Tue, Feb 3, 2026
[Archive]
Volume 11, Issue 4 (November 2024)
IJML 2024, 11(4): 345-352 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Rabeie N, Vajihinejad M, Eslami S. Clinicopathological Characteristics and Survival Outcomes of Signet-Ring Cell Carcinoma: A Retrospective Study. IJML 2024; 11 (4) :345-352
URL: http://ijml.ssu.ac.ir/article-1-548-en.html
URL: http://ijml.ssu.ac.ir/article-1-548-en.html
Department of Pathology, Shahid Sadoughi Hospital, Shool of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
Full-Text [PDF 267 kb]
(452 Downloads)
| Abstract (HTML) (219 Views)
Discussion
References
Full-Text: (41 Views)
Introduction
Signet-ring cell carcinoma (SRCC) is a highly malignant subtype of adenocarcinoma characterized by the presence of signet-ring cells. Signet-ring cells are mucin-secreting tumor cells that have a unique morphology due to the accumulation of mucin. Since SRCC has a high potential for metastasis, especially in the peritoneal region, it is considered highly aggressive and often has a poor prognosis [1]. The epidemiology of SRCC indicates that it is relatively rare in the colorectal region, occurring in only 0.1% to 2.4% of all colorectal cancers [2]. However, when SRCC is diagnosed, it often presents at advanced stages. Therefore, a significant proportion of patients exhibit metastatic disease upon diagnosis [3].
SRCC mostly originates from the stomach or colon, but other organs such as the pancreas and ovary can also be the source of carcinoma [4]. SRCC from gastric or colorectal origins can metastasize to various organs, including the liver, peritoneum, and bones [5]. However, there are some reports of SRCC metastasizing to the breast, ovaries, and lungs, highlighting its potential for hematogenous spread [6, 7].
Additionally, studies have shown that the cumulative five-year survival rate for gastric SRCC is significantly lower compared to non-signet-ring cell carcinoma (NSRCC) of the stomach [1, 8]. Specifically, patients with gastric SRCC often experience a median survival time of less than one year after diagnosis, especially when diagnosed at an advanced stage [8]. Colorectal SRCC also exhibits poor survival rates. Research indicates that patients with colorectal cancers containing a signet-ring cell component have worse outcomes than those without this histological feature [3]. A retrospective study highlighted that even a minor signet-ring cell component in colorectal cancer is associated with significantly poorer survival rates [9].
In this study, we aimed to determine the clinical and pathological characteristics and anatomical sites involved in patients with SRCC and the survival time after the diagnosis of SRCC in a population of patients in Shahid Sadoughi Hospital. Recognizing the clinical symptoms and characteristics of SRCC can help physicians achieve early diagnosis, allowing for better intervention and patient outcomes.
Materials and Methods
Study design and included patients
The study employed a descriptive cross-sectional design on patients diagnosed with SRCC who were referred to the University Hospital of Shahid Sadoughi, Yazd, Iran, from January 2011 to December 2021. Patients above 18 years of age with a confirmed diagnosis of SRCC by an expert pathologist were enrolled. Patients with serious comorbidities, such as advanced heart disease, severe pulmonary conditions, or other chronic illnesses that could lead to death, were excluded from the study. Additionally, individuals who had been admitted to the intensive care unit and those with immunocompromised status were not included. Medical records with significant missing data were also excluded to ensure the integrity of the results.
Procedures
Two independent investigators reviewed the patients’ medical records and extracted their demographic data, anatomical position of the carcinoma, and their clinical symptoms. Then a third investigator, our validated pathologist, finalized the extracted data and reached consensus with the two primary investigators. One preassigned staff member called the patients or their relatives using the phone number in their medical record and asked them about the duration of survival after the primary diagnosis at the hospital.
Statistical analysis
All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) version 24.0 for Windows (SPSS, Chicago, IL, USA). Descriptive statistics were used to evaluate the frequency distribution of patients’ characteristics according to the anatomical position of the carcinoma. The Kaplan-Meier method was used to calculate survival probabilities, and the survival curves were compared using the log-rank test.
Results
Clinicopathological characteristics
143 patients (53.84% men and 46.15% women) were included in this study. Clinical characteristics of the study population are presented in Table 1. The mean age of all patients was 55.15±16.29, ranging from 20 to 92 years. The highest proportion of cases across all anatomical sites was observed in the 41–60 and >60 age groups, except for those involving the rectum. The most frequent site of SRCC in the study population was the stomach, accounting for 76.92% of the cases. Esophagus (9.1%), lung (7.7%), colon (7%), rectum (3.4%), breast (3.4%), and intestine (1.4%) were other sites involved with SRCC. Abdominal pain was the most commonly reported symptom, affecting 36.3% of the patients. Dyspepsia and gastrointestinal (GI) obstruction were the next two most frequent, reported by 16.8% and 14% of the patients, respectively, while vomiting was the least observed clinical symptom, present in only 1.39% of cases.
Survival analysis
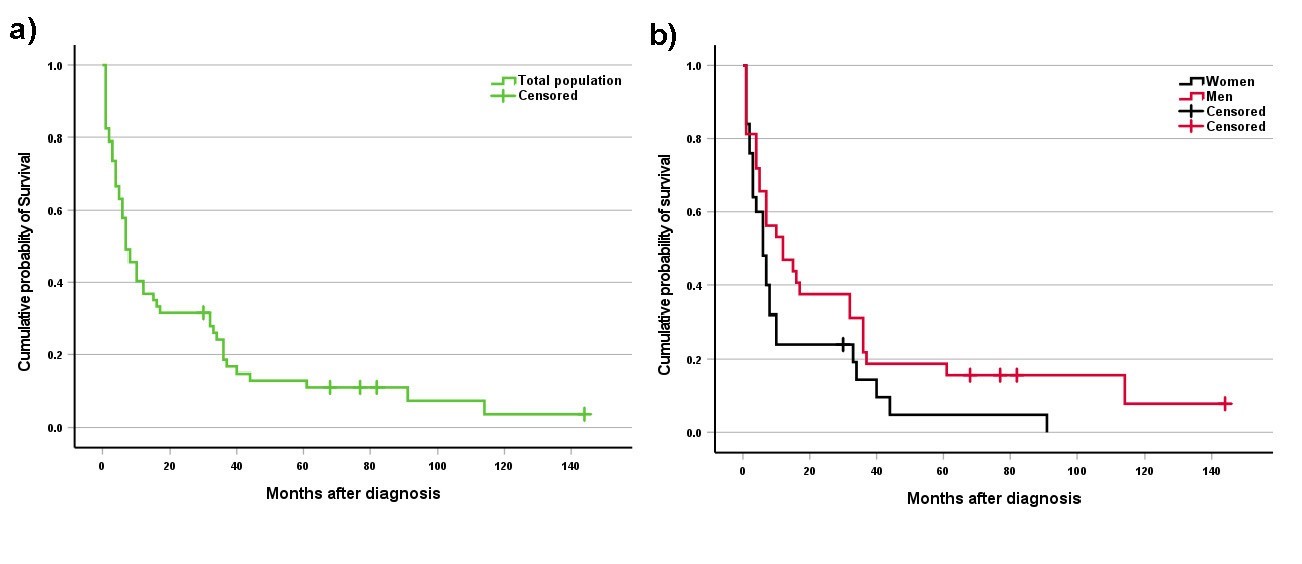
The survival data were not available for 86 participants. The survival duration following primary diagnosis was recorded for 57 patients. The overall median survival time was 7 months after the initial diagnosis of SRCC. The median survival time was 12 and 6 months for subgroups of men and women, respectively. There was a notable difference between men and women; however, this difference did not reach statistical significance (p =0.07). Analysis revealed that the survival rates were 40% at 1 year, 32% at 3 years, and 24% at 5 years. The survival curves of the SRCC-diagnosed patients are presented in Figure 1.
SRCC mostly originates from the stomach or colon, but other organs such as the pancreas and ovary can also be the source of carcinoma [4]. SRCC from gastric or colorectal origins can metastasize to various organs, including the liver, peritoneum, and bones [5]. However, there are some reports of SRCC metastasizing to the breast, ovaries, and lungs, highlighting its potential for hematogenous spread [6, 7].
Additionally, studies have shown that the cumulative five-year survival rate for gastric SRCC is significantly lower compared to non-signet-ring cell carcinoma (NSRCC) of the stomach [1, 8]. Specifically, patients with gastric SRCC often experience a median survival time of less than one year after diagnosis, especially when diagnosed at an advanced stage [8]. Colorectal SRCC also exhibits poor survival rates. Research indicates that patients with colorectal cancers containing a signet-ring cell component have worse outcomes than those without this histological feature [3]. A retrospective study highlighted that even a minor signet-ring cell component in colorectal cancer is associated with significantly poorer survival rates [9].
In this study, we aimed to determine the clinical and pathological characteristics and anatomical sites involved in patients with SRCC and the survival time after the diagnosis of SRCC in a population of patients in Shahid Sadoughi Hospital. Recognizing the clinical symptoms and characteristics of SRCC can help physicians achieve early diagnosis, allowing for better intervention and patient outcomes.
Materials and Methods
Study design and included patients
The study employed a descriptive cross-sectional design on patients diagnosed with SRCC who were referred to the University Hospital of Shahid Sadoughi, Yazd, Iran, from January 2011 to December 2021. Patients above 18 years of age with a confirmed diagnosis of SRCC by an expert pathologist were enrolled. Patients with serious comorbidities, such as advanced heart disease, severe pulmonary conditions, or other chronic illnesses that could lead to death, were excluded from the study. Additionally, individuals who had been admitted to the intensive care unit and those with immunocompromised status were not included. Medical records with significant missing data were also excluded to ensure the integrity of the results.
Procedures
Two independent investigators reviewed the patients’ medical records and extracted their demographic data, anatomical position of the carcinoma, and their clinical symptoms. Then a third investigator, our validated pathologist, finalized the extracted data and reached consensus with the two primary investigators. One preassigned staff member called the patients or their relatives using the phone number in their medical record and asked them about the duration of survival after the primary diagnosis at the hospital.
Statistical analysis
All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) version 24.0 for Windows (SPSS, Chicago, IL, USA). Descriptive statistics were used to evaluate the frequency distribution of patients’ characteristics according to the anatomical position of the carcinoma. The Kaplan-Meier method was used to calculate survival probabilities, and the survival curves were compared using the log-rank test.
Results
Clinicopathological characteristics
143 patients (53.84% men and 46.15% women) were included in this study. Clinical characteristics of the study population are presented in Table 1. The mean age of all patients was 55.15±16.29, ranging from 20 to 92 years. The highest proportion of cases across all anatomical sites was observed in the 41–60 and >60 age groups, except for those involving the rectum. The most frequent site of SRCC in the study population was the stomach, accounting for 76.92% of the cases. Esophagus (9.1%), lung (7.7%), colon (7%), rectum (3.4%), breast (3.4%), and intestine (1.4%) were other sites involved with SRCC. Abdominal pain was the most commonly reported symptom, affecting 36.3% of the patients. Dyspepsia and gastrointestinal (GI) obstruction were the next two most frequent, reported by 16.8% and 14% of the patients, respectively, while vomiting was the least observed clinical symptom, present in only 1.39% of cases.
Survival analysis
The survival data were not available for 86 participants. The survival duration following primary diagnosis was recorded for 57 patients. The overall median survival time was 7 months after the initial diagnosis of SRCC. The median survival time was 12 and 6 months for subgroups of men and women, respectively. There was a notable difference between men and women; however, this difference did not reach statistical significance (p =0.07). Analysis revealed that the survival rates were 40% at 1 year, 32% at 3 years, and 24% at 5 years. The survival curves of the SRCC-diagnosed patients are presented in Figure 1.
Table 1. Clinical characteristics of the study population based on the anatomical position of Signet-ring cell carcinoma
| Variable | Anatomical position | |||||||
| Esophagus | Stomach | Small intestine | Colon | Rectum | Lung | Breast | Total | |
| N | 13 | 97 | 2 | 10 | 5 | 11 | 5 | 143 |
| Age | ||||||||
| ≤40 | 1 (3.6) | 20 (71.4) | 0 (0) | 2 (7.1) | 3 (10.7) | 2 (7.1) | 0 (0) | 28 |
| 41-60 | 3 (5.4) | 40 (71.4) | 1 (1.8) | 5 (8.9) | 1 (1.8) | 3 (5.4) | 3 (5.4) | 56 |
| >60 | 9 (15.3) | 37 (62.7) | 1 (1.7) | 3 (5.1) | 1 (1.7) | 6 (10.2) | 2 (3.4) | 59 |
| Sex | ||||||||
| Male | 7 (9.1) | 54 (70.1) | 0 (0) | 5 (6.5) | 3 (3.9) | 8 (10.4) | 0 (0) | 77 |
| Female | 6 (9.1) | 43 (65.2) | 2 (3) | 5 (7.6) | 2 (3.0) | 3 (4.5) | 5 (7.5) | 66 |
| Clinical symptom | ||||||||
| Dysphagia | 12 (100) | - | - | - | - | - | - | 12 |
| Gastrointestinal bleeding | - | 7 (41.2) | - | 5 (29.4) | 5 (29.4) | - | - | 17 |
| Gastrointestinal obstruction | - | 20 (100) | - | - | - | - | - | 20 |
| Vomiting | - | 2 (100) | - | - | - | - | - | 2 |
| Abdominal pain | - | 45 (86.5) | 2 (3.8) | 5 (9.6) | - | - | - | 52 |
| Dyspepsia | 1 (4.2) | 23 (95.8) | - | - | - | - | - | 24 |
| Dyspnea | - | - | - | - | - | 8 (100) | - | 8 |
| Cough | - | - | - | - | - | 3 (100) | - | 3 |
| Mass palpation | - | - | - | - | - | - | 5 (100) | 5 |
Data are presented as a number (%)

Fig. 1. Kaplan-Meier Survival Analysis of Signet Ring Cell Carcinoma patients. a) Overall survival probability for the total study population. b) Comparative survival analysis between male and female patients.
Discussion
Our study included 143 patients diagnosed with SRCC, with a mean age of 55.15 years and 53.84% being male. Similar age and sex distributions have been reported in previous studies. For example, Kown et al. described the clinicopathological features of 51 patients with gastric SRCC, reporting a mean age of 55.5 ± 11.5 years and a male proportion of 51% [10]. Given the relative rarity of SRCC, conducting studies with large sample sizes remains a significant challenge.
Our findings revealed that most patients were over 60 years of age; however, a significant portion of colorectal SRCC cases occurred in individuals younger than 40. This finding contrasts with earlier studies, which typically reported a mean or median age between 50 and 70 years for colorectal SRCC patients [11]. Despite this, recent literature suggests a shifting trend. Several studies have shown that SRCC, particularly in the stomach and colon, tends to present at a younger age compared to NSRCCs in the same anatomical regions [12, 13]. Moreover, a study by Tawadros et al. reported a 3.6% annual increase since 2010 in the incidence of colorectal SRCC among patients under 40 years of age [14], which may partially explain the younger age distribution observed in our study. Although this trend is not universally supported, it highlights a possible evolving epidemiological pattern that needs further investigation.
In our study, the stomach was identified as the most commonly affected site, accounting for 76.92% of cases, followed by the esophagus, lung, colon, rectum, breast, and small intestine. Similarly, Wu et al. analyzed a large cohort of over 24,000 patients and reported that the stomach was the primary site of SRCC in 63.4% of cases [15]. Their study also documented a higher proportion of colorectal SRCC cases compared to ours and identified less common sites such as the pancreas, bladder, and gallbladder. These discrepancies are likely attributable to the significantly larger sample size in their study, which allowed for the identification of a broader range of anatomical sites affected by SRCC. Despite these insights, the origin and underlying molecular mechanisms driving SRCC development remain poorly understood. However, consistent with previous literature, our findings reinforce that gastrointestinal organs, particularly the stomach, are the most frequently involved sites, and that metastases often arise from these primary locations [1, 4, 15]. Further research into the molecular pathways involved in SRCC is essential to better understand its origin and pathogenesis.
In our study, the most commonly observed clinical symptoms of SRCC were abdominal pain, dyspepsia, and GI obstruction. Gastric SRCC typically presented with abdominal pain, dyspepsia, and GI obstruction, while SRCC involving the colon, intestine, and esophagus was often associated with abdominal pain or GI bleeding. These findings are consistent with previous studies, which have shown that gastric SRCC often presents with abdominal pain, dysphagia, early satiety, and weight loss [16, 17]. Tumors located in the rectum may cause rectal bleeding, changes in bowel habits, and tenesmus, while SRCC lesions in the colon are associated with abdominal discomfort, weight loss, and anemia [18, 19]. Since the clinical symptoms of SRCC are closely tied to tumor location and progression stage, many patients exhibit minimal or no symptoms in the early phases, delaying diagnosis until the disease has advanced [4]. These findings highlight the critical importance of early detection and diagnosis in improving outcomes for SRCC patients.
The survival rates of patients after 1, 3, and 5 years were 40%, 32%, and 24%, respectively. The median length of survival was 7, 12, and 6 months for the total population, men, and women, respectively. The subgroup of men had a longer survival time compared to women; however, statistical significance was not reached. One previous study reported the median survival rate of SRCC patients was 12 months, and 1-, 3-, and 5-year survival rates were 49.5%, 28.9% and 24%, respectively [15]. Recent evidence indicates that SRCC of the stomach and colon has a worse prognosis and less survival time compared to NSRCC [20, 21]. Overall, the prognosis for SRCC across its various types remains challenging, characterized by high mortality rates and limited survival time, particularly in advanced cases. Effective management strategies focused on early detection and tailored chemotherapy have been shown to slightly improve survival rates [22].
Although we reviewed the patients’ records over 10 years, we encountered several limitations in this study. First, our sample size was constrained due to the rarity of SRCC, and studies on bigger populations can be performed. Second, in the case of survival analysis, we couldn’t access a significant number of patients due to missing or incorrect phone numbers. Third, this study lacks a comparative analysis of SRCC with other types of carcinomas, and future studies can be carried out using this method.
Conclusion
This retrospective study provides a descriptive overview of the clinicopathological characteristics, anatomical distribution, clinical presentation, and survival outcomes of patients diagnosed with SRCC over ten years. Our findings confirm that the stomach is the most commonly affected organ, and abdominal pain is the predominant symptom. The disease predominantly affects individuals over the age of 60, with a slight male predominance. The overall prognosis for SRCC remains poor, with a median survival time of 7 months and a 5-year survival rate of only 24%. These results highlight the aggressive nature of SRCC and underscore the need for improved strategies in early detection and treatment. Future studies with larger cohorts and comparative analyses are necessary to further understand the behavior of SRCC and to optimize patient management.
Ethical Considerations
The study was performed according to the Declaration of Helsinki, and the study design was approved by Shahid Sadoughi University's ethics committee (IR.SSU.MEDICINE.REC.1401.161). We obtained informed consent from each patient or their relatives to use their data for educational or research purposes. No personal data was used at the time of data extraction.
Funding Statement
This study was conducted without any external funding or financial support.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
We would like to express our deepest gratitude to our colleagues at Shahid Sadoughi Hospital, especially the Department of Pathology, for their invaluable support. We are also profoundly thankful to all participants for agreeing to take part in this study. Additionally, we acknowledge the use of a large language model (ChatGPT, developed by OpenAI, GPT-4, 2025) for assistance with grammar correction during manuscript preparation; the tool was not used for data analysis, interpretation, or the generation of original scientific content.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Authors' Contributions
N.R. was responsible for assembling data, conducting the literature review, providing feedback on the manuscripts. M.V. was responsible for writing the manuscript and improving the interpretation of the results. S.E. was responsible for designing the review protocol, writing the manuscript, assembling data, analyzing data, and interpreting analyses.
Our findings revealed that most patients were over 60 years of age; however, a significant portion of colorectal SRCC cases occurred in individuals younger than 40. This finding contrasts with earlier studies, which typically reported a mean or median age between 50 and 70 years for colorectal SRCC patients [11]. Despite this, recent literature suggests a shifting trend. Several studies have shown that SRCC, particularly in the stomach and colon, tends to present at a younger age compared to NSRCCs in the same anatomical regions [12, 13]. Moreover, a study by Tawadros et al. reported a 3.6% annual increase since 2010 in the incidence of colorectal SRCC among patients under 40 years of age [14], which may partially explain the younger age distribution observed in our study. Although this trend is not universally supported, it highlights a possible evolving epidemiological pattern that needs further investigation.
In our study, the stomach was identified as the most commonly affected site, accounting for 76.92% of cases, followed by the esophagus, lung, colon, rectum, breast, and small intestine. Similarly, Wu et al. analyzed a large cohort of over 24,000 patients and reported that the stomach was the primary site of SRCC in 63.4% of cases [15]. Their study also documented a higher proportion of colorectal SRCC cases compared to ours and identified less common sites such as the pancreas, bladder, and gallbladder. These discrepancies are likely attributable to the significantly larger sample size in their study, which allowed for the identification of a broader range of anatomical sites affected by SRCC. Despite these insights, the origin and underlying molecular mechanisms driving SRCC development remain poorly understood. However, consistent with previous literature, our findings reinforce that gastrointestinal organs, particularly the stomach, are the most frequently involved sites, and that metastases often arise from these primary locations [1, 4, 15]. Further research into the molecular pathways involved in SRCC is essential to better understand its origin and pathogenesis.
In our study, the most commonly observed clinical symptoms of SRCC were abdominal pain, dyspepsia, and GI obstruction. Gastric SRCC typically presented with abdominal pain, dyspepsia, and GI obstruction, while SRCC involving the colon, intestine, and esophagus was often associated with abdominal pain or GI bleeding. These findings are consistent with previous studies, which have shown that gastric SRCC often presents with abdominal pain, dysphagia, early satiety, and weight loss [16, 17]. Tumors located in the rectum may cause rectal bleeding, changes in bowel habits, and tenesmus, while SRCC lesions in the colon are associated with abdominal discomfort, weight loss, and anemia [18, 19]. Since the clinical symptoms of SRCC are closely tied to tumor location and progression stage, many patients exhibit minimal or no symptoms in the early phases, delaying diagnosis until the disease has advanced [4]. These findings highlight the critical importance of early detection and diagnosis in improving outcomes for SRCC patients.
The survival rates of patients after 1, 3, and 5 years were 40%, 32%, and 24%, respectively. The median length of survival was 7, 12, and 6 months for the total population, men, and women, respectively. The subgroup of men had a longer survival time compared to women; however, statistical significance was not reached. One previous study reported the median survival rate of SRCC patients was 12 months, and 1-, 3-, and 5-year survival rates were 49.5%, 28.9% and 24%, respectively [15]. Recent evidence indicates that SRCC of the stomach and colon has a worse prognosis and less survival time compared to NSRCC [20, 21]. Overall, the prognosis for SRCC across its various types remains challenging, characterized by high mortality rates and limited survival time, particularly in advanced cases. Effective management strategies focused on early detection and tailored chemotherapy have been shown to slightly improve survival rates [22].
Although we reviewed the patients’ records over 10 years, we encountered several limitations in this study. First, our sample size was constrained due to the rarity of SRCC, and studies on bigger populations can be performed. Second, in the case of survival analysis, we couldn’t access a significant number of patients due to missing or incorrect phone numbers. Third, this study lacks a comparative analysis of SRCC with other types of carcinomas, and future studies can be carried out using this method.
Conclusion
This retrospective study provides a descriptive overview of the clinicopathological characteristics, anatomical distribution, clinical presentation, and survival outcomes of patients diagnosed with SRCC over ten years. Our findings confirm that the stomach is the most commonly affected organ, and abdominal pain is the predominant symptom. The disease predominantly affects individuals over the age of 60, with a slight male predominance. The overall prognosis for SRCC remains poor, with a median survival time of 7 months and a 5-year survival rate of only 24%. These results highlight the aggressive nature of SRCC and underscore the need for improved strategies in early detection and treatment. Future studies with larger cohorts and comparative analyses are necessary to further understand the behavior of SRCC and to optimize patient management.
Ethical Considerations
The study was performed according to the Declaration of Helsinki, and the study design was approved by Shahid Sadoughi University's ethics committee (IR.SSU.MEDICINE.REC.1401.161). We obtained informed consent from each patient or their relatives to use their data for educational or research purposes. No personal data was used at the time of data extraction.
Funding Statement
This study was conducted without any external funding or financial support.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
We would like to express our deepest gratitude to our colleagues at Shahid Sadoughi Hospital, especially the Department of Pathology, for their invaluable support. We are also profoundly thankful to all participants for agreeing to take part in this study. Additionally, we acknowledge the use of a large language model (ChatGPT, developed by OpenAI, GPT-4, 2025) for assistance with grammar correction during manuscript preparation; the tool was not used for data analysis, interpretation, or the generation of original scientific content.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Authors' Contributions
N.R. was responsible for assembling data, conducting the literature review, providing feedback on the manuscripts. M.V. was responsible for writing the manuscript and improving the interpretation of the results. S.E. was responsible for designing the review protocol, writing the manuscript, assembling data, analyzing data, and interpreting analyses.
References
- Mizushima T, Nomura M, Fujii M, Akamatsu H, Mizuno H, Tominaga H, et al. Primary colorectal signet-ring cell carcinoma: Clinicopathological features and postoperative survival. Surgery Today 2010; 40(3): 234-38.
- Tanaka Y, Chino O, Kajiwara H, Hanashi T, Nakamura T, Makuuchi H. A rare case of esophageal metastasis from signet-ring cell carcinoma of the cecum. Surgical Case Reports 2023; 9(1): 186.
- Bhawani S, Mubashira H. Increasing incidence of colorectal carcinoma in younger age group in our population. J Muhammad Med Coll. 2016; 6(2): 34-7.
- Shoji T, Takeshita R, Saito T, Aida T, Sasou S, Baba T. A case of primary ovarian signet-ring cell carcinoma treated with S-1/CDDP therapy. Journal of Ovarian Research 2020; 13(1): 33.
- Iwai N, Okuda T, Harada T, Oka K, Hara T, Inada Y, et al. Gastric metastasis from colorectal cancer mimicking a submucosal tumor. Case Reports in Gastroenterology 2020; 14(2): 338-45.
- Buerba-Vieregge HH, Fernández-Ferreira R, Soberanis-Piña PD, Ildefonso Roberto De la P-L, Navarro-García LM, Macari-Jorge A. Breast metastasis of gastric signet ring cell carcinoma: A case report and literature review. Case Reports in Oncology 2021; 14(1): 165-72.
- Castro CY, Moran CA, Flieder DG, Suster S. Primary signet ring cell adenocarcinomas of the lung: A clinicopathological study of 15 cases. Histopathology 2001; 39(4): 397-401.
- Jiang C, Wang Z, Sun Z, Liu F, Yu M, Xu H. Clinicopathologic characteristics and prognosis of signet ring cell carcinoma of the stomach: Results from a Chinese mono‐institutional study. Journal of Surgical Oncology 2011; 103(7): 700-703.
- Tan Y, Fu J, Li X, Jiao Y, Jiang M, Ding K, et al. A minor signet-ring cell component associated with poor prognosis in colorectal cancer patients: A 26-year retrospective study in China. Plos One 2015; 10(3): 121944.
- Kwon KJ, Shim KN, Song EM, Choi JY, Kim SE, Jung HK, et al. Clinicopathological characteristics and prognosis of signet ring cell carcinoma of the stomach. Gastric Cancer 2014; 17(1): 43-53.
- An Y, Zhou J, Lin G, Wu H, Cong L, Li Y, et al. Clinicopathological and molecular characteristics of colorectal signet ring cell carcinoma: a review. Pathology and Oncology Research 2021; 27: 1609859.
- Kumar M, Tata MD, Lah NA. A case of rectal cancers in teenager: A conundrum of genetics and clinical medicine. Annals of Medicine and Surgery 2021; 65: 102353.
- Weng MT, Chao KH, Tung CC, Chang HC, Shih IL, Lin BR, et al. Characteristics of primary signet ring cell carcinoma of colon and rectum: A case control study. BMC Gastroenterol. 2022; 22(1): 173.
- Tawadros PS, Paquette IM, Hanly AM, Mellgren AF, Rothenberger DA, Madoff RD. Adenocarcinoma of the rectum in patients under age 40 is increasing: Impact of signet-ring cell histology. Diseases of the Colon & Rectum 2015; 58(5): 474-78.
- Wu SG, Chen XT, Zhang WW, Sun JY, Li FY, He ZY, et al. Survival in signet ring cell carcinoma varies based on primary tumor location: A surveillance, epidemiology, and end results database analysis. Expert Review of Gastroenterology & Hepatology 2018; 12(2): 209-14.
- Lu M, Yang ZY, Feng Q, Mei Y, Zhang Y, Mao C, et al. The characteristics and prognostic value of signet ring cell histology in gastric cancer. Medicine 2016; 95(27): 4052.
- Hass HG, Smith U, Jäger C, Schäffer M, Wellhäußer U, Hehr T, et al. Signet ring cell carcinoma of the stomach is significantly associated with poor prognosis and diffuse gastric cancer (Lauren’s): Single-center experience of 160 cases. Onkologie 2011; 34(12): 682-86.
- Shi T, Huang M, Han D, Tang X, Chen Y, Li Z, et al. Chemotherapy is associated with increased survival from colorectal signet ring cell carcinoma with distant metastasis: A Surveillance, epidemiology, and end results database analysis. Cancer Medicine 2019; 8(4): 1930-940.
- Hugen N, Verhoeven RHA, Lemmens VEPP, Aart CJV, Elferink MAG, Radema SA, et al. Colorectal signet-ring cell carcinoma: benefit from adjuvant chemotherapy but a poor prognostic factor. International Journal of Cancer 2014; 136(2): 333-39.
- Zhao S, Lv L, Zheng K, Tian Y, Zheng JC, Jiang CG. Prognosis and biological behavior of gastric signet-ring cell carcinoma better or worse: A meta-analysis. Frontiers in Oncology 2021; 11: 603070.
- Fadel MG, Malietzis G, Constantinides V, Pellino G, Tekkis P, Kontovounisios C. Clinicopathological factors and survival outcomes of signet-ring cell and mucinous carcinoma versus adenocarcinoma of the colon and rectum: A systematic review and meta-analysis. Discover Oncology 2021; 12(1): 5-12.
- Yang L, Wang M, He P. Clinicopathological characteristics and survival in colorectal signet ring cell carcinoma: A population-based study. Scientific Reports 2020; 10(1): 10460.
Type of Study: Research |
Subject:
Pathology
Received: 2025/05/20 | Accepted: 2025/08/19 | Published: 2024/10/31
Received: 2025/05/20 | Accepted: 2025/08/19 | Published: 2024/10/31
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






