng, have also been shown to stimulate GLP-1 secretion [39, 43]. Chronic inflammation can contribute to insulin resistance and impaired glucose metabolism. Exercise has anti-inflammatory effects, and some herbal products, such as turmeric and ginger, have also been shown to have anti-inflammatory properties [44, 45]. Mitochondria are the energy-producing organelles in cells, and impaired mitochondrial function can contribute to insulin resistance and impaired glucose metabolism. Exercise has been shown to improve mitochondrial function, and some herbal products, such as ashwagandha, have also been shown to enhance mitochondrial function [46, 47].
The anti-diabetic effect of PFRE can be attributed to the effective compounds of this plant, including quercetin and apigenin. Quercetin, a natural flavonoid, stimulates GLP-1 release through TAS2R38-mediated PLC signaling from enteroendocrine NCI-H716 cells. Quercetin significantly enhanced GLP-1 secretion, which was attenuated by TAS2R38 siRNA and PLC inhibitors [48].
Quercetin decreases blood glucose levels, improves glucose tolerance, and enhances pancreatic β-cell function via various mechanistic pathways, such as AMPK, which regulates GLUT4 expression in adipose tissue and muscles. It also regulates glycaemia by reducing GLUT2 expression and sodium-dependent glucose uptake in the gut, as well as lowering glucose absorption. It also inhibits the release of pro-inflammatory mediators, such as interleukin-1β, interleukin-4, interleukin-6, and tumor necrosis factor-α., preventing pancreatic β-cell damage. Quercetin has been shown to improve insulin sensitivity, glucose metabolism, and insulin secretion in diabetic animal models by promoting pancreatic β-cell proliferation. It inhibits the α-glucosidase and dipeptidyl peptidase-IV enzymes, which prolong the half-life of GLP-1 and glucose-dependent insulinotropic polypeptide [49].
Apigenin, a natural bioflavonoid, possesses the ability to inhibit α-glucosidase activity, cause stimulation of insulin action and secretion, Amplifies glucose-stimulated insulin secretion through the PKA-MEK signaling cascade without directly affecting K-ATP channels [50]. It can be concluded that each of the effective compounds of PFRE in lowering blood sugar can affect lowering blood sugar through one or more pathways, of which GLP-1 gene expression is one of these pathways. Therefore, PFRE can be effective in lowering blood sugar by increasing GLP-1 gene expression.
Despite the efforts to control disturbing and interfering factors, some things were out of the researcher's control. One of these factors was the stress caused by the treadmill and the nature of the animals' activity on the treadmill, which may have affected the results of the present study. Another factor was the injury caused by the friction between the animal's foot and the conveyor belt, as well as the tail injury of the animals in the training group, which probably caused inflammation in the body of these animals and may have affected the results of the present study. Gavage of animals is another thing that causes stress to animals.
It is suggested that to better investigate the mechanism of blood sugar reduction and the effect of GLP-1 gene expression on it, the expression of other genes, including TCF7L2, which affect regulating GLP-1 activity, should be used. Also, given that the differences in the results of studies investigating the effect of exercise on GLP-1 gene expression can be attributed to the intensity, duration, volume, and type of exercise, it is suggested that exercise training be performed with different intensities and durations, because in this study, due to the obesity of the animals, the training started with a lower intensity than the original protocol.
Conclusion
In general, the results of the present study showed that intermittent exercise, PFRE consumption, and the combination of 2 interventions reduce blood sugar. Also, this decrease in blood sugar decreased the expression of GLP-1 through a feedback mechanism. However, further research is needed to fully understand the mechanisms underlying this effect and to determine the optimal combination of exercise and herbal products for maximizing GLP-1 expression.
Ethical Considerations
After the study, the animals were euthanized easily using standard conventional methods. Moreover, all experiments were done according to the Animal Ethics Committee Guidelines of Islamic Azad University - Science & Research Branch of Ahvaz, Iran.
Funding
The corresponding author covered the experiment and other associated costs.
Conflict of Interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the Laboratory Animal Breeding and Reproduction Center, Jundishapur University of Ahvaz, and the Faculty of Botany, Shahid Chamran University of Ahvaz.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Authors' Contributions
Z.K. and M.G. were responsible for designing the study protocol, conducting the literature review, providing feedback on the manuscripts, writing the manuscript and improving the interpretation of the results. M.G were responsible for analyzing data. Z.K was responsible for writing the manuscript, assembling data, and interpreting analyses.
References
- Magliano DJ, Islam RM, Barr EL, Gregg EW, Pavkov ME, Harding JL, et al. Trends in incidence of total or type 2 diabetes: systematic review. BMJ 2019; 366:151-65.
- Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X, et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Scientific Reports 2020; 10(1): 1-11.
- Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, et al. Pathophysiology of type 2 diabetes mellitus. International journal of molecular sciences. 2020; 21(17): 627-40.
- Balaji R, Duraisamy R, Kumar M. Complications of diabetes mellitus: A review. Drug Invention Today. 2019; 12(1): 255-70.
- Zimmet PZ, Magliano DJ, Herman WH, Shaw JE. Diabetes: a 21st century challenge. The Lancet Diabetes & Endocrinology 2014; 2(1): 56-64.
- Nauck MA, Meier JJ. Incretin hormones: their role in health and disease. Diabetes, Obesity and Metabolism 2018; 20:5-21.
- Müller TD, Finan B, Bloom S, D'Alessio D, Drucker DJ, Flatt P, et al. Glucagon-like peptide 1 (GLP-1). Molecular Metabolism 2019; 30:72-130.
- D'Alessio D. Is GLP‐1 a hormone: Whether and When? Journal of Diabetes Investigation 2016; 7: 50-55.
- Seino Y, Fukushima M, Yabe D. GIP and GLP‐1, the two incretin hormones: similarities and differences. Journal of Diabetes Investigation 2010; 1(1‐2): 8-23.
- Ramos LS, Zippin JH, Kamenetsky M, Buck J, Levin LR. Glucose and GLP-1 stimulate cAMP production via distinct adenylyl cyclases in INS-1E insulinoma cells. The Journal of General Physiology 2008; 132(3): 329-38.
- Magalhães DA, Kume WT, Correia FS, Queiroz TS, Allebrandt EW, Santos MP, et al. High-fat diet and streptozotocin in the induction of type 2 diabetes mellitus: a new proposal. Anais da Academia Brasileira de Ciências 2019; 91:102-15.
- Furman BL. Streptozotocin‐induced diabetic models in mice and rats. Current Protocols in Pharmacology 2015; 70(1): 5.47. 1-5.
- Shakya AK. Medicinal plants: Future source of new drugs. International Journal of Herbal Medicine 2016; 4(4): 59-64.
- Hajinezhad M, Esmaeilzadeh Bahabadi S, Miri H, Davari S, Darvish Sargazi M. Effect of hydroalcoholic extract of prosopis farcta pod on liver histopathology and malondialdehyde level in streptozotocin diabetic rats. Ofogh-e-Danesh 2015; 21(1): 31-6.
- Dashtban M, Sarir H, Omidi A. The effect of Prosopis farcta beans extract on blood biochemical parameters in streptozotocin-induced diabetic male rats. Advanced Biomedical Research 2016; 5:23-31.
- Bazi Shad A, Miri HR, Esmaeilzadeh Bahabadi S, Hajinezhad MR, Sabori H, Hassanzadeh M. The effect of hydro-alcoholic extract of Prosopis farcta on weight, blood glucose and gene expression of Pyruvate Kinase in Diabetic Rats (Type1]. Journal of Torbat Heydariyeh University of Medical Sciences 2017; 4(4): 1-9.
- Mirzahosseini-pourranjbar A, Karimabad MN, Hajizadeh MR, Khoshdel A, Fahmidehkar MA, Mohammad-Sadeghipour M, et al. The effect of Prosopis farcta extract on the expression of some key genes of the glycolysis pathway and the genes involved in insulin signaling in HepG2 cells. Gene Reports 2019; 17: 100494.
- Dela F, von Linstow ME, Mikines KJ, Galbo H. Physical training may enhance β-cell function in type 2 diabetes. American Journal of Physiology-Endocrinology and Metabolism 2004; 287(5): 1024-1031.
- Rawal S, Huang H, Novikova L, Hamedi T, Smirnova I, Stehno Bittel L. Effects of exercise on pancreatic islets in zucker diabetic fatty rats. J Diabetes Metab S. 2013; 10(2): 211-18.
- Tabibirad S, Aabednatanzi H, Nik Bakht H, Ghazalian F, Gholami M. Comparison of the effect of aerobic and resistance training on glucagon-like peptide-1 and insulin resistance in obese women with type 2 diabetes. Daneshvar Medicine 2020; 28(4): 46-56.
- Lee SS, Yoo JH, So YS. Effect of the low-versus high-intensity exercise training on endoplasmic reticulum stress and GLP-1 in adolescents with type 2 diabetes mellitus. Journal of Physical Therapy Science 2015; 27(10): 3063-3068.
- Ueda SY, Miyamoto T, Nakahara H, Shishido T, Usui T, Katsura Y, et al. Effects of exercise training on gut hormone levels after a single bout of exercise in middle-aged Japanese women. Springerplus 2013; 2:1-9.
- Charrin E, Dubé JJ, Connes P, Pialoux V, Ghosh S, Faes C, et al. Moderate exercise training decreases inflammation in transgenic sickle cell mice. Blood Cells, Molecules, and Diseases 2018; 69:45-52.
- Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ. A practical model of low‐volume high‐intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. The Journal of Physiology 2010; 588(6): 1011-22.
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007; 132(6): 2131-57.
- Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014; 37(S1): 81-90.
- Hamasaki H. Exercise and glucagon-like peptide-1: Does exercise potentiate the effect of treatment? World Journal of Diabetes 2018; 9(8): 138-50.
- Abolfathi F, Ranjbar R, Shakerian S, Yazdanpanah L. The effect of eight weeks aerobic interval training on adiponectin serum levels, lipid profile and HS-CRP in women with type II diabetes. Iranian Journal of Endocrinology and Metabolism 2015; 17(4): 342-50.
- Chen X, Sun X, Wang C, He H. Effects of exercise on inflammatory cytokines in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. Oxidative Medicine and Cellular Longevity 2020; 2020: 122-38.
- Al-Ishaq RK, Abotaleb M, Kubatka P, Kajo K, Büsselberg D. Flavonoids and their anti-diabetic effects: Cellular mechanisms and effects to improve blood sugar levels. Biomolecules. 2019; 9(9): 430.
- Pegah A, Abbasi-Oshaghi E, Khodadadi I, Mirzaei F, Tayebinia H. Probiotic and resveratrol normalize GLP-1 levels and oxidative stress in the intestine of diabetic rats. Metabolism Open 2021; 10: 93-112.
- Heidar Lashkari SHL, Sepehri G, Emadi L, Motaghi S. The effects of methanolic extract of prosopis farcta seed on blood glucose in streptozocin induced diabetic rats. Journal of Kerman University of Medical Sciences 2017; 24(3): 200-208.
- Dashtban M, Sarir H, Omidi A. The effect of Prosopis farcta beans extract on blood biochemical parameters in streptozotocin-induced diabetic male rats. Advanced Biomedical Research 2016; 5(1): 15-24.
- Heydari M, Sarir H, Ghiasi SE, Farhangfar H. Effects of Prosopis farcta fruit hydroalcoholic extract on serum concentrations of glucose and lipids in insulin resistance model of rats. Zahedan Journal of Research in Medical Sciences 2018; 20(1): 1-7.
- Feyzmand S, Shahbazi B, Marami M, Bahrami G, Fattahi A, Shokoohinia Y. Mechanistic in vitro evaluation of Prosopis farcta roots potential as an antidiabetic folk medicinal plant. Pharmacognosy Magazine 2017; 13(4): 852-63.
- Shahbazi B, Feyzmand S, Jafari F, Ghiasvand N, Bahrami G, Fattahi A, et al. Antidiabetic potential of Prosopis farcta roots: In vitro pancreatic beta cell protection, enhancement of glucose consumption, and bioassay-guided fractionation. Evidence-Based Complementary and Alternative Medicine 2020; (1): 125-33.
- Ghalavand A, Motamedi P, Delaramnasab M, Khodadoust M. The Effect of interval training and nettle supplement on glycemic control and blood pressure in men withtype 2 diabetes. Int J Basic Sci Med. 2022; 2(1): 33-40.
- Nouri A, Farzanegi P, Azarbayjani MA. Effects of resveratrol supplementation and exercise on apoptosis, lipid profile, and expression of farnesoid X receptor, liver X receptor and sirtuin 1 genes in the liver of type 1 diabetic rats. Medical Laboratory Journal 2022; 16(4): 39-46.
- Fujiwara Y, Eguchi S, Murayama H, Takahashi Y, Toda M, Imai K, et al. Relationship between diet/exercise and pharmacotherapy to enhance the GLP‐1 levels in type 2 diabetes. Endocrinology, Diabetes & Metabolism 2019; 2(3): 68-79.
- Frøsig C, Richter EA. Improved insulin sensitivity after exercise: focus on insulin signaling. Obesity 2009; 17(S3): 15-20.
- Saadeldeen FS, Niu Y, Wang H, Zhou L, Meng L, Chen S, et al. Natural products: regulating glucose metabolism and improving insulin resistance. Food Science and Human Wellness 2020; 9(3): 214-28.
- Heidari H, Azarbayjani MA, Peeri M, Farzanegi P, Hosseini SA. Health-boosting effects of aerobic exercise training and berberine on diabetes: A brief overview. Thrita 2021; 10(2): 25-38.
- Yaribeygi H, Jamialahmadi T, Moallem SA, Sahebkar A. Boosting GLP-1 by natural products. Adv Exp Med Biol. 2021; 1328: 513-522.
- Ghasemian M, Owlia S, Owlia MB. Review of anti-inflammatory herbal medicines. Advances in Pharmacological and Pharmaceutical Sciences 2016; 2016:59-71.
- Pedersen BK. Anti‐inflammatory effects of exercise: role in diabetes and cardiovascular disease. European Journal of Clinical Investigation 2017; 47(8): 600-11.
- Memme JM, Erlich AT, Phukan G, Hood DA. Exercise and mitochondrial health. The Journal of Physiology 2021; 599(3): 803-17.
- Trumbeckaite S, Benetis R, Bumblauskiene L, Burdulis D, Janulis V, Toleikis A, et al. Achillea millefolium L. sl herb extract: Antioxidant activity and effect on the rat heart mitochondrial functions. Food Chemistry 2011; 127(4): 1540-8.
- Wang C, Zhou J, Liu X, Zhu F, Li J, Xu W, et al. Quercetin enhances GLP‐1 secretion via TAS2R38‐mediated PLC signaling in enteroendocrine L‐Cells. Molecular Nutrition & Food Research 2025: 70109.
- Ansari P, Choudhury ST, Seidel V, Rahman AB, Aziz MA, Richi AE, et al. Therapeutic potential of quercetin in the management of type-2 diabetes mellitus. Life 2022; 12(8): 1146.
- Shahab F, Hameed A, Ali A, Imad R, Hafizur RM. Apigenin potentiates glucose-stimulated insulin secretion through the PKA-MEK kinase signaling pathway independent of K-ATP channels. Biomedicine & Pharmacotherapy 2024; 177: 116986.
Type of Study: Research |
Subject:
Genetics/ Biotechnology
Received: 2025/05/5 | Accepted: 2024/08/31 | Published: 2024/10/31
Received: 2025/05/5 | Accepted: 2024/08/31 | Published: 2024/10/31
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






